1. Introduction
Bovine rotaviruses (BRVs) are major causative agents of severe diarrhea in newborn calves, leading to dehydration, electrolyte imbalances, and potentially death [
1]. Rotaviruses are classified in the
Reoviridae family, consisting of a non-enveloped virion with a double-stranded nucleic acid of positive polarity and 11 segmented RNAs. These viruses have six structural proteins (VP 1-2-3-4-6-7) and five or six non-structural proteins (NSP 1-2-3-4-5/6). Rotaviruses are grouped into 10 categories (A–J) based on antigenic relationships with VP6. Most commonly, groups A, B, and C infect humans and animals. Outer capsids, endowed with VP4 (protease-sensitive P protein) and VP7 (G glycoprotein) and targeted by neutralizing antibodies, are used for the classification and identification of rotavirus strains. Rotavirus groups have been further described by the dual classification system with G-type and P-type groups based on capsid proteins, and combinations of these types are used for strain identification [
2,
3].
Rotaviral gastroenteritis in the cattle industry is responsible for significant economic losses due to calf mortality, reduced productivity, and increased medication costs [
4]. BRV infection prevalence in diarrheic calves ranges from 20% to 60% [
5]. BRV is primarily transmitted through the fecal–oral route, specifically targeting the enterocytes (absorptive cells of the small intestine) in the calf’s gastrointestinal tract. This leads to nutritional deficits in calves and hampers water uptake in other parts of the digestive system. Ultimately, it triggers episodes of calf scour or neonatal diarrhea in newborn calves [
6]. The development of novel and effective antiviral agents against BRVs is crucial for improving animal health and welfare.
Rotavirus activation is facilitated by trypsin [
7], a serine protease enzyme found in the digestive system of many vertebrates, including humans and animals. It is produced in the pancreas and secreted into the small intestine [
8]. We hypothesized that inactivation of trypsin using
Ecballium elaterium (
E. elaterium) extract enriched with protease inhibitors may help reduce rotavirus-induced viral activation and subsequent infection. One notable application of antiviral protease inhibitors is their utility in the management of HIV, where protease inhibitors such as combinations of darunavir–ritonavir or lopinavir–ritonavir have become integral components of combination therapy for HIV/AIDS patients, significantly ameliorating their clinical outcomes and overall quality of life [
9]. Furthermore, protease inhibitors have shown promise in the treatment of other viral infections, including hepatitis C, influenza, and COVID-19 [
10]. For instance, rotaviral infections have proven to be effectively mitigated through the application of antiviral protease inhibitors. Oral administration of some cysteine protease inhibitors and a serine protease inhibitor, such as aprotinin (extracted from bovine pancreases), significantly decreased the diarrhea ratio in suckling mice infected with human rotavirus, suggesting that these inhibitors could be protective agents against human rotavirus infection [
11].
The natural source of inhibitors of serine protease enzymes can be found in some plants, especially in plant seeds [
12].
E. elaterium, also known as squirting cucumber or exploding cucumber, is a plant native to the Mediterranean region [
13]. It has been used in traditional medicine for various purposes, mainly due to its active components, such as cucurbitacins, flavonoids, and sterols, and is a perennial plant from the
Cucurbitaceae family known for its diverse phytochemical constituents, including
E. elaterium trypsin isoinhibitors [
13,
14,
15,
16]. Plant protease inhibitors are used for cancer treatment, inflammation, and neurodegenerative diseases [
17,
18]. Additionally, a bioinformatics study showed that a similar plant-derived trypsin inhibitor may be effective for hemostasis [
19].
In this study, we aimed to investigate the potential efficacy of E. elaterium seed and fruit juice extract against bovine rotavirus infections in vitro. Our objective posited that E. elaterium plant extract can inhibit trypsin activity, thereby preventing rotavirus activation and infection. To test this hypothesis, we initially conducted an ethanol extraction from E. elaterium seeds while plant fruit juice was being filtered. Subsequently, we infected MA-104 cells with a bovine rotavirus isolate in the presence of the E. elaterium extract. Our findings revealed a decrease in the rotavirus titer in vitro upon treatment with the plant extract, indicating its potential therapeutic application. These results support the hypothesis that E. elaterium plants can inhibit trypsin activity, possibly via E. elaterium trypsin isoinhibitors (EETIso), and rotavirus replication, offering a promising approach for the development of alternative treatments to prevent bovine rotavirus-induced diarrhea in newborn calves. The potential use of derivatives from E. elaterium may contribute to a reduction in calf mortality, minimized medication costs, and an overall improvement in economic value in cattle farming.
2. Materials and Methods
2.1. Sample Collection and Extraction
E. elaterium plant fruits were collected from the Ödemiş region of İzmir, Türkiye in 2018 and were subsequently stored at +4 °C until the plant extracts could be isolated. To obtain the fruit juice, the fruits were squeezed by hand, and the resulting juice was clarified by centrifugation at 6000 rpm at +4 °C for 30 min. The supernatant was then filtered through a 0.45 µm filter (Millipore, SLHVR33RS; Darmstadt, Germany,) attached to a syringe to ensure sterility, and the filtrates were stored at −20 °C until they were used in cytotoxicity assays and anti-trypsin efficiency experiments.
For the seed extracts, the fruit seeds were soaked in water at 37 °C overnight and washed under running tap water. After draining the water, the seeds were gently rubbed with a paper towel to remove the seed coat, and then washed again in a mesh under running tap water. The seeds were then air-dried overnight at room temperature. For the extraction, an ethanolic extraction method was carried out as described before [
20] with some modifications as follows: seeds were ground into a powder using a porcelain mortar and the powder was dried at 37 °C for 24 h. A total of 35 g of the dried powder was mixed with 200 mL of 80% ethanol and stirred at room temperature for 24 h using a magnetic stirrer. Insoluble particles were removed by centrifuging at 10,000 rpm at +4 °C for 10 min. Supernatants were collected and transferred to Petri dishes, and 50 mL was dried at 37 °C overnight. The dried powder was reconstituted in sterile distilled water to a concentration of 200 mg/mL and stored at −20 °C until use.
2.2. Cell Culture
MA-104 cell lines (ATCC, CRL-2378.1; Manassas, VA, USA) were used for cytotoxicity assays of plant extractions and antiviral experiments. The cells were maintained by adding 10% heat-inactivated fetal bovine serum (Sigma, F0926; St. Louis, MO, USA) to M199 medium (Gibco, 11150059; Carlsbad, CA, USA) and supplemented with 100 U/mL penicillin and 100 mg/mL streptomycin (Sigma, A5955; St. Louis, MO, USA). MA-104 cells were maintained at 37 °C in a 5% CO2 incubator environment.
2.3. Virus
The BRV/ERU_2018 strain was aliquoted at 3 × 10
7 tissue culture infectious titer 50 per mL (TCID
50/mL) and stored at −80 °C [
21]. Bovine rotavirus virus propagation was performed as described before [
22].
2.4. In Vitro Cytotoxicity Study
MA-104 cells were seeded on 96-well plates with a 3 × 103 cells/well density. The plates were incubated at 37 °C with 5% CO2 for 24 h in the incubator. The next day, serial dilutions of E. elaterium fruit juice and seed extracts in a 1% FBS-containing M199 medium were prepared, ranging from Log 10 base (−1 to −10). Eight wells were filled with each dilution and 16 wells were left untreated for control, but the media was replaced as used for the dilutions of fruit juice and seed extract. Plates were returned to the incubator for another 24 h. In vitro cytotoxicity was assessed using an inverted tissue culture microscope, crystal violet staining, and MTT cytotoxicity analysis for quantitative results. The same procedures were applied to the cell control group.
2.4.1. Determination of Cytotoxicity Using Crystal Violet Staining
The plates incubated with extract dilutions were fixed with neutral formalin buffer (1:10 formalin) for 30 min at room temperature without removing the medium. After fixation, the wells were washed under running water five times by soaking, and the medium/formalin residues were removed. The remaining liquid was removed by tapping the plate on a paper towel. A 0.2% solution of crystal violet in 2% ethanol-containing phosphate-buffered saline (PBS) was prepared and added to the wells (50 μL per well) and incubated at room temperature for 30 min. The wells were washed five times as described before to completely remove dye residues, and then the cell staining was evaluated.
2.4.2. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Cytotoxicity Analysis
The MTT analysis, which is based on the measurement of mitochondrial metabolic activity, was performed to determine cell viability in cultures exposed to the fruit juice and seed extracts. For this purpose, the culture medium was removed from the plates, and the cells were washed twice with 200 μL PBS. Then, 100 μL of MA-104 containing a 5 mg concentration of MTT (Thermo Fisher, M6494; Waltham, MA, USA) was added to each well and incubated for an additional 4 h at 37 °C. At the end of this period, the amount of formazan crystals formed was determined by removing the medium and incubating it with DMSO (SERVA, 67-68-5; Heidelberg, Germany) for 30 min more. The absorbance at the 570 nm wavelength was measured using a microplate reader, and the inhibitor concentration 50 (IC50) of the test compounds was determined.
2.5. Virus Infection and Antiviral Activity of Plant Extracts
In order to assess the efficacy of both plant extracts in reducing the viral titer, we conducted an in vitro antiviral study. MA104 cells were seeded in a 96-well plate with a density of 3 × 104 cells per well and incubated at 37 °C with 5% CO2 for 24 h. The following day, both plant extraction liquids were diluted in M199 media containing trypsin (1 µg/mL; Sigma, T1426; St. Louis, MO, USA) at their respective IC50 rates.
The diluted plant extracts mixed with trypsin-containing media were transferred to tubes, with each tube containing a volume of 450 µL. To initiate the experiment, a 50 µL aliquot of rotavirus at a concentration of 3 × 107 TCID50/mL was added to the first tube, resulting in a 10−1 dilution. Subsequently, serial dilutions were performed up to a 10−7 dilution based on a Log10 dilution scheme. After one hour of rotavirus activation at 37 °C in a water bath, 100 µL from each dilution step was added sequentially to quadruplicate wells of the 96-well plate.
The last two columns of the plate were allocated as the control group for rotavirus titration. In this case, the virus was similarly diluted in a Log10 manner, but only in the M199 medium. After the same procedures, 100 µL from each dilution was transferred to separate quadruplicate wells containing MA104 cells. Additional quadruplicate wells were set aside for virus and cell controls. The plate was then incubated at 37 °C with 5% CO2, and daily observations were made using a tissue culture microscope for 5 days.
The results were calculated using the TCID
50 method according to the Spearman–Karber method [
23,
24], which enables the determination of viral titers.
2.6. Statistical Analysis
GraphPad PRISM was utilized to visualize the results. For the cytotoxicity analysis, a dose–response curve was constructed using non-linear fit curve analysis after data transformation to determine the LogIC50 and R2 values. Cell viability was assessed by normalizing the values based on the optical density (OD) measurements of control wells in the MTT assay. The normalized values were then transformed and expressed as percentages. To identify significant differences between groups, a Mann–Whitney nonparametric statistical analysis test was performed.
3. Results
E. elaterium, a perennial plant, has a sprawling stem, cordate to triangular leaves that are quite fleshy, and flowers with yellowish petals. The fruit is green, has white hairs, and is oval in shape. As the fruit matures, it turns from green to yellowish-green, violently expelling its seeds and juice (
Figure 1A). The seeds are 4–5 mm in size and are brown and egg-shaped (
Figure 1B).
3.1. Crystal Violet Staining and MTT Cytotoxicity Assessment
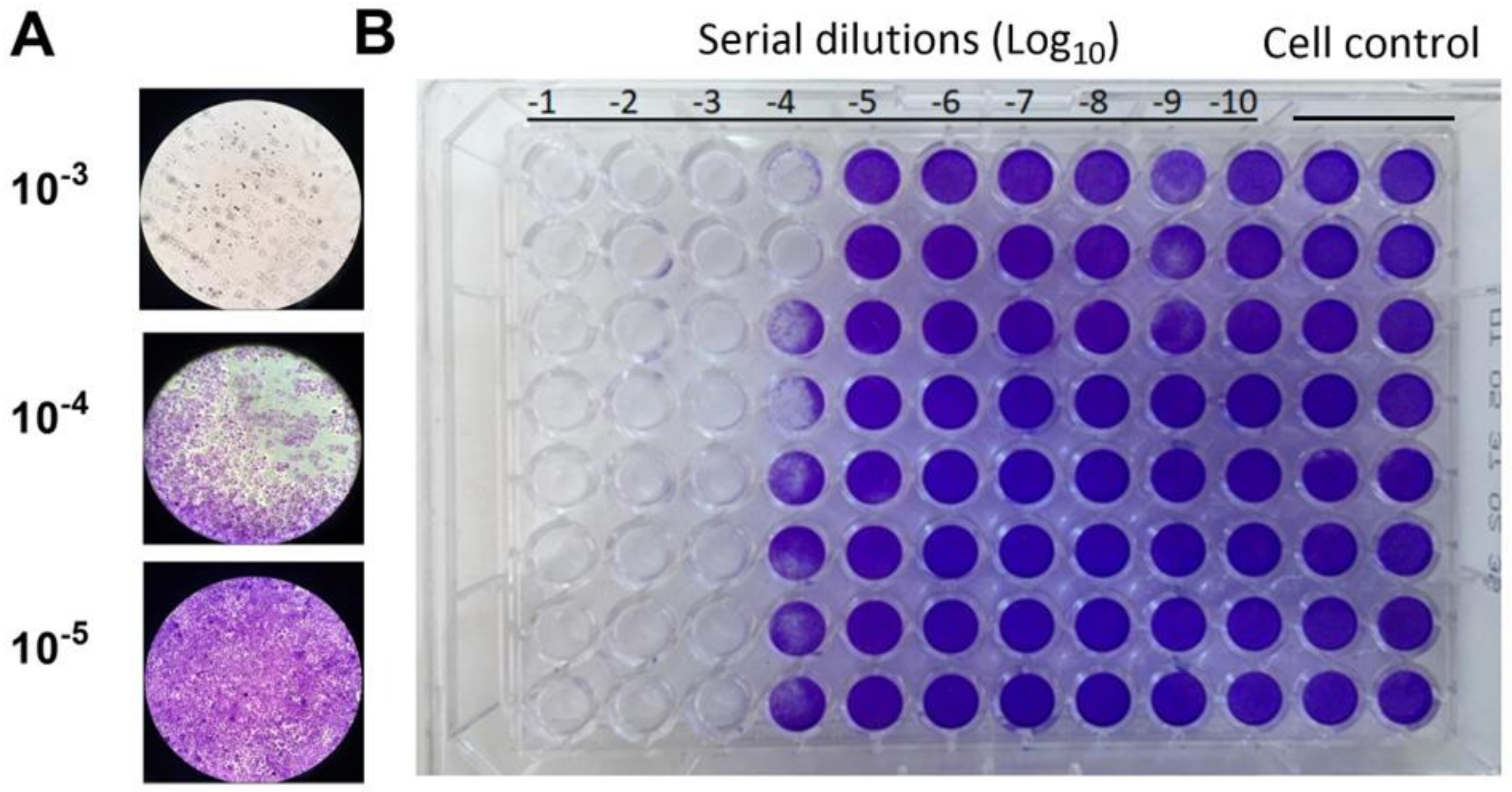
Through the crystal violet staining method, we observed notable changes in cell morphology, such as cell loss and reduced dye uptake, in the wells containing higher concentrations of the extract. These observations further substantiate the cytotoxic effects of the E. elaterium extracts on the MA-104 cell lines. Compared to the control cell group which displayed normal cell morphology, the extracts induced cytotoxicity.
The fruit juice caused complete cell death in dilutions ranging from 10
−1 to 10
−3. In the 10
−4 dilution, half of the wells did not absorb the crystal violet dye. However, all other wells with dilutions ranging from 10
−5 to 10
−10 showed dye uptake similar to the control wells. This suggests that the maximal non-cytotoxic fruit juice dilution, which is the highest concentration of a substance, is approximately 1 × 10
−5 (
Figure 2A,B).
Interestingly, we observed that the seed extract did not interfere with dye uptake in the wells with dilutions from 10−7 to 10−10. However, cells were completely lost in wells containing higher concentrations of the extract (10−1 to 10−4). The crystal violet staining results showed that there was more toxicity and cell death in the seed extract compared to the fruit juice. The fruit juice and seed extracts from the E. elaterium plant demonstrated varied cytotoxic effects on the MA-104 cell lines, and dose-dependent cellular toxicity was higher in the seed extract.
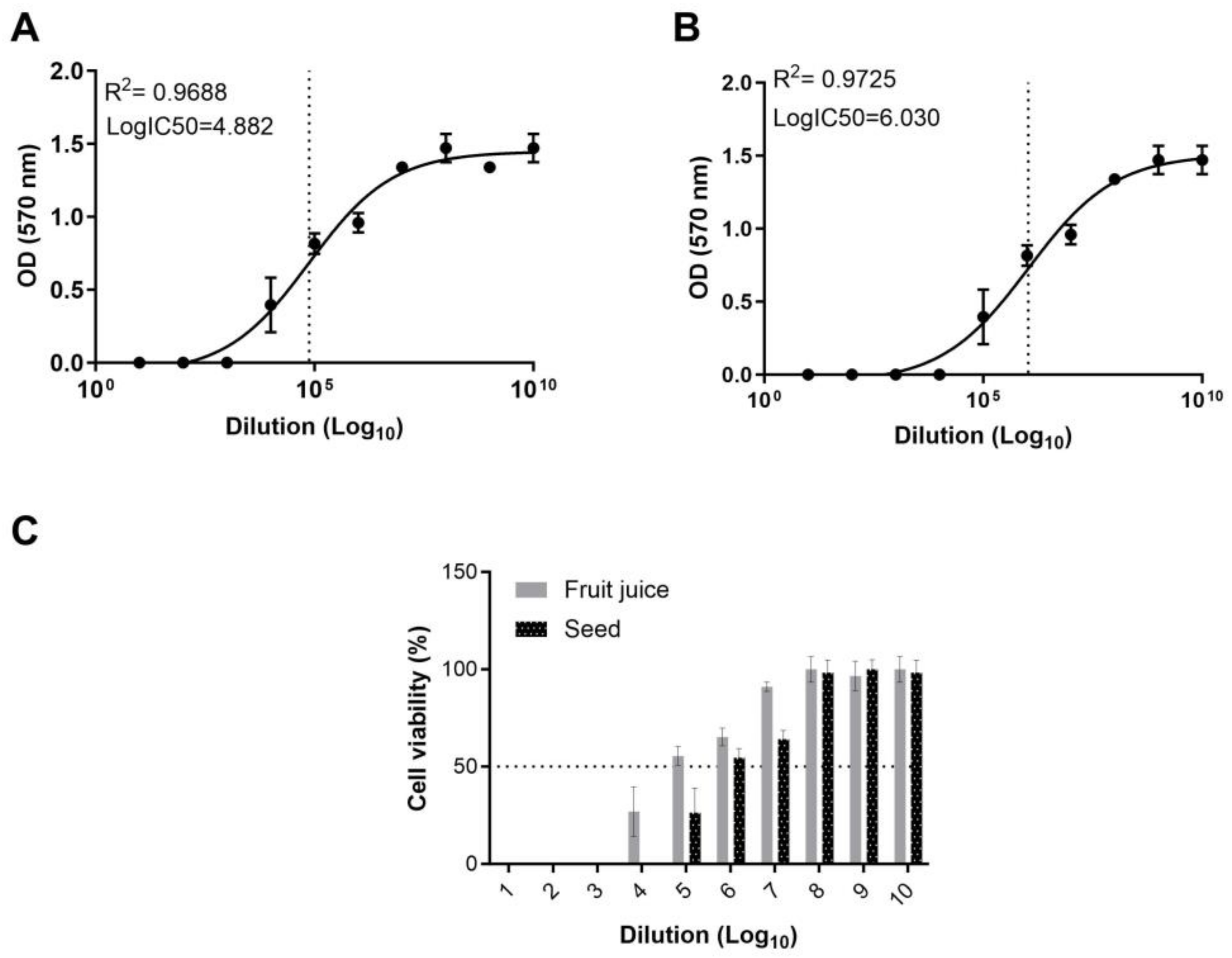
To assess the cytotoxic potential and ascertain the half-maximal inhibitor concentration (IC
50) of the fruit juice and seed extract of
E. elaterium, we employed an MTT assay. Using the absorbance values, the IC
50 of the fruit juice and seed extracts was calculated. Our results indicate that the fruit juice exhibits a LogIC
50 of 4.8 on MA104 cells, while the seed extract presents a substantially higher LogIC
50 of 6.03. The IC
50 of the fruit juice extract was found to be higher than that of the seed extract, indicating a more potent cytotoxic effect of the latter (
Figure 3A,B).
To determine cellular viability, we normalized the cell viability in the treated wells against the OD values measured in the control wells. In wells treated with the fruit juice, cell viability could not be determined for dilutions from 10
−1 to 10
−3. However, for dilutions ranging from 10
−4 to 10
−7, the cell viability was observed to be 26.8%, 55.4%, 65.2%, and 91%, respectively (
Figure 3C). In the wells treated with different concentrations of the seed extract, cell viability was undetectable in the 10
−1 to 10
−4 range. However, in dilutions between 10
−5 and 10
−7, cell viability was recorded as 26.4%, 54.5%, and 64.1%. For both the fruit juice and seed extract, cell viability in the range of 96.6% to 100% was observed in the wells treated with dilutions varies from 10
−8 to 10
−10 (
Figure 3C). Results reveal a dose-dependent response in both fruit juice- and seed extract-treated cells, where higher extract concentrations resulted in increased cytotoxicity compared to the untreated controls. This observation further corroborates that the cytotoxic effects can be directly attributed to the
E. elaterium fruit juice and extracts. The MTT assay results revealed that the
E. elaterium fruit juice and seed extracts induced a significant reduction in cell viability at higher concentrations, further supporting the cytotoxicity assessment.
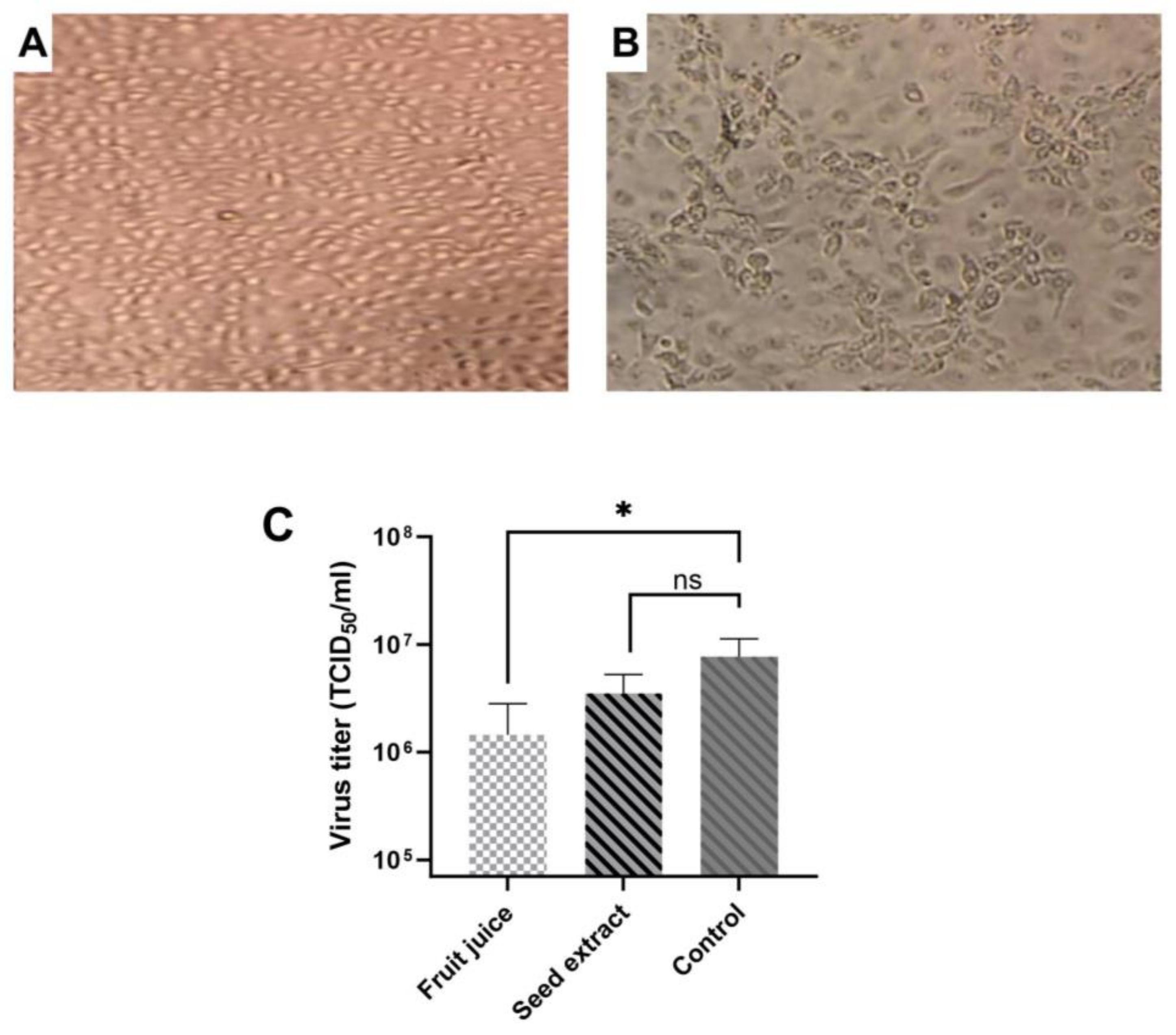
3.2. Antiviral Activities of E. elaterium Extracts
Following the determination of the cytotoxicity and IC
50 of plant extracts, we performed an antiviral activity assay. The fruit juice extract was diluted at a concentration of 10
−5, while the seed extract was diluted at a concentration of 10
−6, which is the maximal non-cytotoxic concentration of compounds. Virus dilution was performed using Log10 in media either containing fruit juice or seed extract. Both untreated and virus-infected cells served as controls. Cells infected with rotavirus showed cytopathic effect (CPE) in MA-104 cells while the control group demonstrated no morphological changes, and TCID
50 was calculated using an inverted microscope according to CPE formations in the treated wells (
Figure 4A,B). Results showed that both fruit juice and seed extract reduced the rotavirus titer, but the reduction was varied. When TCID
50 results were compared between control and treated cells (
Figure 4C), there was a statistically significant difference between the control and fruit juice (
p = 0.013) while the result was not significant between the seed extract and control (
p = 0.075). Results indicate that
E. elaterium plant juice has a more inhibitory effect on rotavirus replication compared to seed extract.
Overall, these results show that fruit juice has less toxicity but its antiviral activity is more pronounced compared to seed extract.
4. Discussion
Bovine rotaviral diarrhea in newborn calves holds significant importance in veterinary and agricultural contexts due to its widespread occurrence and economic implications. The disease poses a considerable threat to calf health, leading to dehydration, malnutrition, and, in severe cases, increased mortality rates. Even though some vaccines have been developed against bovine rotaviral diarrhea in newborns, results are variable, and prevention is limited in the field. Due to the absence of effective vaccines against bovine rotaviral diarrhea, there is a need for antiviral drugs to treat infections, especially in cases where vaccination is not feasible or when breakthrough infections occur. In this study, we tested the cellular cytotoxicity and potential inhibitory effects of E. elaterium seed and fruit juice extracts against bovine rotavirus infections in vitro. The findings of this study support the potential therapeutic efficacy of extracts derived from E. elaterium against bovine rotavirus infections. Treatment with the E. elaterium plant extracts resulted in a decrease in the rotavirus titer, indicating its potential as a therapeutic agent. These results have implications for the development of alternative treatments to prevent bovine rotavirus-induced diarrhea in newborn calves, leading to reduced calf mortality, minimized medication costs, and improved economic outcomes in cattle farming.
In a study, researchers investigated the effects of various protease inhibitors on neonatal mice infected with the MO strain of human rotavirus [
11]. Notably, neonatal mice infected with the trypsin-activated human rotavirus strain MO and subsequently administered cysteine or serine protease inhibitors at 12 and 24 h post-infection yielded significant findings. Suckling mice receiving a high dose (300 µg) of the cysteine inhibitor, Ovocystatin, developed no diarrhea, whereas only 20% of those receiving a low dose (38 µg) of Ovocystatin experienced diarrhea. In another group, mice given a high dose (300 µg) of aprotinin, a serine protease inhibitor, exhibited a 26.7% incidence of diarrhea, in contrast to a 14.3% incidence among neonatal mice administered with a low dose (150 µg). These results indicate that protease inhibitors can be effectively used in the treatment and prevention of rotaviral-induced diarrhea.
Antiviral protease inhibitors can be derived from various sources, including animal origins, recombinant expressions, and chemical synthesis. A significant proportion of these inhibitors can be naturally extracted from plants, such as
E. elaterium, as demonstrated in the present study. Our research further establishes the efficacy of fruit juice in suppressing rotavirus replication in vitro, offering promise for preventive therapy against rotaviral diarrhea in newborn calves. The fruit juice of
E. elaterium is commonly used for sinusitis treatment, while some parts obtained from
E. elaterium are externally applied for tumor and eczema treatments, showing some levels of success in therapy [
25,
26]. Previous studies have demonstrated that the fruit juice exhibits increasing anti-inflammatory activity in a dose-dependent manner, which has been attributed to the presence of cucurbitacin B, another compound enriched in the
E. elaterium [
16]. Cingi et al. [
27] reported a 50% improvement in patients with chronic sinusitis when liquid obtained from the fruits of
E. elaterium was applied as nasal drops, and Sezik et al. detected a 71% improvement in another study [
28]. Various studies have reported different levels of effectiveness against
Candida albicans,
Aspergillus fumigatus, and
Escherichia coli in extracts from different parts of the
E. elaterium fruit [
20,
29]. However, a study investigating the antiviral activity of different parts of the
E. elaterium plant found no anti-HSV-1/2 effect and only a low level of antiretroviral activity against HIV in vitro [
30]. It is essential to note that exposure to undiluted
E. elaterium juice can be highly toxic, with the potential for serious side effects, including lethality [
31]. Considering the potential detrimental effects, caution should be exercised in the use of such plant extracts due to their dose-dependent cytotoxicity [
32]. While certain studies have indicated a beneficial effect against infection, the precise antiviral mechanisms are still not completely understood.
A limitation of this study is that the specific antiviral properties obtained from the plant extracts, whether attributed to cucurbitacin B or EETIso as a protease inhibitor, remain unknown. Further investigation is needed to elucidate the specific mechanisms of action and identify the active compounds responsible for the observed antiviral effects. This information would provide a more comprehensive understanding of the therapeutic potential of the E. elaterium extracts against bovine rotavirus infections. Other limitations of our study included the absence of a suitable murine model to study viral pathogenesis and the effects of E. elaterium extracts treatment in vivo. Currently, there are no specific animal models for treating diarrhea caused by rotavirus infection. Various laboratory animal models have been employed to study RV infection for vaccine development; however, viral challenges or primary infections did not manifest similar symptomatic infection. While newborn mice can be infected with bovine rotavirus and develop some intestinal damage, they typically do not exhibit diarrhea.