1. Introduction
Climate change and rising global temperatures are among the reasons for the gradual transition to green energy, including hydrogen.
One of the main and cheapest ways to produce hydrogen on an industrial scale is steam methane reformation, realized at 800–950 °C. This method produces 90–95% of all hydrogen, which is estimated in millions of tons (1):
Equation (1) shows that, when hydrogen is produced, carbon dioxide (CO2) is released in amounts equivalent to methane consumption, and a large amount of fuel is burned during the process, which leads to additional CO2 emissions.
Nevertheless, over the next 10–15 years, natural gas will be the best and cheapest solution to the transition to hydrogen energy problem.
In the future, hydrogen is planned to be obtained using the heat of nuclear reactors with a helium coolant, in which required temperatures of ~1000 °C are realized, but these reactors have not yet gone beyond the walls of design bureaus [
1].
The introduction of nuclear power complexes does not solve the problem of a large number of CO
2 emissions in general since the same reaction is the basis for hydrogen production (1). It is estimated that, in the case of the production of 500,000,000 m
3 of hydrogen (normal conditions), about 130 thousand tons of CO
2 will enter the atmosphere [
2].
In nature, the task of CO
2 absorption is successfully performed by photosynthetic microorganisms and plants that convert up to 100 Gt/year of carbon into biomass [
3].
The prospects of using microalgae for CO
2 elimination are due to the fact that the photosynthesis utilization rate for microalgae is in the range of 3–8%, whereas in terrestrial plants, it does not exceed 0.8% [
4]. This is due to the fact that algae grow 6–10 times faster than most terrestrial plants, and therefore, the total amount of CO
2 absorbed by them is correspondingly greater.
It is obvious that the use of phototrophic microalgae for CO2 elimination is a promising and environmentally friendly method of CO2 elimination, which also makes it possible to obtain microalgae biomass rich in proteins, carbohydrates, fats, and vitamins, which can be used as fertilizers, feed for farm animals, biofuel production, etc.
An analysis of the literature data [
5,
6,
7,
8] on biomass accumulation and CO
2 fixation showed that algae of the
Chlorella and
Scenedesmus genera are most often used for these purposes. The research results obtained by different authors [
5,
6,
7,
8] vary greatly since different types of microalgae, nutrient media, modes, irradiation intensities, temperatures, etc., are used.
For example, the optimal CO
2 concentration for vital activity, according to various data, ranges from 0.8 to 10% [
9].
In connection with the above, we have attempted to develop a green integrated process for the efficient utilization of waste discharge (CO2 as a carbon and energy source) for the co-production of microalgae biomass feedstock, in the waste-to-value chain, and in waste management for sustainable future development.
The main goal of this process is to solve the problem of carbon dioxide utilization with an increase in the efficiency of carbon dioxide biofixation caused by microalgae in a closed photobioreactor. The available data spread does not make it possible to identify which microalgae, under comparable modes, have the highest biomass productivity and eliminate the largest CO2 amount.
Most of the available data on the parameters (temperature, pH) of microalgae cultivation refer to laboratory studies or are aimed at improving the growth conditions of microalgae. The purpose of this work is to study the optimal growth parameters to increase the efficiency of carbon dioxide absorption with the concomitant production of biomass.
Consequently, the focus of this study is on the concept of “waste to value” and an assessment of the possibility of using various types of microalgae to capture (utilize) CO2 in closed photobioreactors in a periodic mode.
Therefore, the objective of the present study was to experimentally study the effect of cultivation temperature on the reproduction intensity of microalgae of the Chlorella vulgaris Beijer, Scenedesmus fuscus, and Scenedesmus obliquus species and their CO2 absorption with other constant factors (the composition of the nutrient medium, the mode and intensity of illumination, medium pH, CO2 concentration) in a tubular photobioreactor with a volume of 2 L during cultivation in continuous culture.
2. Materials and Methods
Strains of microalgae of the Chlorella vulgaris Beijer, Scenedesmus fuscus, and Scenedesmus obliquus species were used as testing cultures (provided by the microalgae and cyanobacteria collection of the Timiryazev Institute of Plant Physiology Russian Academy of Sciences, which kindly provided cultures from the microalgae collection of the IPP RAS (IPPAS) (LSRF CMC IPPAS IGF RAS).
Microalgal cultures were grown on modified Tamiya medium of the following compositions (g/L): KNO3—5.0; MgSO4·7H2O—2.5; KH2PO4—1.25; EDTA—0.037; FeSO4·7H2O—0.009; agar-agar—20; trace element solution—1 mL. The optimal pH value of the medium for normal microalgae growth was maintained in a range of 7.0–8.4.
A streak microalgal culture from test tubes with slant agar was plated in Petri dishes with Tamiya medium and placed in a thermostat equipped with a light source (cooled halogen incandescent lamp, KGM 24–150). The incubation time was determined based on the results of the visual control. Then, the culture from the Petri dishes was reinoculated into flasks with a liquid Tamiya medium (without agar-agar), where the microalgal culture was kept for 7 days to adapt to the conditions of submerged cultivation. When the concentration of microalgae cells in the suspension reached 500,000 cells/mL, they were reinoculated into a laboratory photobioreactor. The cell concentration was determined via direct counting in a Goryaev chamber. Mobile microalgae were immobilized with alcohol before counting in the Goryaev chamber. For reliability, the cells were counted in three repetitions.
The inoculum for the photobioreactor was 25% of the total reactor volume.
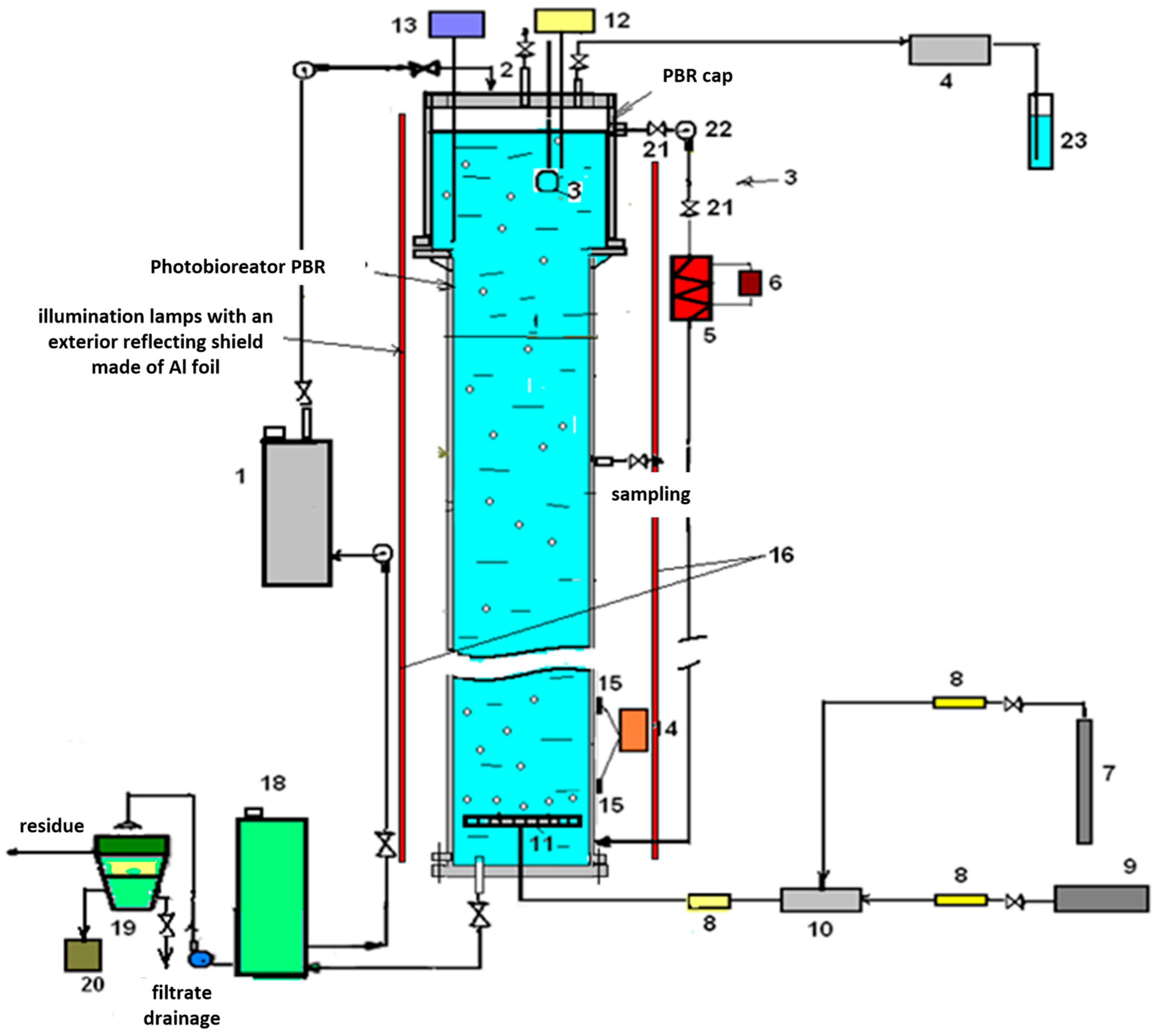
Further cultivation was performed in the laboratory setup shown in
Figure 1.
The microalgae cultivation was carried out in a periodic (cumulative) mode, wherein the inoculum is introduced into the nutrient medium at the beginning of the process and further cultivation takes place with periodic monitoring of the biomass content in the suspension until its maximum concentration is reached and it transitions to the death phase.
LED strips glued to the inner surface of a cylindrical reflector covered with aluminum foil were used as light sources.
The microalgae suspension was illuminated by 4 lamps/LEDs, providing a light intensity of 20 klk.
The photoperiod consisted of 12 h of illumination and a 12 h dark phase.
Mixing of the suspension was performed with a gas–air mixture with a flow rate of 0.3 L/min at a CO2 concentration of 4% in the mixture.
Algae cultivation was carried out at a temperature of 30 °C and around-the-clock aeration with a gas–air mixture.
The pH of the culture fluid was monitored on average once every five hours. Titration was carried out manually with 2 solutions of HNO3 and KOH.
The consumption of CO
2 and air was calculated using Equation (2):
where P—gas pressure, with pressure gauge readings on the gearbox of the CO
2 supply branch and on the compressor supplying air, Pa;
d—the inner diameter of the pipeline through which the gas supply to the photobioreactor is carried out, m;
ρ—gas density, kg/m
3; V—gas flow rate, m
3/s.
The gas consumption was calculated using Equation (3):
The required concentration of CO2 in the gas–air mixture was set by adjusting the pressure at the outlet of the CO2 bulb and compressor.
The concentration of microalgae biomass was determined using a B-1100 spectrophotometer by sampling every 3–5 h according to the optical density of the suspension, followed by conversion to dry weight using a calibration curve. For working microalgae strains, calibration curves were constructed during preliminary experiments on increasing biomass.
A MAG-6 P-T gas analyzer with built-in sensors and a compressor was used to monitor the gas environment based on O2 and CO2. For a controlled gas supply, MASS-VIEW MV-302 electronic rotameters with a supply range of 0.01 to 2 L/min of gas were used. A microprocessor pH/S meter, HI2210-02, was used to measure pH.
The efficiency of CO2 fixation was determined using a gas analyzer based on the difference in the concentrations of CO2 gas between the inlet and outlet of the photobioreactor.
3. Results and Discussion
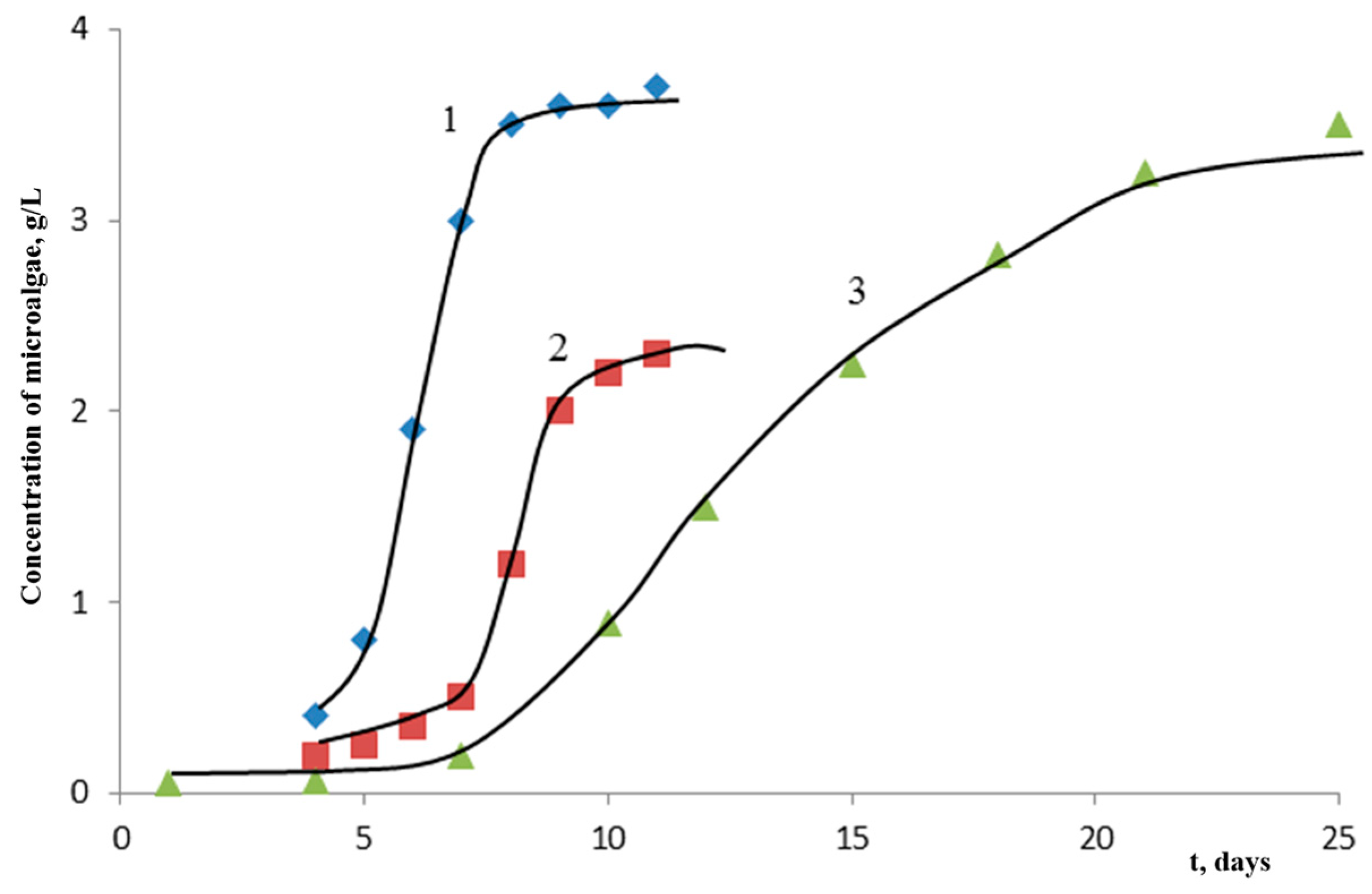
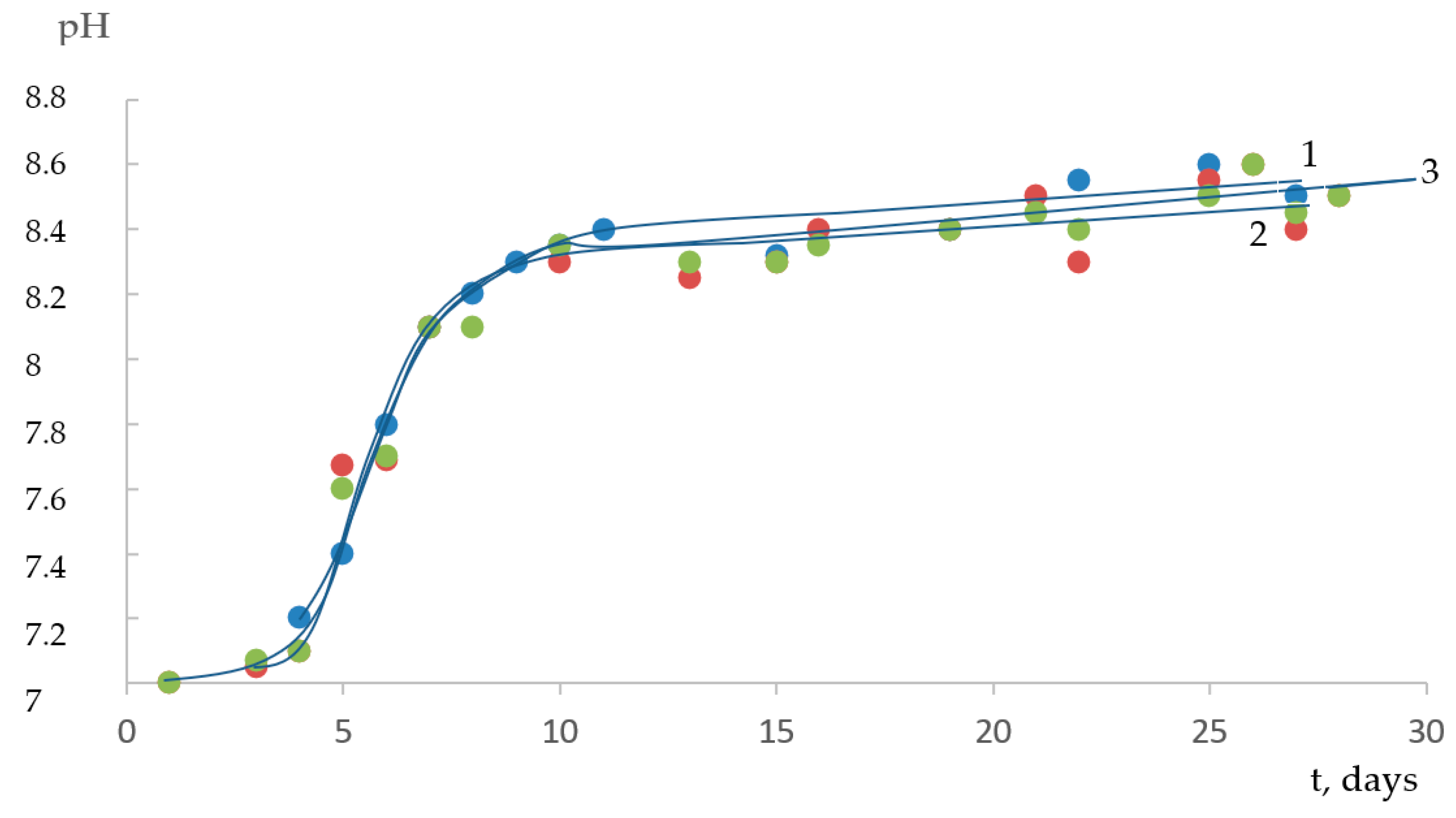
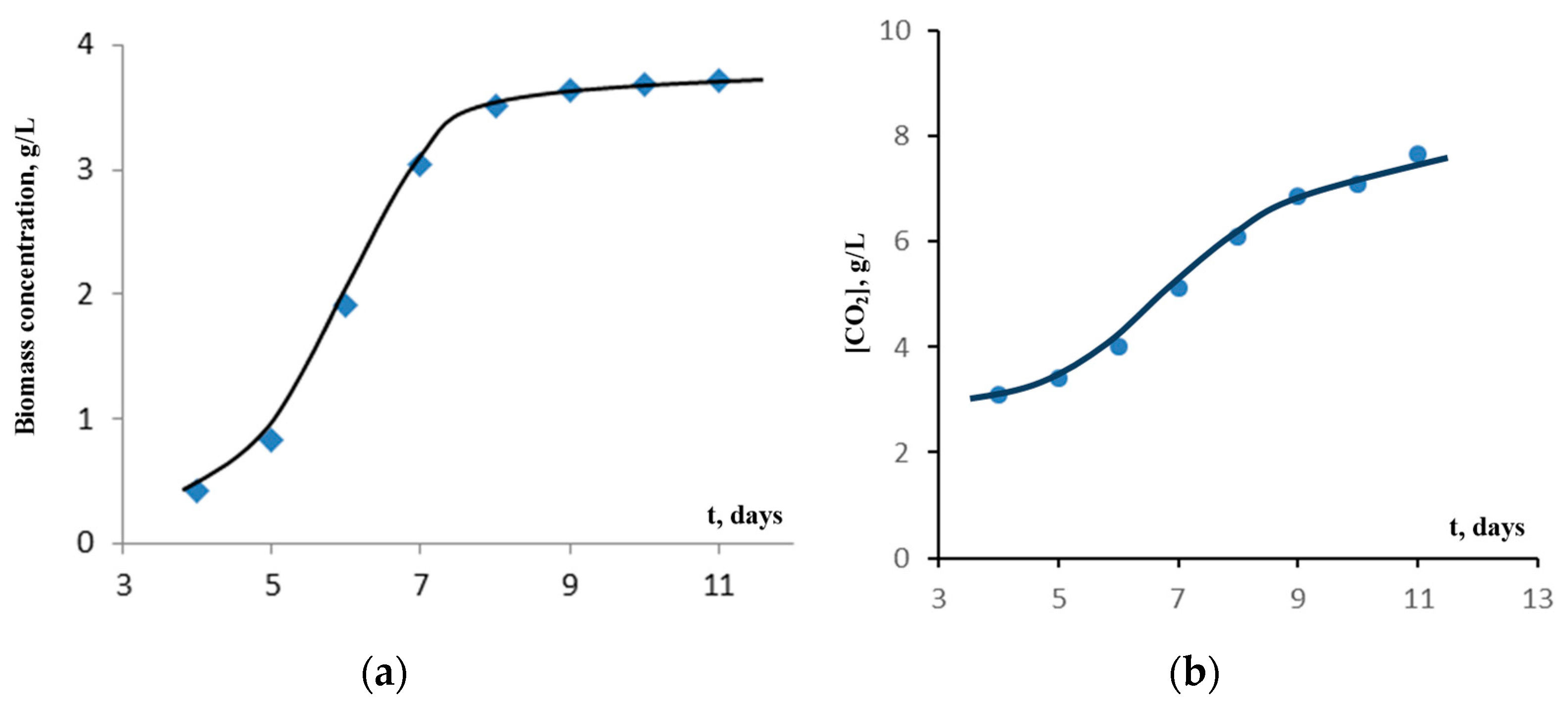
Initially, the experiments on the accumulation of algae biomass were carried out on a Tamiya medium with nitrate nitrogen (nitrogen is supplied in terms of nitrate) with an initial pH value of ≈7 (
Figure 2 and
Figure 3).
In
Figure 2 and
Figure 3, it can be seen that, in this case, the accumulation of biomass for up to 5 days is characterized by an “incubation period” (lag phase)—the acclimatization time of algae—after which, exponential growth, inhibition of the process, and cell death, (not shown in
Figure 2 and
Figure 3) are realized.
Note that with the growth of the culture, the concentration of OH
− ions in the medium increases, and after 8 days, the pH reaches values of 8.0–8.2 units. This is due to the fact that the zinc-containing enzyme carbonic anhydrase, which catalyzes photosynthesis, plays an essential role in carbon assimilation. As a result of the enzymatic work, one HCO
3− ion is subsequently formed from one CO
2 molecule and one hydroxyl ion OH
− [
10].
Then, CO
2 is used in the Calvin cycle, in which CO
2 is converted into carbohydrates, and OH
− ions are removed from the cell into the external environment, by increasing the pH of the solution [
11].
With a further increase in pH > 9, algae cells increasingly begin to switch to carbonate nutrition, which is less energetically beneficial. At the same time, the photosynthetic activity of the cells is limited because of the alkalinization of the medium and, possibly, a lack of energy in the cells since, in an environment with pH = 11, one mole of ATP is consumed to remove one mole of OH
− from the cell [
12].
In the plateau region, the number of microalgae cells practically does not increase because of a decrease in the concentration of biogenic elements in the nutrient medium, the accumulation of microalgae waste products, and a decrease in the flow of light passing through an optically denser culture.
It is known that, at a pH of ≈7.0, about 20% of CO
2 is in the form of free CO
2, and 80% is in the form of HCO
3− and at a pH of ≈8.4; the HCO
3− content corresponds to 100%, and at these pHs, the greatest productivity in biomass and the utilization of CO
2 can be observed [
13].
It has been observed that the initial increase in pH to a level of ~8.4 in the nutrient medium and the introduction of NaHCO3 into it leads to a reduction in the incubation period and the growth of biomass. The shortening of the incubation period shows that HCO3− ions apparently contribute to a faster adaptation of microalgae to new conditions.
Thus, the data indicate that the growth of microalgae is facilitated by an increase in the [HCO3−] medium and not the carbonate ion of CO32− or the free amount of CO2 since a decrease in pH from 8 to 5.5 leads to the inhibition of biomass accumulation at a constant concentration of CO2 in the gas phase. At pH < 5.0, the photosynthetic growth of the biomass actually stops.
This conclusion is confirmed by data from a study performed by [
14], in which it was shown that the microalgae
Chlorella vulgaris and
Scenedesmus grow well in media containing HCO
3−; however, the mechanism of HCO
3− transportation into the cell is still unclear.
Thus, it is clear that HCO3−, in the process of photosynthesis, is better absorbed by microalgae than free CO2 dissolved in water.
CO2 chemisorption means that the change in the carrier gas (air, pure nitrogen, etc.) has little effect on the solubility of CO2 since its absorption by the solution is much greater than the solubility of air, oxygen, or nitrogen in water: at 30 °C, the solubility of CO2 is equal to 1250 mg/L, and the solubility values of oxygen, air, and nitrogen are equal to 35.9, 19.0, and 18.8 mg/L, respectively.
At the same time, the exclusion of nitrogen from nutrient or gas media quickly leads to a slowdown in the growth of microalgae as protein synthesis decreases [
15].
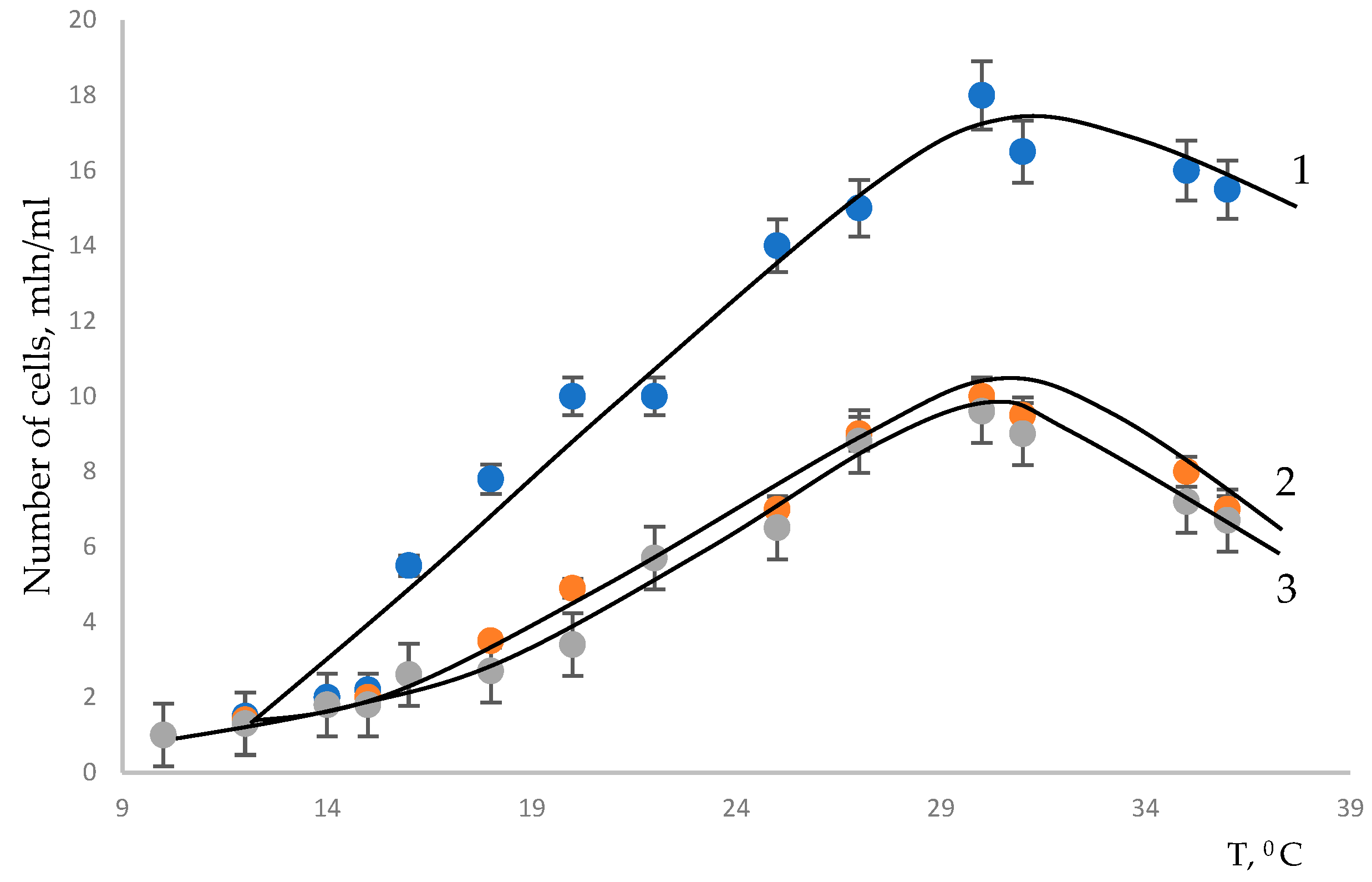
A temperature of 30 °C was chosen on the basis of studies aimed at optimizing the photosynthesis process depending on temperature (
Figure 4).
It was found that the maximum increase in biomass for all the studied cultures was at a temperature of about 28–33 °C.
In
Figure 2 and
Figure 4, it can be seen that the microalga
Chlorella vulgaris has twice the biomass productivity of the microalgae
Scenedesmus fuscus and
Scenedesmus obliquus.
It follows from
Figure 5 that there is a direct relationship between the absorption of CO
2 and the accumulation of
Chlorella vulgaris biomass at a photosynthesis time of ≥ 6 days in the ratio of MCO
2/Mbiomass ≈ 2.0. Close ratios between CO
2 and biomass in the plateau region of 2.0 and 2.2 were observed for
Scenedesmus fuscus and
Scenedesmus obliquus, respectively, which are consistent with the data obtained for other microalgae where the ratio under discussion varies from 1.8 to 2.3 [
16,
17].
In the study in [
18], an experimental approach was used to simulate microalgal growth kinetics and CO
2 fixation over time with respect to inorganic carbon dissolving patterns at different suspension illumination levels. It was shown that, at optimal illumination intensity, depending on the amount of dissolved carbon for
Chlorella vulgaris, the amount of biomass was 2.303 g/L, and the amount of absorbed CO
2 was 4.385 g/L, both of which also correspond to the ratio of MCO
2/Mbiomass = 1.9.
It follows from
Figure 2 and
Figure 4 that the microalgae
Scenedesmus fuscus and
Scenedesmus obliquus had lower rates of biomass accumulation and CO
2 absorption capacity under the studied cultivation parameters than the algae
Chlorella vulgaris. Nevertheless, the prospects of using microalgae of the
Scenedesmus genus are of considerable interest in solving problems regarding CO
2 elimination since they remain operable at higher temperatures and concentrations of carbon dioxide.
4. Conclusions
Microalgae cultivation is one of the most efficient approaches to removing CO2, and each cultivation system has its advantages and drawbacks. Thus, the selection of the best strategy strongly depends on the purpose, scale, microalgae species, and cost.
The effects of two essential process variables, viz., temperature and culture time, on biomass productivity and CO2 biofixation were evaluated using Chlorella vulgaris, Scenedesmus fuscus, and Scenedesmus obliquus. Under the conditions employed in this study, when using a Chlorella vulgaris culture, the increase in biomass under comparable conditions was greater than when cultivating the microalgae Scenedesmus fuscus and Scenedesmus obliquus.
The experimental analysis showed improvement in the microalgae growth, biomass productivity, and CO2 fixation rate of the two algae (Chlorella and Scenedesmus) at a cultivation temperature equal to 29–32 °C and a medium pH of 8.4. A ratio of ~2.0 was observed between CO2 absorption and the increase in biomass for different microalgae.
To increase the performance of the photobioreactor, in order to increase the rate of biomass accumulation and, consequently, CO2 biofixation, we recommend bringing the pH of the nutrient medium to 8.0–8.5 initially.