First Occurrence of Coffee Leaf Rust Caused by Hemileia vastatrix on Coffee in Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Morphological Identification
2.2. PCR Amplification and Sequencing
2.3. Phylogenetic Analysis
2.4. Pathogenicity Tests
3. Results
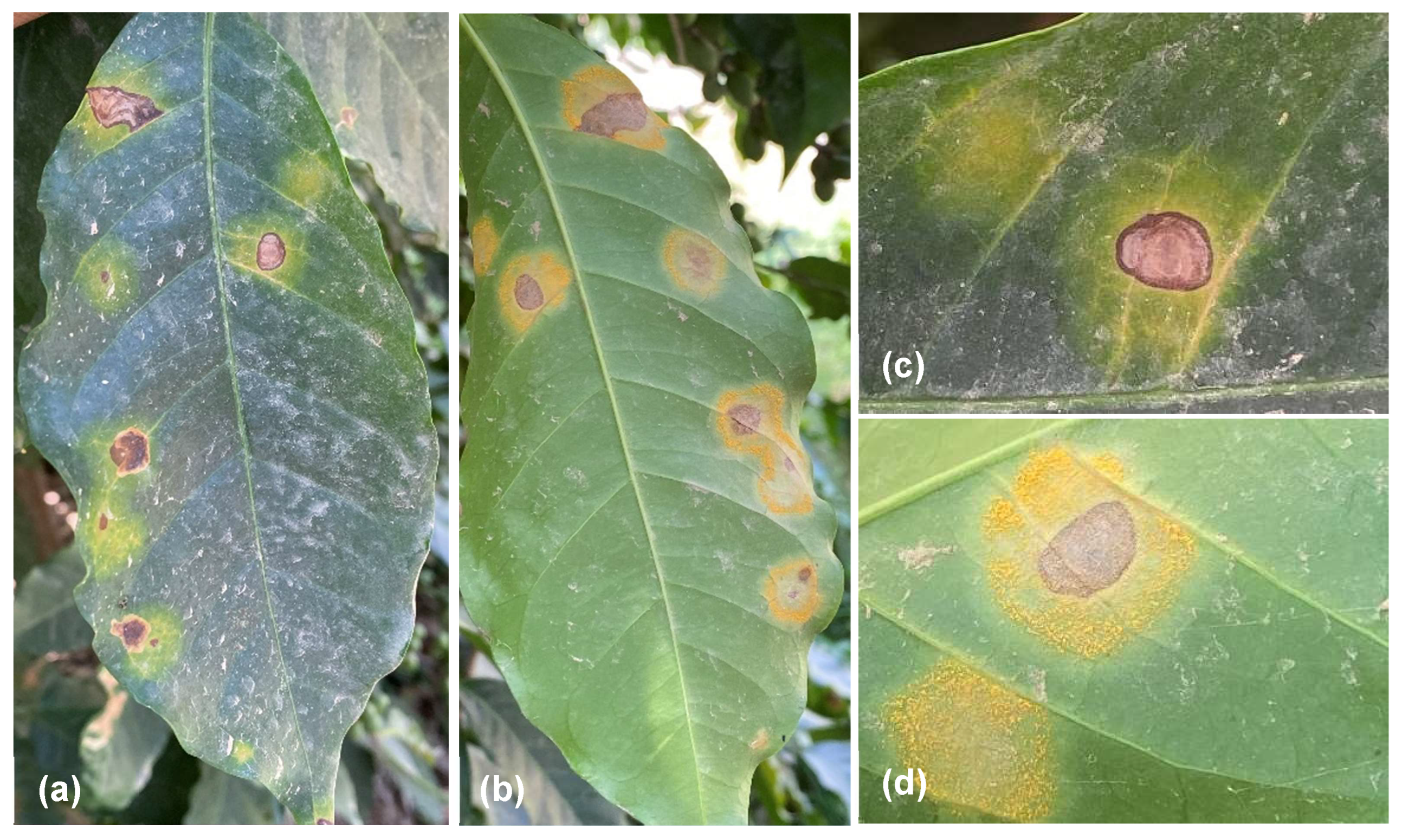
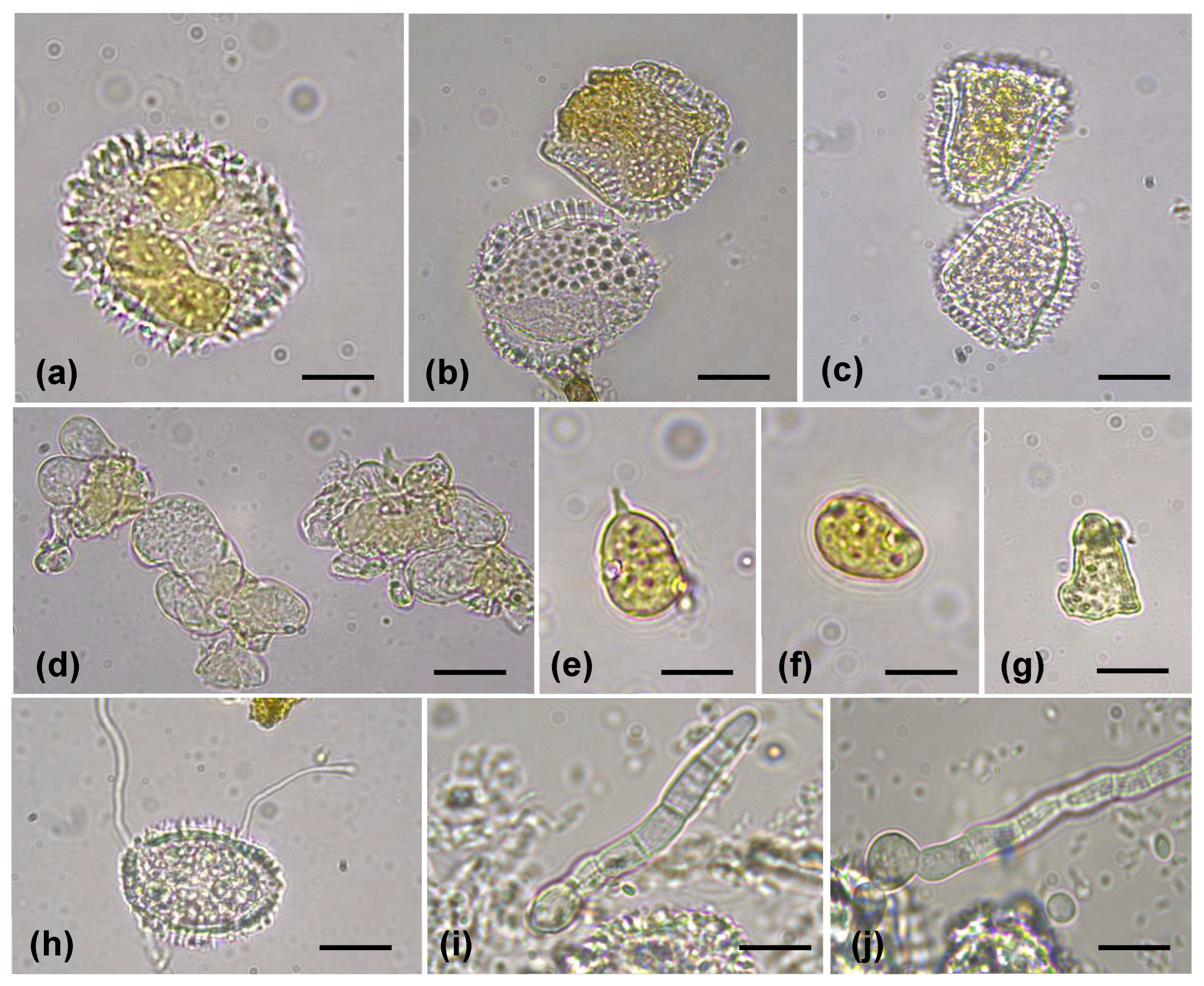
3.1. Survey and Morphological Identification
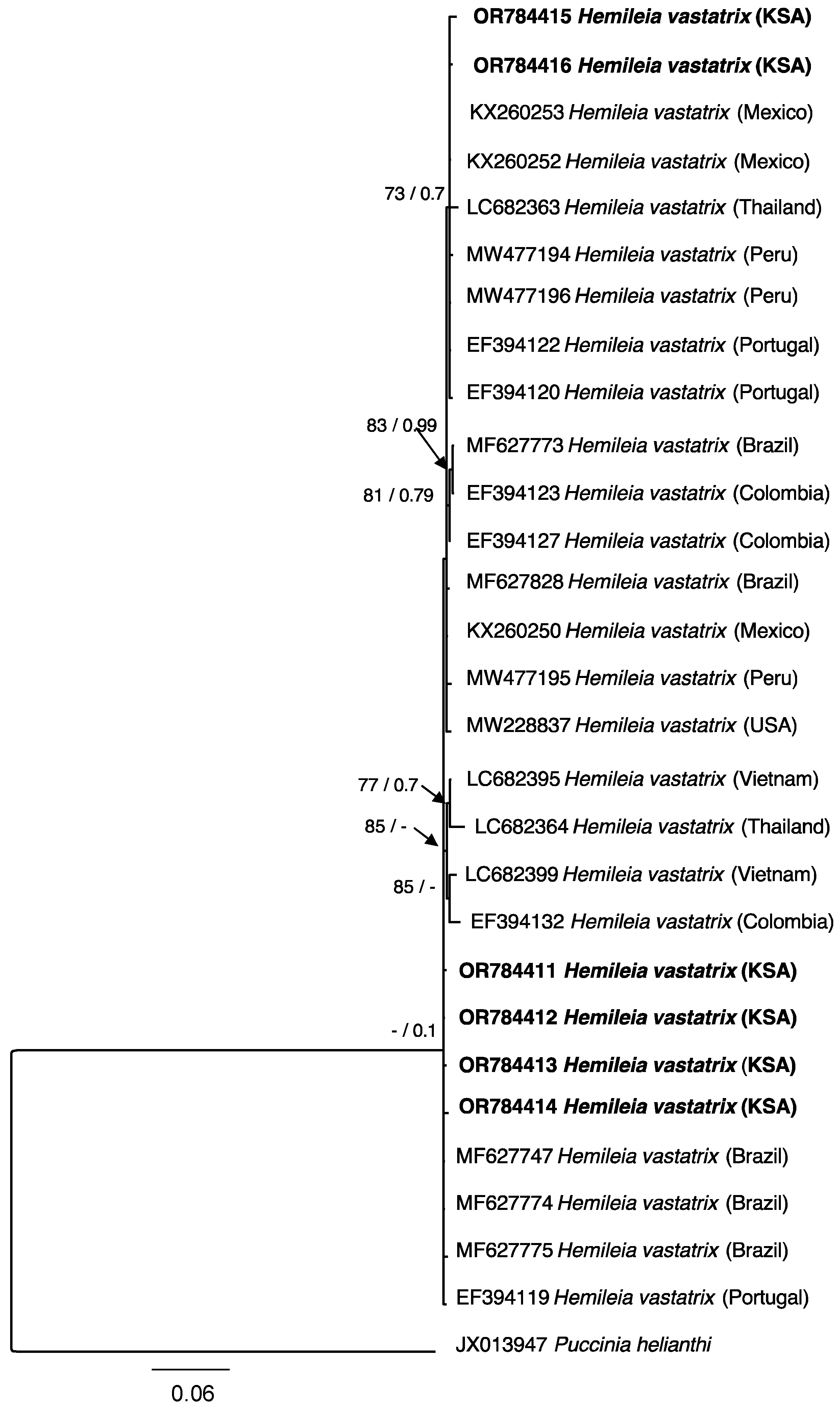
3.2. Phylogenetic Analysis
3.3. Pathogenicity Tests
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govaerts, R.; Ruhsam, M.; Andersson, L.; Robbrecht, E.; Bridson, D.M.; Davis, A.P.; Schanzer, I.; Sonké, B. World Checklist of Rubiaceae. Royal Botanic Gardens, Kew. 2011. Available online: http://www.kew.org/wcsp/rubiaceae (accessed on 22 September 2023).
- Sahachairungrueng, W.; Meechan, C.; Veerachat, N.; Thompson, A.K.; Teerachaichayut, S. Assessing the Levels of Robusta and Arabica in Roasted Ground Coffee Using NIR Hyperspectral Imaging and FTIR Spectroscopy. Foods 2022, 11, 3122. [Google Scholar] [CrossRef] [PubMed]
- Montagnon, C.; Rossi, V.; Guercio, C.; Sheibani, F. Vernacular Names and Genetics of Cultivated Coffee (Coffea arabica) in Yemen. Agronomy 2022, 12, 1970. [Google Scholar] [CrossRef]
- Tounekti, T.; Mosbah, M.; Al-Turki, T.; Khemira, H. Genetic diversity analysis of Coffee (Coffea arabica L.) germplasm accessions growing in the Southwestern Saudi Arabia using quantitative traits. Nat. Resour. 2017, 8, 321–336. [Google Scholar]
- Al-Asmari, K.; Abu Zeid, I.; Al-Attar, A. Coffee Arabica in Saudi Arabia: An Overview. Int. J. Pharm. Phytopharm. Res. 2020, 10, 71–78. [Google Scholar]
- Lewin, B.; Giovannucci, D.; Varangis, P. Coffee Markets: New Paradigms in Global Supply and Demand; (SSRN Scholarly Paper No. ID 996111); Social Science Research Network: Rochester, NY, USA, 2004. [Google Scholar]
- Al-Turki, T.A. An initiative in exploration and management of plant genetic diversity in Saudi Arabia. In Managing Plant Genetic Diversity, Proceedings of the International Conference, Kuala Lumpur, Malaysia, 12–16 June 2000; CABI Publishing: Wallingford, UK, 2002; pp. 339–349. [Google Scholar]
- Tounekti, T.; Mahdhi, M.; Al-Turki, T.A.; Khemira, H. Water relations and photo-protection mechanisms during drought stress in four coffee (Coffea arabica) cultivars from southwestern Saudi Arabia. S. Afr. J. Bot. 2018, 117, 17–25. [Google Scholar] [CrossRef]
- Harvey, C.A.; Rakotobe, Z.L.; Rao, N.S.; Dave, R.; Razafimahatratra, H.; Rabarijohn, R.H.; Rajaofara, H.; MacKinnon, J.L. Extreme vulnerability of smallholder farmers to agricultural risks and climate change in Madagascar. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130089. [Google Scholar] [CrossRef] [PubMed]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Chang. 2019, 156, 609–630. [Google Scholar] [CrossRef]
- Waller, J.M. Coffee rust-epidemiology and control. Crop Prot. 1982, 1, 385–404. [Google Scholar] [CrossRef]
- Waller, J.M.; Bridge, P.D.; Black, R.; Hakiza, G. Characterization of the coffee berry disease pathogen, Colletotrichum kahawae sp. nov. Mycol. Res. 1993, 97, 989–994. [Google Scholar] [CrossRef]
- Adugna, G.; Million, A.; Hindorf, H.; Zeru, A.; Teferi, D.; Jefuka, C. Coffee wilt Disease in Ethiopia; CAB International: London, UK, 2010. [Google Scholar]
- Talhinhas, P.; Batista, D.; Diniz, I.; Vieira, A.; Silva, D.N.; Loureiro, A.; Tavares, S.; Pereira, A.P.; Azinheira, H.G.; Guerra-Guimarães, L.; et al. The coffee leaf rust pathogen Hemileia vastatrix: One and a half centuries around the tropics. Mol. Plant Pathol. 2017, 18, 1039–1051. [Google Scholar] [CrossRef]
- Berkeley, M.J.; Broome, C.E. Hemileia vastatrix. Gard. Chron. 1869, 6, 1157. [Google Scholar]
- Morris, D. Note on the structure and habit of Hemileia vastatrix, the coffee-leaf disease of Ceylon and Southern India 1880. J. Linn. Soc. Bot. 1880, 17, 512–517. [Google Scholar] [CrossRef]
- Gichuru, E.; Alwora, G.; Gimase, J.; Kathurima, C. Coffee Leaf Rust (Hemileia vastatrix) in Kenya—A Review. Agronomy 2021, 11, 2590. [Google Scholar] [CrossRef]
- Alhudaib, K.; Ismail, A.M.; Magistà, D. Multi-Locus Phylogenetic Analysis Revealed the Association of Six Colletotrichum Species with Anthracnose Disease of Coffee (Coffea arabica L.) in Saudi Arabia. J. Fungi 2023, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- AL-Faifi, Z.; Alsolami, W.; Abada, E.; Khemira, H.; Almalki, G.; Modafer, Y. Fusarium oxysporum and Colletotrichum musae Associated with Wilt Disease of Coffea arabica in Coffee Gardens in Saudi Arabia. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3050495. [Google Scholar] [CrossRef] [PubMed]
- El-Komy, M.H.; Alsubaie, M.; Ibrahim, Y.E.; Sharafaddin, A.H.; Al-Saleh, M.A. First Report of Colletotrichum gloeosporioides and Colletotrichum siamense Causing Anthracnose Disease on Coffee Trees in Saudi Arabia. Plant Dis. 2023, 107, 3297. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, L.F.; Johnson, M.A. Monitoring Coffee Leaf Rust (Hemileia vastatrix) on Commercial Coffee Farms in Hawaii: Early Insights from the First Year of Disease Incursion. Agronomy 2022, 12, 1134. [Google Scholar] [CrossRef]
- Gamarra Gamarra, D.P.; Torres Suarez, G.; Villar Quiñonez, C.M.; McTaggart, A.R.; Carrasco Lozano, E.C. Phylogenetic relationship of coffee leaf rust in the central jungle of Peru. Acta Agronómica 2021, 70, 155–162. [Google Scholar] [CrossRef]
- Le, C.T.M.; Okane, I.; Ono, Y.; Tsuda, Y.; Yamaoka, Y. Incidence of Coffee Leaf Rust in Vietnam, Possible Original Sources and Subsequent Pathways of Migration. Front. Plant Sci. 2022, 13, 872877. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A Plant DNA Minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. Guid. Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.M.; Sugiyama, L.S.; Brill, E.; Adams, B.L.; Fukada, M.; Hoffman, K.M.; Ocenar, J.; Kawabata, A.; Kong, A.T.; McKemy, J.M.; et al. First report of coffee leaf rust caused by Hemileia vastatrix on coffee (Coffea arabica) in Hawaii. Plant Dis. 2022, 106, 761. [Google Scholar] [CrossRef] [PubMed]
- Julca-Otiniano, A.; Borjas-Ventura, R.; Alvarado-Huamán, L.; Julca-Vera, N.; Bello-Amez, S.; Castro-Cepero, V. Relationship between the incidence and severity of coffee rust (Hemileia vastatrix) in San Ramón, Chanchamayo, Peru. J. Sci. Res. 2019, 4, 1–9. [Google Scholar]
- Várzea, V.; Pereira, A.P.; Silva, M.d.C.L.d. Screening for Resistance to Coffee Leaf Rust. In Mutation Breeding in Coffee with Special Reference to Leaf Rust; Ingelbrecht, I.L., Silva, M.d.C.L.d., Jankowicz-Cieslak, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Lopez-Bravo, D.F.; de Virginio-Filho, E.M.; Avelino, J. Shade is conducive to coffee leaf rust as compared to full sun exposure under standardized fruit load conditions. Crop Prot. 2012, 38, 21–29. [Google Scholar] [CrossRef]
- Bekele, K.B.; Senbeta, G.A.; Garedew, W.; Caixeta, E.T.; Ramírez-Camejo, L.A.; Aime, M.C. Genetic diversity and population structure of Hemileia vastatrix from Ethiopian Arabica coffee. Arch. Phytopathol. Plant Prot. 2022, 55, 1483–1503. [Google Scholar] [CrossRef]
- Ward, H.M. On the morphology of Hemileia vastatrix Berk. and Br. (the fungus of the Coffee disease of Ceylon). Q. J. Microsc. Sci. 1889, 22, 1–11. [Google Scholar] [CrossRef]
- Fernandes, R.D.C.; Evans, H.C.; Barreto, R.W. Confirmation of the occurrence of teliospores of Hemileia vastatrix in Brazil with observations on their mode of germination. Trop. Plant Pathol. 2009, 34, 108–113. [Google Scholar] [CrossRef]
- Coutinho, T.A.; Rijkenberg, F.H.J.; Van Asch, M.A.J. Teliospores of Hemileia vastatrix. Mycol. Res. 1995, 99, 932–934. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, B.; Li, L.; Huang, X.; Liang, Y.; Zheng, J.; Wang, Q.; Li, R.; He, C.; Yi, K. The rDNA-ITS sequences analysis and phylogenetic relationships of Hemileia vastatrix. Chin. J. Trop. Crops 2018, 39, 2250–2258. [Google Scholar]
- Quispe-Apaza, C.S.; Mansilla-Samaniego, R.C.; López-Bonilla, C.F.; Espejo-Joya, R.; Villanueva-Caceda, J.; Monzón, C. Genetic diversity of Hemileia vastatrix of two coffee producing areas in Peru. Rev. Mex. Fitopatol. 2017, 35, 418–436. [Google Scholar]
- Cabral, P.G.C.; Maciel-Zambolim, E.; Oliveira, S.A.S.; Caixeta, E.T.; Zambolim, L. Genetic diversity and structure of Hemileia vastatrix populations on Coffea spp. Plant Pathol. 2016, 65, 196–204. [Google Scholar] [CrossRef]
- Maia, T.A.; Maciel-Zambolim, E.; Caixeta, E.T.; Mizubuti, E.S.G.; Zambolim, L. The population structure of Hemileia vastatrix in Brazil inferred from AFLP. Australas. Plant Pathol. 2013, 42, 533–542. [Google Scholar] [CrossRef]
- Schieber, E. Economic impact of coffee rust in Latin America. Annu. Rev. Phytopathol. 1972, 10, 491–510. [Google Scholar] [CrossRef]
- Bowden, J.; Gregory, P.H.; Johnson, C.G. Possible wind transport of coffee leaf rust across the Atlantic Ocean. Nature 1971, 224, 500–501. [Google Scholar] [CrossRef]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Silva, M.D.C.; Várzea, V.; Guerra-Guimarães, L.; Gil Azinheira, H.; Fernandez, D.; Petitot, A.-S.; Bertrand, B.; Lashermes, P.; Nicole, M. Coffee resistance to the main diseases: Leaf rust and coffee berry disease. Braz. J. Plant Physiol. 2006, 18, 119–147. [Google Scholar] [CrossRef]
- Sylvain, P.G. Some observations on Coffea arabica L. in Ethiopia. Turrialba 1958, 5, 37–54. [Google Scholar]
- Singh, R.P.; Hodson, D.P.; Jin, Y.; Huerta-Espino, J.; Kinyua, M.G.; Wanyera, R.; Njau, P.; Ward, R.W. Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CABI Rev. 2006, 2006, 1–13. [Google Scholar]
- Al-Ghamedi, K.; Alaraidh, I.; Afzal, M.; Mahdhi, M.; Al-Faifi, Z.; Oteef, M.D.Y.; Tounekti, T.; Alghamdi, S.S.; Khemira, H. Assessment of Genetic Diversity of Local Coffee Populations in Southwestern Saudi Arabia Using SRAP Markers. Agronomy 2023, 13, 302. [Google Scholar] [CrossRef]
- Quispe-Apaza, C.; Mansilla-Samaniego, R.; Espejo-Joya, R.; Bernacchia, G.; Yabar-Larios, M.; López-Bonilla, C. Spatial and temporal genetic diversity and population structure of Hemileia vastatrix from Peruvian Coffee Plantations. Plant Pathol. J. 2021, 37, 280. [Google Scholar] [CrossRef]
| Species Identity | Isolate No. | Host | Country of Origin | Accession Numbers |
|---|---|---|---|---|
| ITS | ||||
| H. vastatrix | PPDU1023 | C. arabica | Saudi Arabia | OR784411 |
| H. vastatrix | PPDU1123 | C. arabica | Saudi Arabia | OR784412 |
| H. vastatrix | PPDU1223 | C. arabica | Saudi Arabia | OR784413 |
| H. vastatrix | PPDU1323 | C. arabica | Saudi Arabia | OR784414 |
| H. vastatrix | PPDU1423 | C. arabica | Saudi Arabia | OR784415 |
| H. vastatrix | PPDU1523 | C. arabica | Saudi Arabia | OR784416 |
| H. vastatrix | Mexico_1 | Coffea arabica L., var. Caturra Rojo | Mexico | KX260253 |
| H. vastatrix | HVV_17.4_2018 | C. arabica | Peru | MW477196 |
| H. vastatrix | Mexico_2 | C. arabica var. Pluma Hidalgo | Mexico | KX260252 |
| H. vastatrix | CIFC_1 | C. arabica var. Caturra | Portugal | EF394122 |
| H. vastatrix | TL_18TH13 | Coffea sp. | Thailand | LC682363 |
| H. vastatrix | HVV_17.2_2018 | C. arabica | Peru | MW477194 |
| H. vastatrix | CIFC_2 | C. arabica var. Caturra | Portugal | EF394120 |
| H. vastatrix | Brazil_1 | C. arabica | Brazil | MF627828 |
| H. vastatrix | HVV_17.3_2018 | C. arabica | Peru | MW477195 |
| H. vastatrix | Haiku | C. arabica | USA | MW228837 |
| H. vastatrix | CMPH-165 | Coffea canephora var. Robusta | Mexico | KX260250 |
| H. vastatrix | Piranga_2 | C. arabica | Brazil | MF627773 |
| H. vastatrix | Colombia_10 | C. arabica var. Caturra | Colombia | EF394123 |
| H. vastatrix | Colombia_6 | C. arabica var. Colombia | Colombia | EF394127 |
| H. vastatrix | VNCR_S1_14 | Coffea sp. | Vietnam | LC682399 |
| H. vastatrix | Colombia_1 | C. arabica var. Caturra | Colombia | EF394132 |
| H. vastatrix | TL_19TH46 | Coffea sp. | Thailand | LC682364 |
| H. vastatrix | VNCR_S1_2 | Coffea sp. | Vietnam | LC682395 |
| H. vastatrix | CIFC_3 | C. arabica var. Caturra | Portugal | EF394119 |
| H. vastatrix | B1_1 | C. arabica | Brazil | MF627747 |
| H. vastatrix | Piranga_3 | C. arabica | Brazil | MF627774 |
| H. vastatrix | Piranga_4 | C. arabica | Brazil | MF627775 |
| Puccinia helianthi | NM-1 | Helianthus annuus L. (Sunflower) | China | JX013947 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhudaib, K.; Ismail, A.M. First Occurrence of Coffee Leaf Rust Caused by Hemileia vastatrix on Coffee in Saudi Arabia. Microbiol. Res. 2024, 15, 164-173. https://doi.org/10.3390/microbiolres15010011
Alhudaib K, Ismail AM. First Occurrence of Coffee Leaf Rust Caused by Hemileia vastatrix on Coffee in Saudi Arabia. Microbiology Research. 2024; 15(1):164-173. https://doi.org/10.3390/microbiolres15010011
Chicago/Turabian StyleAlhudaib, Khalid, and Ahmed Mahmoud Ismail. 2024. "First Occurrence of Coffee Leaf Rust Caused by Hemileia vastatrix on Coffee in Saudi Arabia" Microbiology Research 15, no. 1: 164-173. https://doi.org/10.3390/microbiolres15010011
APA StyleAlhudaib, K., & Ismail, A. M. (2024). First Occurrence of Coffee Leaf Rust Caused by Hemileia vastatrix on Coffee in Saudi Arabia. Microbiology Research, 15(1), 164-173. https://doi.org/10.3390/microbiolres15010011






