Response of Biofortified Green Bean Plants to Colletotrichum lindemuthianum
Abstract
:1. Introduction
2. Materials and Methods
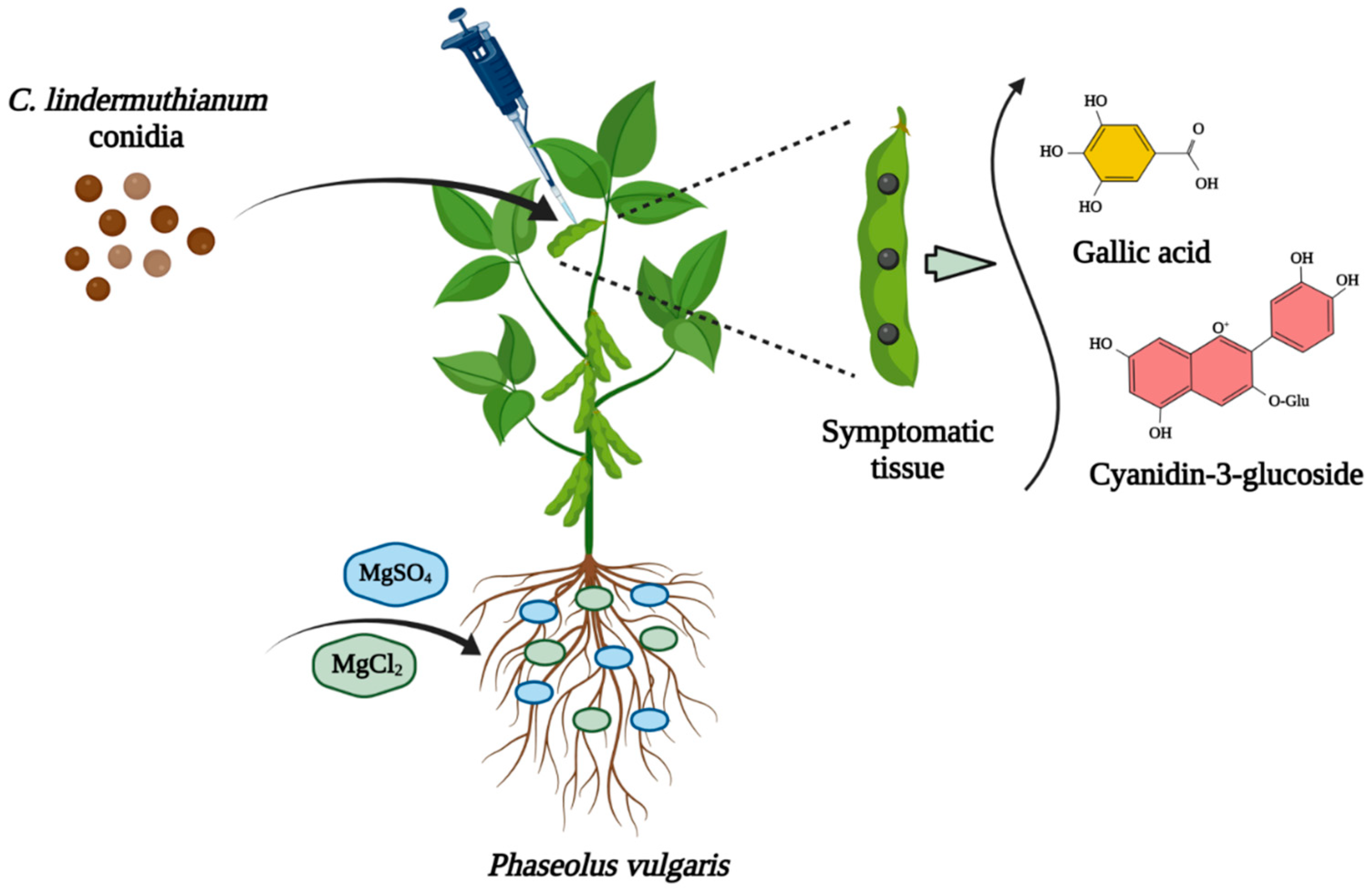
2.1. Biofortification with Magnesium
2.2. C. lindemuthianum Inoculation and Disease Assessment
2.3. Bioactive Compounds (BCs)
2.3.1. Total Anthocyanins
2.3.2. Total Phenols
2.3.3. Total Flavonoids
2.4. Statistical Analysis
3. Results and Discussion
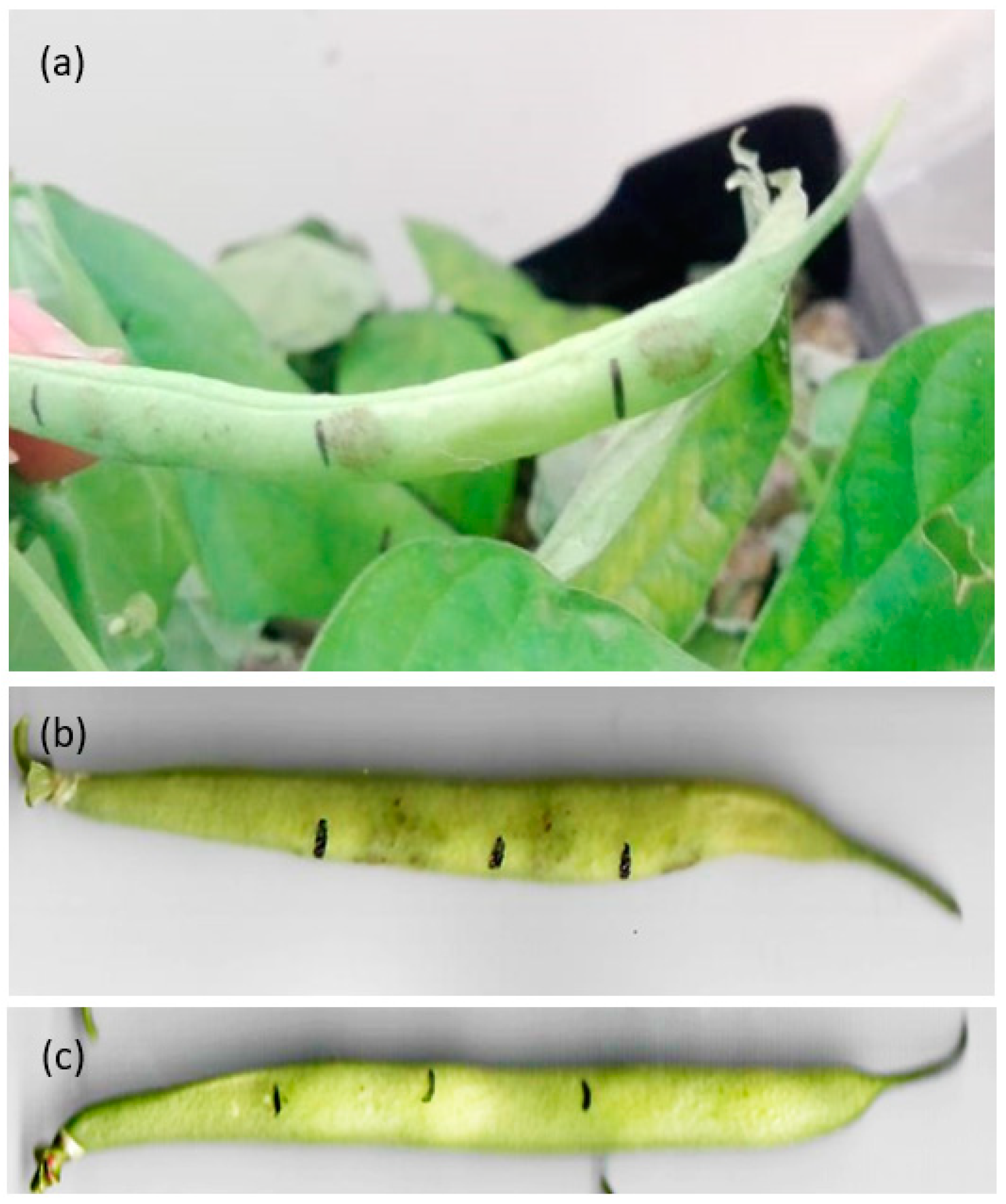
3.1. Symptoms on Green Bean Pods
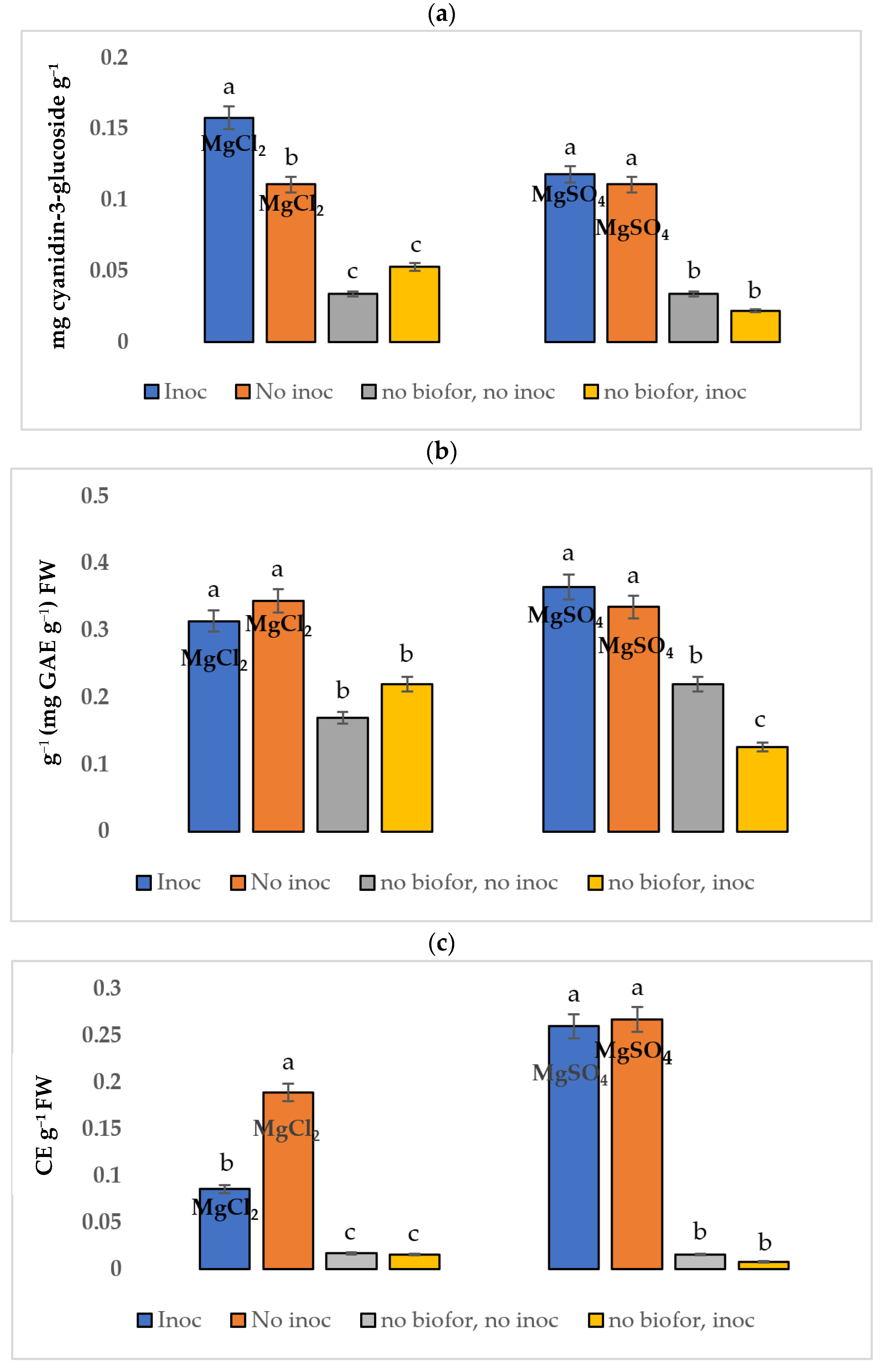
3.2. Bioactive Compounds in Healthy and Diseased Tissues
3.2.1. Total Anthocyanin Content
3.2.2. Total Phenols
3.2.3. Total Flavonoids
3.3. C. lindemuthianum in Mg-Biofortified Green Bean Pods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva-Rojas, H.V.; Ávila-Quezada, G.D. Phylogenetic and morphological identification of Colletotrichum boninense: A novel causal agent of anthracnose in avocado. Plant Pathol. 2011, 60, 899–908. [Google Scholar] [CrossRef]
- García-Ávila, C.; Valenzuela-Tirado, G.A.; Florencio-Anastasio, J.G.; Ruiz-Galván, I.; Moreno-Velázquez, M.; Hernández-Macías, B.; López-Buenfil, J.A.; Bravo-Pérez, D.; Pineda-Ríos, J.M.; Quezada-Salinas, A.; et al. Organisms associated with damage to post-harvest potato tubers. Mex. J. Phytopathol. 2018, 36, 308–320. [Google Scholar]
- Martins, S.J.; de Faria, A.F.; Pedroso, M.P.; Cunha, M.G.; da Rocha, M.R.; de Medeiros, F.H.V. Microbial volatiles organic compounds control anthracnose (Colletotrichum lindemuthianum) in common bean (Phaseolus vulgaris L.). Biol. Control. 2019, 131, 36–42. [Google Scholar] [CrossRef]
- Cakmak, I.; White, P.J. Magnesium in crop production and food quality. Plant Soil 2020, 457, 1–3. [Google Scholar] [CrossRef]
- Mathew, A.A.; Panonnummal, R. Magnesium the master cation as a drug possibilities and evidences. Biometals 2021, 34, 955–986. [Google Scholar] [CrossRef]
- Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. Magnesium (Mg2+) deficiency, not well-recognized non-infectious pandemic: Origin and consequence of chronic inflammatory and oxidative stress-associated diseases. Cell Physiol. Biochem. 2023, 57, 1–23. [Google Scholar]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral biofortification of vegetables as a tool to improve human diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil 2013, 368, 73–85. [Google Scholar] [CrossRef]
- Marra, R.; Lombardi, N.; Piccolo, A.; Bazghaleh, N.; Prashar, P.; Vandenberg, A.; Woo, S. Mineral biofortification and growth stimulation of lentil plants inoculated with Trichoderma strains and metabolites. Microorganisms 2021, 10, 87. [Google Scholar] [CrossRef]
- Avila-Quezada, G.D.; Ingle, A.P.; Golińska, P.; Rai, M. Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks. Nanotech. Rev. 2022, 11, 2123–2140. [Google Scholar] [CrossRef]
- Ciscomani-Larios, J.P.; Sánchez-Chávez, E.; Jacobo-Cuellar, J.L.; Sáenz-Hidalgo, H.K.; Orduño-Cruz, N.; Cruz-Alvarez, O.; Avila-Quezada, G.D. Biofortification efficiency with magnesium salts on the increase of bioactive compounds and antioxidant capacity in snap beans. Ciência Rural 2021, 51, e20200442. [Google Scholar] [CrossRef]
- Ali, M.; Li, Q.H.; Zou, T.; Wei, A.M.; Gombojab, G.; Lu, G.; Gong, Z.H. Chitinase gene positively regulates hypersensitive and defense responses of pepper to Colletotrichum acutatum infection. Int. J. Mol. Sci. 2020, 21, 6624. [Google Scholar] [CrossRef]
- Schulman, P.; Ribeiro, T.H.; Fokar, M.; Chalfun-Junior, A.; Lally, R.D.; Paré, P.W.; de Medeiros, F.H. A microbial fermentation product induces defense-related transcriptional changes and the accumulation of phenolic compounds in Glycine max. Phytopathol. 2022, 112, 862–871. [Google Scholar] [CrossRef]
- Abbey, J.; Jose, S.; Percival, D.; Jaakola, L.; Asiedu, S.K. Modulation of defense genes and phenolic compounds in wild blueberry in response to Botrytis cinerea under field conditions. BMC Plant Biol. 2023, 23, 117. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Amara, A.A.A.F. Recent approaches towards control of fungal diseases in plants: An updated review. J. Fungi 2021, 7, 900. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Jakopic, J.; Cunja, V.; Veberic, R.; Munda, A.; Stampar, F. Phenolic compounds as defence response of pepper fruits to Colletotrichum coccodes. Physiol. Mol. Plant Pathol. 2013, 84, 138–145. [Google Scholar] [CrossRef]
- Slatnar, A.; Petkovsek, M.M.; Halbwirth, H.; Stampar, F.; Stich, K.; Veberic, R. Polyphenol metabolism of developing apple skin of a scab resistant and a susceptible apple cultivar. Trees 2012, 26, 109–119. [Google Scholar] [CrossRef]
- Yu, D.; Wei, W.; Fan, Z.; Chen, J.; You, Y.; Huang, W.; Zhan, J. VabHLH137 promotes proanthocyanidin and anthocyanin biosynthesis and enhances resistance to Colletotrichum gloeosporioides in grapevine. Hortic. Res. 2023, 10, uhac261. [Google Scholar] [CrossRef]
- Somalraju, A.; Mccallum, J.L.; Main, D.; Peters, R.D.; Fofana, B. Foliar selenium application reduces late blight severity and incidence in potato and acts as a pathogen growth inhibitor and elicitor of induced plant defence. Can. J. Plant Pathol. 2022, 44, 39–55. [Google Scholar] [CrossRef]
- Márquez-Quiroz, C.; De-la-Cruz-Lázaro, E.; Osorio-Osorio, R.; Sánchez-Chávez, E. Biofortification of cowpea beans with iron: Iron’s influence on mineral content and yield. J. Soil Sci. Plant Nutr. 2015, 15, 839–847. [Google Scholar] [CrossRef]
- Sida-Arreola, J.P.; Sánchez-Chávez, E.; Ávila-Quezada, G.D.; Zamudio-Flores, P.B.; Acosta, M.C. Iron biofortification and its impact on antioxidant system, yield and biomass in common bean. Plant Soil Environ. 2015, 61, 573–576. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents, Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Trousdale, E. Caftaric acid disappearance and conversion to products of enzymatic oxidation in grape must and wine. Am. J. Enol. Vitic. 1985, 36, 50–56. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Tian, C. Mucin Msb2 cooperates with the transmembrane protein Sho1 in various plant surface signal sensing and pathogenic processes in the poplar anthracnose fungus Colletotrichum gloeosporioides. Mol. Plant Pathol. 2021, 22, 1553–1573. [Google Scholar] [CrossRef]
- Miranda-Gómez, B.; García-Hernández, A.; Muñoz-Castellanos, L.; Ojeda-Barrios, D.L.; Avila-Quezada, G.D. Pectate lyase production at high and low pH by Colletotrichum gloeosporioides and Colletotrichum acutatum. Afr. J. Microbiol. Res. 2014, 8, 1948–1954. [Google Scholar]
- Ferreira, J.J.; Campa, A.; Kelly, J.D. Organization of genes conferring resistance to anthracnose in common bean. In Translational Genomics for Crop Breeding; Varshney, R.K., Tuberosa, R., Eds.; John Wiley & Sons Inc.: Iowa City, IA, USA, 2013; pp. 151–181. [Google Scholar]
- Harshman, J.M.; Jurick, W.M.; Lewers, K.S.; Wang, S.Y.; Walsh, C.S. Resistance to Botrytis cinerea and quality characteristics during storage of raspberry genotypes. HortScience 2014, 49, 311–319. [Google Scholar] [CrossRef]
- Bassolino, L.; Zhang, Y.; Schoonbeek, H.-J.; Kiferle, C.; Perata, P.; Martin, C. Accumulation of anthocyanins in tomato skin extends shelf life. New Phytol. 2013, 200, 650–655. [Google Scholar] [CrossRef]
- Duan, Y.; Hao, S.; Luo, R.; Lu, Y.; Li, G.; Zhang, J.; Tian, J.; Yao, Y. Antioxidant defense against rust infection in the leaf tissue of Malus crabapple. Acta Physiol. Plant. 2019, 41, 58. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, V.; Alkan, N. Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharvest Biol. Technol. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Ghorbi, M.; Momeni, H.; Rashidi, V.; Ahmadzadeh, A.; Yarnia, M. Changes in phenolic acid levels in wheat cultivars inoculated with Pyrenophora tritici-repentis race 1. J. Agric. Sci. Technol. 2023, 25, 185–198. [Google Scholar] [CrossRef]
- Schovankova, J.; Opatova, H. Changes in phenols composition and activity of phenylalanine-ammonia lyase in apples after fungal infections. Hortic. Sci. 2011, 38, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, R.; Tian, Y.; Wang, H.; Ma, F.; Liu, C.; Liang, W.; Li, C. Exogenous chitosan enhances the resistance of apple to Glomerella leaf spot. Sci. Hortic. 2023, 309, 111611. [Google Scholar]
- Morcillo, M.; Sales, E.; Corredoira, E.; Martínez, M.T.; Segura, J.; Arrillaga, I. Effect of methyl jasmonate in gene expression, and in hormonal and phenolic profiles of Holm oak embryogenic lines before and after infection with Phytophthora cinnamomi. Front. Plant Sci. 2022, 13, 824781. [Google Scholar] [CrossRef]
- Pérez Gómez, L.; Mendoza Rodríguez, J.; Quirós Molina, Y.; Leiva-Mora, M.; Martinez-Montero, M.E.; Acosta-Suarez, M.; Ferrer Serrano, A.; Trujillo Sánchez, R.; Pérez Martínez, A.T. Antifungal activity of Mosiera bullata (Britton & P. Wilson) extract against phytopathogenic fungi. Vegetos 2022, 1–10. [Google Scholar] [CrossRef]
- Rana, B.; Chahal, K. Phenolic compounds under stress. In Plant Metabolites under Environmental Stress; Desai, N.M., Patil, M., Pawar, U.R., Eds.; Apple Academic Press: New York, NY, USA, 2023; pp. 203–218. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK; Elsevier: London, UK, 2012; 650p. [Google Scholar]
- Zhang, M.; Zhang, Y.; Li, Y.; Bi, Y.; Mao, R.; Yang, Y.; Jiang, Q.; Prusky, D. Cellular responses required for oxidative stress tolerance of the necrotrophic fungus Alternaria alternata, causal agent of pear black spot. Microorganisms 2022, 10, 621. [Google Scholar] [CrossRef]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Madadkar Haghjou, M.; Najafi-Kakavand, S.; Charagh, S.; Osman, H.S.; Albaqami, M.; Zhuang, Y.; et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 961872. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The key roles of ROS and RNS as a signaling molecule in plant–microbe interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant secondary metabolites as a tool to investigate biotic stress tolerance in plants: A review. Gesunde Pflanzen 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Kawa, D.; Brady, S.M. Root cell types as an interface for biotic interactions. Trends Plant Sci. 2022, 11, 1173–1186. [Google Scholar] [CrossRef]
- Xia, Z.; He, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. Sex-specific interactions shape root phenolics and rhizosphere microbial communities in Populus cathayana. For. Ecol. Manag. 2022, 504, 119857. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Ávila-Quezada, G.D.; Balandrán-Quintana, R.R.; Glossman-Mitnik, D.; Ruiz-Cruz, S. Characterization of the semiquinones and quinones of (−)-epicatechin by means of computational chemistry. J. Mol. Struct. Theochem 2009, 897, 6–11. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Micaelli, F.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A.; Quartacci, M.F. Elevated CO2 and salinity are responsible for phenolics-enrichment in two differently pigmented lettuces. Plant Physiol. Biochem. 2017, 115, 269–278. [Google Scholar] [CrossRef]
- Gonçalves-Vidigal, M.C.; Cruz, A.S.; Lacanallo, G.F.; Vidigal Filho, P.S.; Sousa, L.L.; Pacheco, C.M.; McClean, P.; Gepts, P.; Pastor-Corrales, M.A. Co-segregation analysis and mapping of the anthracnose Co-10 and angular leaf spot Phg-ON disease-resistance genes in the common bean cultivar Ouro Negro. Theor. Appl. Genet. 2013, 126, 2245–2255. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S. Role of iron in rhizobacteria-mediated suppression of root-infecting fungi and root-knot nematodes in tomato. Nematol. Mediterr. 2003, 31, 11–14. [Google Scholar]
- Guerrero-Prieto, V.M.; Berlanga-Reyes, D.I.; Jacobo-Cuéllar, J.L.; Guigón-López, C.; Ojeda-Barrios, D.; Ávila-Quezada, G.D.; Núñez-Barrios, A.; Hernández-Rodríguez, A. Calcium content on apple fruit influences the severity of Penicillium expansum. Phyton-Int. J. Experim. Bot. 2017, 86, 74–78. [Google Scholar]
- Krawczyk, K.K.; Smoleń, S.; Wisła-Świder, A.; Kowalska, I.; Kiełbasa, D.; Pitala, J.; Krzeminska, J.; Wasniowska, J.; Koronowicz, A. Kale (Brassica oleracea L. var. sabellica) biofortified with iodoquinolines: Effectiveness of enriching with iodine and influence on chemical composition. Sci. Hortic. 2024, 323, 112519. [Google Scholar] [CrossRef]
- Sindireva, A.; Golubkina, N.; Bezuglova, H.; Fedotov, M.; Alpatov, A.; Erdenotsogt, E.; Sekara, A.; Murariu, O.C.; Caruso, G. Effects of high doses of selenate, selenite and nano-selenium on biometrical characteristics, yield and biofortification levels of Vicia faba L. cultivars. Plants 2023, 12, 2847. [Google Scholar] [CrossRef]
- Malka, M.; Du Laing, G.; Bohn, T. Separate effects of foliar applied selenate and zinc oxide on the accumulation of macrominerals, macronutrients and bioactive compounds in two pea (Pisum sativum L.) seed varieties. Plants 2022, 11, 2009. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Montiel, L.G.; Ciscomani-Larios, J.P.; Sánchez-Chávez, E.; Vargas-Arispuro, I.; Hashem, A.; Abd_Allah, E.F.; Avila-Quezada, G.D. Response of Biofortified Green Bean Plants to Colletotrichum lindemuthianum. Microbiol. Res. 2023, 14, 2067-2078. https://doi.org/10.3390/microbiolres14040139
Hernández-Montiel LG, Ciscomani-Larios JP, Sánchez-Chávez E, Vargas-Arispuro I, Hashem A, Abd_Allah EF, Avila-Quezada GD. Response of Biofortified Green Bean Plants to Colletotrichum lindemuthianum. Microbiology Research. 2023; 14(4):2067-2078. https://doi.org/10.3390/microbiolres14040139
Chicago/Turabian StyleHernández-Montiel, Luis G., Juan P. Ciscomani-Larios, Esteban Sánchez-Chávez, Irasema Vargas-Arispuro, Abeer Hashem, Elsayed F. Abd_Allah, and Graciela D. Avila-Quezada. 2023. "Response of Biofortified Green Bean Plants to Colletotrichum lindemuthianum" Microbiology Research 14, no. 4: 2067-2078. https://doi.org/10.3390/microbiolres14040139
APA StyleHernández-Montiel, L. G., Ciscomani-Larios, J. P., Sánchez-Chávez, E., Vargas-Arispuro, I., Hashem, A., Abd_Allah, E. F., & Avila-Quezada, G. D. (2023). Response of Biofortified Green Bean Plants to Colletotrichum lindemuthianum. Microbiology Research, 14(4), 2067-2078. https://doi.org/10.3390/microbiolres14040139









