Abstract
The current study evaluated the biogenic synthesis of nickel oxide nanoparticles (SP-NiONPs) from the root extract of (Salvadora persica) S. persica and their biological properties. The nanoparticles were characterized using spectroscopic and microscopic techniques and then evaluated for their antimicrobial properties against 10 oral pathogens. The ultraviolet-visible (UV–Vis) spectra exhibited a distinctive resonance spectrum at 334 nm for the SP-NiONPs produced from S. persica. The fourier transform infrared (FTIR) analysis revealed the presence of functional groups of biomolecules of S. persica that served as reducing and capping agents of the SP-NiONPs. The scanning electron microscope (SEM) and transmission electron microscopy (TEM) analyses showed that the nanoparticles were spherical-shaped, tightly packed, and ranged in size from 18.20 nm to 45.12 nm. The energy dispersive x-ray (EDX) analysis confirmed 69.9% of the nickel (Ni) content by weight, and the X-ray diffraction (XRD) results showed the face-centered cubic (FCC) crystalline structure of the formed SP-NiONPs. The antioxidant activity of the SP-NiONPs exhibited a dose-dependent profile with an IC50 value of 51.45 ± 0.65 and a 54.13 ± 0.98 DPPH• and ABTS•+ radical scavenging activity, respectively. The SP-NiONPs showed an antibacterial activity against all the test strains; however, E. cloacae was found to be the most sensitive strain, with an inhibition zone of 31 ± 0.50 mm. The SEM image of the E. cloacae cells treated with SP-NiONPs showed irregular shapes and ruptured, destroyed cell membranes. Our findings revealed that SP-NiONPs could be used as excellent antibacterial agents against oral pathogens.
1. Introduction
The oral cavity provides a variety of distinct habitats for microbial colonization, including the teeth, gingival sulcus, attached gingiva, tongue, cheek, lips, and hard and soft palate. These oral habitats support the growth of diverse microbial communities and make up a highly heterogeneous ecological system [1]. Microorganisms can flourish in the warm, moist environment of the mouth, which provides host-derived nutrients such as salivary proteins, glycoproteins, and gingival crevicular fluid [2]. In addition, the teeth offer exceptional opportunities for extensive biofilm formation and microbial persistence [3]. More than 600 prokaryotic taxa have been found in the oral cavity so far, many of which are difficult to isolate using conventional culture techniques [4]. With the increased prevalence of oral diseases, several therapeutic strategies were considered for microbial control, including mechanical and chemical methods. However, the current strategies to prevent dental diseases are not completely free of side effects. This has led to the development of alternative nanomaterials using natural products with antimicrobial activity to treat oral diseases.
Bio-nanoparticle technology is considered to be more effective than conventional nanoparticle technology and is employed in the procedures of dental filling, enamel cleaning for the prevention of caries, and implantation technology as nano-implant particles [5]. The ability of nanomaterials to prevent bacterial growth and the production of bacterial biofilms is a crucial aspect of the nano-strategy [6]. Nanotechnology is gaining tremendous popularity among individuals as it is affordable, time saving, and has the potential to prevent major dental surgery [7]. Among the various types of metallic and non-metallic nanoparticles, there are several benefits from adopting nickel oxide nanoparticles as the type of nanoparticle [8]. Nickel oxide nanomaterials are durable, chemically more stable, small sized, easy to synthesize, and exhibit pronounced thermal resistance with a long shelf life compared to other forms of organic or bulk oxide nanomaterials, including silver oxide, copper oxide, and zinc oxide nanoparticles [9]. An extensive spectrum of fruitful applications for nickel oxide nanoparticles (NiONPs) in photocatalysis, catalytic analysis, magnetic materials, batteries, sensors, and superconductors as well as their uses in the medicinal industry such as antibiotic, hygiene and cosmetic products, nano-carriers for the release and transfer of drugs at the targeted cells, and cancer treatment, have been documented [10,11]. Several studies have demonstrated that NiONPs have a very low selective toxicity in contrast to other types of nanoparticles and showed minimal toxicity effects on human cells [12]. These nanostructures are reported to possess antimicrobial effects against various gram-negative and gram-positive bacterial strains as well as fungal spores that are often resistant to high temperatures and pressures [13,14,15,16,17]. The advancement of modern nanotechnology, due to its efficacy and precision, can address a variety of dental issues. However, the efficiency of nanomaterials can be further enhanced by mixing them with bioactive constituents extracted from natural phyto-extracts that have promising medicinal properties and that are traditionally utilized for cleaning and whitening teeth enamel [12]. Nanobiotechnology based on the phyto-mediated synthesis of nanoparticles has become a growing trend in green chemistry due to its easy, affordable, and non-toxic nature for overcoming the problem of toxic chemicals and non-ecofriendly methods. Plant-based syntheses for nanomaterials have a major advantage over chemical methods since no hazardous chemicals are needed for the reduction in and capping of the nanomaterials. Various parts of plants, such as leaves, bark, roots, and fruit peels, have been used in previous studies [13]. The texture of the leaves and petals of plants act as a bio-template thereby controlling the size of the nanoparticles and hindering the agglomeration of the particles [14].
Salvadora persica (S. persica), the desert plant family of Salvadoraceae, has a long history as a medicinal herb and is commonly used as a tooth-cleaning stick in many African and Middle Eastern countries [15]. It contains carbohydrates, flavonoids, volatile oils, alkaloids, steroids, terpenoids, and saponin [16,17]. The sticks from S. persica also contain a substantial amount of three lignin glycosides [18,19] and minor components such as volatile oils and flavonoids [20]. Toxicity studies using laboratory animals showed that the aqueous extract of S. persica is quite safe [21]. S. persica exhibits antimicrobial, anti-inflammatory, antipyretic, antioxidant, anticancer, and other activities [22,23,24]. Given the negative consequences of chemical agents, herbal medicine with unique features such as being natural, inexpensive, readily available and accessible, and well accepted without any significant negative effects has recently received a lot of attention. Various anti-inflammatory, antibacterial, antiviral, antifungal, and antioxidant characteristics have been attributed to medicinal plants [25]. Herbal medicine and medicinal plants have also long been recognized as safe treatments for dental and oral problems.
Therefore, the aim of this study was to use a green synthesis approach for the synthesis of biogenic SP-NiONPs using S. persica and to evaluate its antioxidant and antimicrobial activity to assess its possible chemo-preventive activity against oral lesions. The characterization step will provide more insight into the effectiveness of the bio-reduction, stability, and size of SP-NiONPs.
2. Materials and Methods
2.1. The Chemical Reagents
Ethanol (80%, LR), nickel (II), nickel hexahydrate (Ni(NO3)2.6H2O), 2,2-Diphenyl-1-picrylhydrazyl (DPPH, 98.81%), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate (K2S2O8, ≥99%, AR), nutrient agar, dextrose agar, Mueller–Hinton broth, phosphate buffered saline (PBS), glutaraldehyde (OHC(CH2)3CHO) solution, and paraformaldehyde (95%) were procured from Sigma-Aldrich, St. Louis, MO, USA.
2.2. Preparation of the S. persica Extract
The roots (miswak chewing sticks) of Salvadora persica were collected from the Jazan region, Saudi Arabia in April 2022. The plant was identified by a taxonomist, and a voucher specimen (SP-678) was deposited at the herbarium, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia, for future reference. The sticks were cleaned, chopped into small pieces, shade dried at an ambient temperature, and then ground to a coarse powder using a domestic blender. The powder (100 g) of S. persica was dipped in 80% of ethanol (3 × 500 mL) at room temperature with continuous shaking for 48 h. The supernatant was collected and filtered through Whatman filter paper (0.45 μm). The extraction procedure was repeated two more times under similar conditions. All the obtained ethanolic extracts were pooled, centrifuged, and concentrated under reduced pressure at 40 °C ± 5 using a rotary evaporator. The obtained residue (24.5 g) was stored in the refrigerator at 4 °C for subsequent experiments.
2.3. Green Synthesis of Nickel Oxide Nanoparticles Using S. persica (SP-NiONPs)
A green synthesis approach was applied for the synthesis of biogenic NiO nanoparticles by obeying the previously described Uddin et al. [26] method with slight modifications. Briefly, 100 mL of the ethanol extract of the S. persica root (20 g) was added to 50 mL of a Ni(NO3)2.6H2O, (5% w/v) precursor solution and heated at 70–80 °C with constant stirring for 4 h to ensure a complete oxidation and reduction process. After thorough stirring, the reaction mixture was placed in the dark to monitor the color change and precipitate formation at the bottom of the flask. The formation of biogenic NiO pellets was confirmed by the alteration in the color. Then, the precipitates were recovered from the bottom of flask after centrifugation at 15,000× g rpm for 30 min. After centrifugation, the pellets were collected, and the clear supernatant was discarded. The obtained pellets were dissolved in deionized water and washed thoroughly several times to get rid of contaminants. The pellets were then dried overnight in an oven at 100 °C and calcined for efficient crystallization at 300 °C. The calcinated nickel oxide nanoparticles (SP-NiONPs) were powdered using a mortar and pestle and stored in glass vials for further analysis.
2.4. Characterization of the SP-NiONPs
Several techniques were applied to characterize the biosynthesized SP-NiONPs. Ultraviolet–visible spectroscopy (UV–Vis) was performed to confirm the formation of the SP-NiONPs at a wavelength of 200–800 nm using a UV–Vis spectrophotometer (UV-1601, Shimadzu, Kyoto, Japan), and the blank was used for comparision. Fourier transform infrared spectroscopy (FTIR, Agilent Technologies, Santa Clara, CA, USA) in the range of 4000–400 cm−1 was used to detect the functional groups and phytoconstituents involved in the reduction in and stabilization of the NiO nanoparticles. The phase of the biogenic SP-NiONPs was characterized using X-Ray diffraction (XRD,) and the spectra were recorded in the 2θ (20–80°) with CuKα radiation (λ = 1.5406 Å) at 40 kV and 30 mA (XRD model 6000 diffractometer Shimadzu, Columbia, SC, USA). The XRD patterns were computed by the XRD-6000 (P/N 215-00283-02) lab software version 2.0 (2018) and compared with the JCPDS Card: ICSD ID 47–1049. The surface morphology and elemental mapping of the biosynthesized NiONPs were studied using scanning electron microscopy (SEM) coupled with an energy-dispersive X-ray (EDX) and carried out on the JEOL-JSM-6390 LA analyzer (JEOL, Tokyo, Japan). The particle morphological features and size of the as-synthesized SP-NiONPs were investigated using transmission electron microscopy (TEM), conducted on JOEL-JEM-1011 (JEOL, Tokyo, Japan). Dynamic light scattering (DLS) and zeta potential (ZI) were performed to examine the particle size, stability, and surface charge of the formed SP-NiONPs using a particle analyzer (Nano ZS Zetasizer, Malvern Instruments, Malvern, UK).
2.5. Antioxidant Activity
2.5.1. DPPH Radical Scavenging Assay
The DPPH• free radical scavenging activity of the ethanol extract of S. persica and the pre-synthesized SP-NiONPs was measured using the proposed method by [27] with slight modification. Briefly, 200 µL of a DPPH• methanol solution was mixed with a varied concentration of the test samples (15, 30, 60, 70, 90, 120, 140 µg mL−1) and incubated for 30 min at 30 °C. After 30 min of incubation, the absorbance of the reaction mixture was noted at a 517 nm wavelength using a microtiter plate reader (SpectraMax Gemini, Molecular Devices, San Jose, CA, USA). The data obtained were estimated using the Pro 3.0 software Softmax (Molecular Devices, Sunnyvale, CA, USA). The minimum antioxidant needed to scavenge 50% of the DPPH• free radicals was known as the IC50 value. The positive control applied was ascorbic acid. The percentage of the DPPH free radical scavenging potential was determined by applying the following equation.
where As and Ac represent the absorbance of the test sample and control, respectively, reacted with the mixture of the DPPH• free radical.
2.5.2. ABTS•+ Radical Cation Scavenging Assay
The ABTS•+ radical cation scavenging was determined using a method proposed by Mohan et al. [28] with minor modifications. The parent ABTS•+ solution was prepared by treating ABTS (7 mM) dissolved in distilled water with potassium persulfate (2.45 mM) at a 2:1 (v/v) ratio. The prepared solution was then placed for 16 h at room temperature in the dark to allow for the ABTS and potassium persulfate to completely react. A stable ABTS•+ radical cation was formed during this reaction. The parental ABTS•+ solution was stable for over two days when maintained at room temperature and was protected from sunlight. The freshly prepared parent ABTS•+ solution was diluted with ethanol to a working ABTS•+ solution concentration of 0.70 ± 0.04 absorption units at a 734 nm wavelength before use. After that, 400 µL of the working ABTS•+ solution was mixed with a varied concentration of the test samples (15, 30, 60, 70, 90, 120, 140 µg mL−1) and incubated for 10 min at an ambient temperature under dark conditions. After 10 min, the absorbance of the reaction mixture was determined at 734 nm in a 96-well plate using a spectrophotometer. The ABTS•+ solution without antioxidants represented 100% free radicals, and the positive control employed was ascorbic acid. The following equation was applied to calculate the percentage of the ABTS•+ radical cation scavenging activity.
where As and Ac represent the absorbance of the test sample and control, respectively, before and after the scavenging reaction.
2.6. Antibacterial Assay
2.6.1. Media Preparation
The medium was prepared by dissolving 28 g of nutrient agar and 37 g of dextrose into 1 L of distilled water. The dissolved media was boiled and autoclaved for 15 min at 121 °C, left to cool, and poured into Petri dishes.
2.6.2. Microbial Strains
The strains used in this study were mostly common oral microbes and were provided by the central laboratory at King Saud University, which included bacterial strains of Staphylococcus epidermidis, Enterococcus faecalis Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli Enterobacter cloacae Klebsiella pneumoniae, and Pseudomonas aeruginosa and fungal strains of Candida albicans and Candida tropicalis. All the bacterial strains were reactivated on nutrient agar plates, and the fungal strains were reactivated on dextrose agar (SDA) plates before use.
2.6.3. Agar Well Diffusion Assay
The antibacterial activity was assayed using the well diffusion method. The bacterial strains cultured in broth were adjusted to an inoculum density of a 100 µL:0.1A600 culture containing 3.2 × 108 colony forming units. Further, 20 µL was spread onto 20 mL of sterile agar plates using a sterile cotton swab. The surface of the medium was allowed to dry for approx. 3 min. Sterile wells were inserted on the plates (6 mm in diameter), and 100 µL of the test solution was poured into the well for the test. The plates were then incubated at 37 °C for 24 h, after which the microbial growth was determined by measuring the diameter of the inhibition zone (mm) using a transparent scale [29]. The assay was carried out in triplicate and the results are reported as the means ± SD.
2.6.4. Determination of the Minimum Inhibitory (MIC) and Minimum Bactericidal Concentrations (MBC)
The minimum inhibitory concentration was carried out using a tube macrodilution assay with a slight modification. The Mueller–Hinton broth was used for all the tests. A serial dilution of nanoparticles was made (ug/mL) in 1 mL of broth in sterile test tubes containing 5 × 105 CFU/mL of bacterial inoculums and incubated for 24 h at 37 °C. The highest dilution (lowest concentration) that showed no visible growth was regarded as the MIC. The MBC was determined as an adjunct to the MIC test.
2.6.5. Scanning Electronic Microscope (SEM) Examination
The most sensitive strain was exposed to SP-NiONPs for 24 h and examined under a SEM. The morphology changes in the bacteria exposed to nanoparticles were compared with those of the control grown in pure media. After incubation, the cells were centrifuged at 5000× g r/min for 10 min. The pellets were washed with a sterile PBS and fixed with 2.5% glutaraldehyde and 2% paraformaldehyde for 4 h at 4 °C with intermittent vortexing. The cell biomass was fixed to a glass coverslip and the morphological changes in the cell wall were viewed under a SEM.
3. Results and Discussion
3.1. Characterization of the SP-NiONPs
The initial confirmation of the SP-NiONPs formation was realized by the visual inception of the reaction mixture, and the appearance of white NiONP precipitates deposited at the bottom of the flask. Meanwhile, the color changes in the reaction suspension were used to evaluate the green-mediated synthesis. The ethanol extract of S. persica was light yellow in color, and after the addition of NiO+, the color of the reaction mixture changed to dark yellow, then dark brown, indicating that NiO+ had been reduced to SP-NiONPs (Figure 1). The reduction in NiO+ to NiONPs and the optical features of the biosynthesized SP-NiONPs were measured using UV spectroscopy in a colloidal solution in the wavelength range of 200–800 nm. The UV spectrum of the biogenic SP-NiONPs displayed a profound peak at 334 (Figure 1a), which was a typical characteristic of NiONPs and corresponded to the SP-NiONPs formation The bandgap energy of the as-synthesized NiONPs was assessed by employing the Tauc equation αhv = A(hv − Eg)n, where α, hυ, Eg, and n represent the absorption coefficient, photon energy, band gap, and ½, respectively, for the direct transitions. A graph plotted between (αhv) vs. hv and the band gap was calculated by extrapolating the linear component of the curve to the hv axis (Figure 1b). The band gap energy was found to be 3.47 eV, which was lower than that of the bulk NiO (4.0 eV) due to the quantum confinement effect. Similar studies have reported band gap energies for NiONPs of 4.6 and 3.5 eV using extracts of Stevia rebaudiana and Punica granatum L. (pomegranate) [30,31]. The variation in the band gap energy may be attributed to the difference in the amount of plant extract utilized, the quantity of precursors, the synthesis temperature, as well as the type of extract employed [32]. Hong et al. [32] reported that the properties of NiONPs were influenced by different synthetic conditions. Another important characteristic shown in Figure 1b was the absence of any additional peaks related to contaminants and structural flaws, which was a good indication of the crystalline nature of the biosynthesized SP-NiONPs.
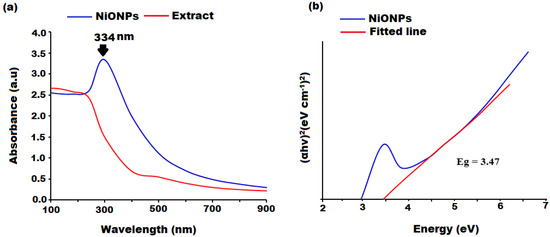
Figure 1.
(a) UV₋Vis absorbance spectra, and (b) the Tauc plot of the green synthesized SP-NiONPs using the S. persica root extract.
The FTIR analysis was performed to identify the functional moieties participating in the reduction, capping, and stabilization of the biosynthesized SP-NiONPs as well as their distribution on the surface of the resulting SP-NiONPs. Figure 2 illustrates the FTIR profile of the dried S. persica root extract and biogenic SP-NiONPs within the 4000 to 400 cm−1 range. The FTIR spectra of the root extract of S. persica (Figure 2a) showed the existence of multiple absorption peaks at 3264.12, 2892.35, 2362.14, 2162.20, 1646.32, 1545.68, 1438.24, 1392.14, and 1146.60 cm−1, which corresponded to O–H stretching (hydroxyl group), C–H stretching (alkane), O=C=O stretching, S–C≡N stretching (thiocyanate), C=C stretching (conjugated alkene or monosubstituted), N–O stretching (nitro compound), O–H bending (carboxylic acid), O–H bending (alcohol), and C–O stretching (tertiary alcohol or aliphatic ether), respectively [33,34]. After the treatment of the Ni(NO3)2·6H2O solution with the bio-extract, the FTIR spectrum exhibited a significant change in its absorption peaks, which were detected at 3436.33, 2926.82, 2092.97, 1625.75, 1459.46, 1385.25, 1112.46, 616.73, 457.27, 443.22, and 418.84 cm−1 for the bio-components adsorbed by the synthesized nanoparticle (Figure 2b). The broad absorption peak that appeared at 3436.33 cm−1 corresponded to the O–H stretching of the free hydroxyl group, which could be attributed to the polyphenolic components of the S. persica extract. The green zinc oxide nanoparticles prepared from a pomegranate peel extract showed an absorption band at 3427.99 cm−1 that corresponded to the O–H stretching of the phenolic component of the extract [35]. Furthermore, two successive absorption peaks were detected at 2926.82 and 2092.97 cm−1 and assigned to the C–H stretching of alkanes and the N=C=S stretching of isothiocyanate, respectively. Whereas the absorption peak observed at 1625.75 cm−1 indicated the presence of the C=C stretching of alkenes. On the other hand, the appearance of peaks at 1459.46, 1385.25, and 1112.46 cm−1 demonstrated the presence of O–H bending of carboxylic acid, C–H bending in alkanes, and C–O stretching in aromatic esters [36]. Moreover, the existence of prominent characteristic peaks at 616.73 cm−1 and 457.27 cm−1 due to the Ni–O bond vibration was indicative of the formation of the metal–oxide (Ni=O) bond, which recognized the configuration of the SP-NiONPs [37]. Overall, the identification of various functional moieties in the biogenic SP-NiONPs, including phenols, alcohols, carboxylic acids, ketones, alkanes, alkenes, amines, and aromatic constituents, increased the possibility they may have contributed to the reduction in Ni(II) and also acted as capping and stabilizing agents for the biogenic SP-NiONPs.
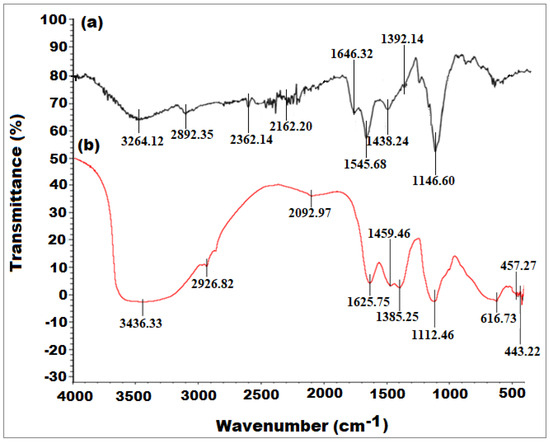
Figure 2.
FTIR spectrum of (a) the S. persica root extract and (b) the synthesized SP-NiONPs using the S. persica root extract.
The crystalline state and phase purity of the formed SP-NiONPs was analyzed using the XRD technique. The XRD analysis revealed the occurrence of four diffraction peaks with 2θ values of 37.2°, 43.2°, 62.7°, and 75.3° (Figure 3), corresponding to the crystal planes of (111), (200), (220), and (311), respectively. These peaks matched the Joint Committee on Powder Diffraction (JCPD) standards, card number (47–1049), for NiO quite well [38]. The existence of the diffracting planes (111), (200), (220), and (311) in the XRD pattern confirmed the face-centered cubic (FCC) crystalline structure of the formed SP-NiONPs. Moreover, the average crystalline size was estimated using the Debye–Scherrer equation, D = kλ/βcosθB, where D, k, λ, β, and θB denoted the crystalline size, with an empirical constant equal to 0.89 or 0.9 depending upon the crystallite shape, wavelength of the X-ray (1.5405 Å), full width at half maximum (FWHM) or integral breath, and Bragg angle, respectively. The average crystallite size of the biogenic SP-NiONPs was found to be 32.4 nm, which was computed from the observed values for the spacing of the (111) plane.
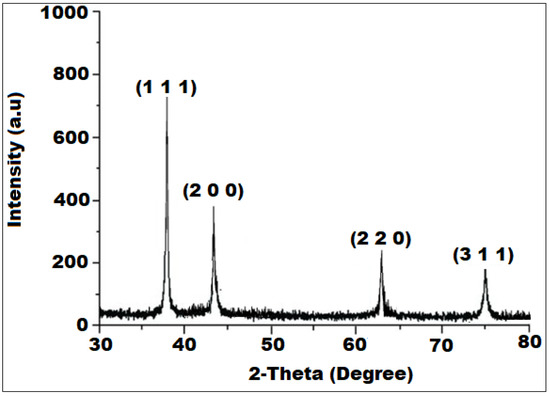
Figure 3.
XRD diffraction patterns of the SP-NiONPs synthesized using the S. persica root extract.
The morphological features of the pre-synthesized SP-NiONPs were investigated using the SEM analysis. The SEM image (Figure 4a) revealed that the nanoparticles were spherical-shaped, tightly packed, highly crystalline, and intercalated with each other due to strong attractive forces. The elemental composition and purity percentage of the green synthesized SP-NiONPs were estimated using the EDX analysis. The analysis revealed the presence of nickel and oxygen, with corresponding mass percentages of 69.96 and 11.21%, respectively (Figure 4b), confirming the formation of NiO nanoparticles. The contaminant peaks, including, Na (12.75%), Si (0.97%), S (3.97%), Cl (0.40%), and Zn (0.79%) observed in the EXD analysis could be attributed to the adsorption of biomolecules on the surface of the SP-NiONPs and impurities from the glass apparatus over which the SP-NiONPs sample was coated. Similarly, the carbon tape used to adhere the sample was responsible for a carbon peak of 0.4 keV.
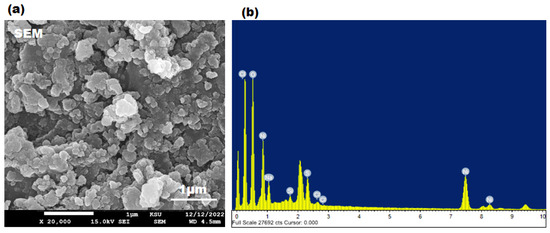
Figure 4.
(a) SEM at ×20,000 magnification; (b) EDX spectrum of the SP-NiONPs synthesized using the S. persica root extract.
The spherical shape of the SP-NiONPs was also confirmed in the TEM image (Figure 5a), and the particle size distribution histogram (Figure 4b) exhibited a size range from 18.20 nm to 45.12 nm, with an average of 25.15 nm and polydispersity index of 0.574, which was within the estimated crystalline size range of the XRD pattern. The zeta potential value was computed to ascertain the surface charge and measure the quantity of the charge of the formed SP-NiONPs. This demonstrated the stability of the nanoparticles in dispersion through the formation of specific charge groups on their surface. The results showed the presence of negatively charged SP-NiONPs with a zeta potential of −28.6 mV (Figure 5c).
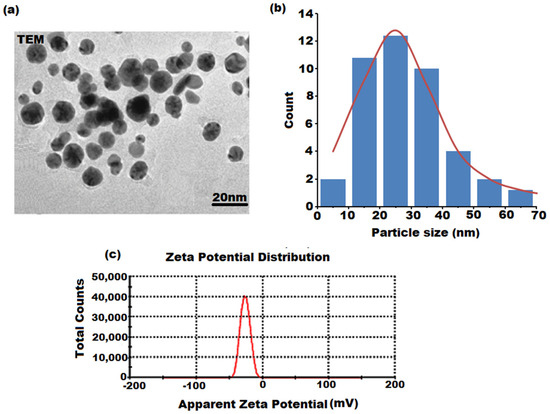
Figure 5.
(a) TEM, (b) particle size distribution histogram with 18.20–45.12 nm size range repsented by red line, and (c) zeta potential measurement (negatively charged, −28.6) of the SP-NiONPs biosynthesized using the S. persica root extract.
3.2. Antioxidant Activity
Antioxidants have been shown in epidemiological studies to slow or stop the course of several chronic illnesses. However, it is challenging to estimate the antioxidant capacity of samples from the assessment of a single antioxidant property. Thus, DPPH• and ABTS•+ were used as the two substrates to assess the antioxidant capability of the biosynthesized SP-NiONPs and S. persica extract. The obtained results revealed that the antioxidant potential of the selected samples increased with the increasing concentration of the samples. The standard antioxidant (ascorbic acid) demonstrated excellent DPPH• and ABTS•+ radical scavenging capabilities of 80.12% and 86.13%, respectively, at 60 μg mL−1 concentrations. The DPPH• and ABTS•+ radical scavenging activities reached 69.14% and 67.26%, respectively, at 90 μg mL−1 concentrations of SP-NiONPs. However, the radical scavenging abilities for DPPH• and ABTS•+ were found to be 84.14% and 72.12%, respectively, at a 100 μg mL⁻1 concentration of SP-NiONPs. Notably, no significant difference was observed for the DPPH• and ABTS•+ radical scavenging potential at 140 μg mL−1 NiONPs in contrast with similar concentrations of ascorbic acid. The findings of our investigation (84.14%) were higher than those published by Uddin et al. [36], who found that nickel oxide nanoparticles (200 μg mL−1) synthesized from a Berberis balochistanica stem extract had radical scavenging capabilities for DPPH of 71.48% [39]. The DPPH• and ABTS•+ radical scavenging activity results demonstrated that the as-prepared SP-NiONPs displayed an efficient antioxidant ability with an IC50 value of 51.45 ± 0.65 and 54.13 ± 0.98, respectively (Table 1). However, the ethanol extract of S. persica showed a significant but lower antioxidant effect compared to the SP-NiONPs for both the DPPH• (IC50 value: 59.12 ± 0.37) and ABTS•+ (IC50 value: 62.25 ± 1.27) radical scavenging activities. Furthermore, in this study, the S. persica extract was chosen as a stabilizing and capping agent for the biosynthesis of the SP-NiONPs due to the presence of an appreciable amount of phytocompounds (phenolic, flavonoid and tannins) and their potent antioxidant properties [40].

Table 1.
IC50 values of the DPPH• and ABTS•+ radical scavenging activity of the S. persica extract and biosynthesized NiONPs.
3.3. Antimicrobial Activity
The antimicrobial activity of the aqueous extract, ethanol extract of S. persica, and biogenic SP-NiONPs at a concentration of 250 μg mL−1 against eight bacterial and two fungal strains was assessed using an agar well diffusion method. This method is widely used to measure the antimicrobial activity of natural products [41]. All the test extracts efficiently suppressed the growth of oral pathogens with variable potency, and the corresponding inhibition zones are shown in Table 2 and Figure 6. As listed in Table 2 and Figure 6, the aqueous and ethanol extracts showed the highest inhibition against E. coli with inhibition zones of 10 ± 0.50 mm and 11 ± 0.40 mm, respectively, whereas the SP-NiONPs highest inhibition zone was 31 ± 0.50 mm against E. cloacae. Since E. cloacae was found to be the most sensitive strain, it was monitored for morphological changes. The MIC and MBC values of the NiO nanoparticles are presented in Table 3. Among the gram-positive bacterial strains, a maximum sensitivity was observed in E. faecalis. E. cloacae was recorded as the most sensitive gram-negative strain, while C tropicalis was among the fungal strains, all with an MIC of 15.6 μg/mL and an MBC of 62.50 μg/mL. N-Benzylbenzamide, extracted from S. persica, had a significant antiseptic effect on E. coli [42]. Sofrata et al. [43] reported a strong antibacterial efficacy of S. persica root chewing sticks against gram-negative oral pathogens due to the presence of benzyl isothiocyanate. Benzyl isothiocyanate, an active component of S. persica, is a well-known bactericidal material that can restrict acid production and the growth of many oral pathogens [44]. The presence of essential oils, sulfur, potassium chloride, sodium chloride, and some organic compounds contribute to its antimicrobial properties [45]. The antibacterial activity of the biogenic SP-NiONPs was higher than the other two extracts from S. persica (Table 2 and Figure 6). ThevSP-NiONPs had a positively charged surface, whereas the bacterial cell wall had a negatively charged surface. As a result, it induced an electromagnetic interaction that caused the bacterial wall and cytoplasm to be destroyed [46]. Furthermore, the SP-NiONPs generated reactive oxygen species (ROS) that caused DNA damage, the oxidation of protein, and killed bacteria [46] The biogenic SP-NiONPs showed a higher inhibitory effect against the gram-negative strains when compared to the gram-positive strains. Similar findings have been reported by the studies [47,48,49]. The difference in the cell wall structure could be the main cause as the gram-negative bacteria contained a layer of lipopolysaccharides and peptidoglycans, which facilitated the entrance of NPs inside the cell. On the other hand, the gram-positive bacteria had a peptidoglycan layer that was covalently bonded with the wall polymers, such as teichoic and teichuronic acids, and acted as a protective layer [50].

Table 2.
Zone of inhibition (mm) of the S. persica aqueous extract, S. persica ethanol extract, and synthesized NPs.
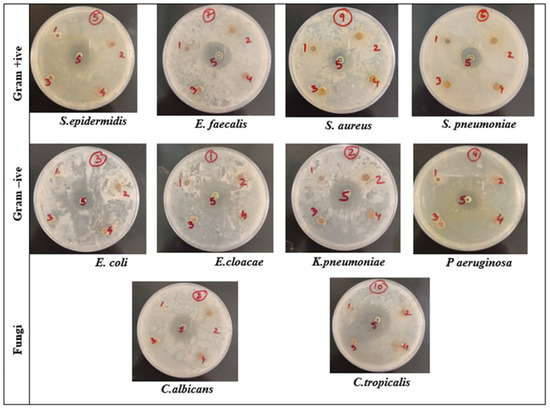
Figure 6.
Well diffusion assay of the S. persica aqueous extract (well 1 and 2), ethanol extract (well 3 and 4), and biosynthesized NiONPs (well 5).

Table 3.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the NiONPs.
The E. cloacae strain was evaluated for morphological changes using the SEM before and after treatment with SP-NiONPs (Figure 7). The SEM image of the control E. cloacae showed uniform smooth rod structures compared to the treated cells with irregular shapes and ruptured, destructed cell membranes. These results pointed toward morphological damage in the bacterial cell membranes through the penetration of SP-NiONPs through the cell wall, which could kill the bacterial cell by releasing intracellular materials. These SEM results supported the growth inhibition in the plates, which showed a good zone of inhibition. The generation of reactive oxygen species (ROS) generated at the NP interface was the principal mechanism behind the antimicrobial activity of SP-NiONPs [51]. The ROS put oxidative stress on the bacterial cell membranes, resulting in membrane damage and cell death [46,52,53].
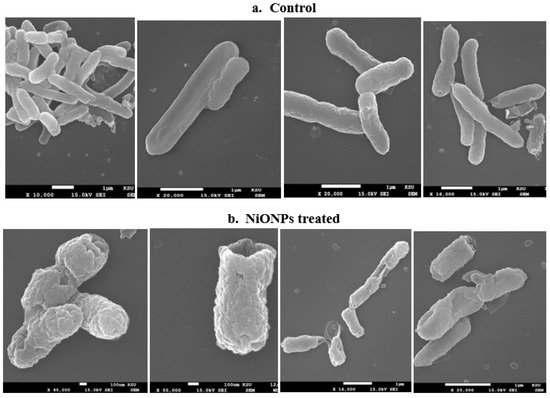
Figure 7.
SEM micrograph showing the Enterobacter cloacae cells treated with NiONPs synthesized from the S. persica extract. (a) Control—shows regular, and smooth rods; (b) treated cells—shows the irregular shape and ruptured and destructed cells.
4. Conclusions
Nickel oxide nanoparticles, with an average size of 32.4 nm, were successfully biosynthesized by an environmentally benign route using an S. persica root extract. The optical properties, crystallinity, morphological features, and composition of the formed SP-NiONPs were determined using various analytical techniques. The root extract of S. persica contained numerous phytoconstituents, including phenolics, flavonoids, steroids, secondary amines, esters, and fatty acids. These bioactive components functioned as reducing and stabilizing agents, which assisted in the reduction in metal ion precursors. The biogenic SP-NiONPs exhibited potent antioxidant and antibacterial activities. The SP-NiONPs efficiently suppressed the growth of all the test microbes with variable potency, but the highest inhibition was recorded against E. cloacae. The SEM images of the SP-NiONPs treated E. cloacae cells showed irregular shapes with ruptured and destructed cell membranes. The findings demonstrate the potential use of biogenic SP-NiONPs in diverse biological applications, especially as antimicrobial agents. Further studies are required to explore their utility at an in vivo level and to ascertain the biochemical processes and mechanisms behind their antioxidant and antimicrobial capabilities.
Author Contributions
Conceptualization, H.B.; visualization and data curation, M.A. and R.S.B.; methodology and formal analysis, M.A. and R.S.B.; validation, H.M.A.-Y.; biological studies, R.S.B.; writing—review and editing, A.E., S.H.A., M.A., R.S.B. and H.B. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project number (RSP 2023/175), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The outcomes of this study support the findings and are included within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, X.; He, J.; Xue, J.; Wang, Y.; Li, K.; Zhang, K.; Guo, Q.; Liu, X.; Zhou, Y.; Cheng, L.; et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ. Microbiol. 2015, 17, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, W.; Veerman, E.C.; Nieuw Amerongen, A.V.; Ligtenberg, A.J. Antimicrobial defense systems in saliva. Monogr. Oral. Sci. 2014, 24, 40–51. [Google Scholar] [PubMed]
- Marsh, P.D.; Devine, D.A. How is the development of dental biofilms influenced by the host? J. Clin. Periodontol. 2011, 38 (Suppl. S11), 28–35. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The potential application of green-synthesized metal nanoparticles in dentistry: A comprehensive review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef]
- Lahiri, D.; Ray, R.R.; Sarkar, T.; Upadhye, V.J.; Ghosh, S.; Pandit, S.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Nag, M.; et al. Anti-biofilm efficacy of green-synthesized ZnO nanoparticles on oral biofilm: In vitro and in silico study. Front. Microbiol. 2022, 13, 939390. [Google Scholar] [CrossRef]
- Sen, D.; Patil, V.; Smriti, K.; Varchas, P.; Ratnakar, R.; Naik, N.; Kumar, S.; Saxena, J.; Kapoor, S. Nanotechnology and Nanomaterials in Dentistry: Present and Future Perspectives in Clinical Applications. Eng. Sci. 2022, 20, 14–24. [Google Scholar] [CrossRef]
- Narender, S.S.; Varma, V.V.S.; Srikar, C.S.; Ruchitha, J.; Varma, P.A.; Praveen, B.V.S. Nickel oxide nanoparticles: A brief review of their synthesis, characterization, and applications. Chem. Eng. Technol. 2022, 45, 397–409. [Google Scholar] [CrossRef]
- Suresh, K.C.; Balamurugan, A. Evaluation of structural, optical, and morphological properties of nickel oxide nanoparticles for multi-functional applications. Inorg. Nano-Met. Chem. 2020, 51, 296–301. [Google Scholar] [CrossRef]
- Danjumma, S.G.; Abubakar, Y.; Suleiman, S. Nickel oxide (NiO) devices and applications: A review. Int. J. Eng. Res. Technol. 2019, 8, 12–21. [Google Scholar]
- Berhe, M.G.; Gebreslassie, Y.T. Biomedical Applications of Biosynthesized Nickel Oxide Nanoparticles. Int. J. Nanomed. 2023, 18, 4229–4251. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Shaheen, R.; Akram, B.; Ahmed, M.N.; Haq, S.; Din, S.U.; Zeb, M.; Khan, M.A. Green synthesis of nickel oxide nanoparticles using Populus ciliata leaves extract and their potential antibacterial applications. South Afr. J. Chem. 2021, 75, 168–173. [Google Scholar] [CrossRef]
- Antunes Filho, S.; Dos Santos, M.S.; Dos Santos, O.A.L.; Backx, B.P.; Soran, M.L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef]
- Al-Zaqri, N.; Umamakeshvari, K.; Mohana, V.; Muthuvel, A.; Boshaala, A. Green synthesis of nickel oxide nanoparticles and its photocatalytic degradation and antibacterial activity. J. Mater. Sci. Mater. Electron. 2022, 33, 11864–11880. [Google Scholar] [CrossRef]
- Anand, G.T.; Nithiyavathi, R.; Ramesh, R.; Sundaram, S.J.; Kaviyarasu, K. Structural and optical properties of nickel oxide nanoparticles: Investigation of antimicrobial applications. Surf. Interfaces 2020, 18, 100460. [Google Scholar] [CrossRef]
- Rheima, A.; Anber, A.A.; Shakir, A.; Salah, H.A.; Hameed, S. Novel method to synthesis nickel oxide nanoparticles for antibacterial activity. Iran. J. Phys. Res. 2020, 20, 51–55. [Google Scholar]
- Sabouri, Z.; Akbari, A.; Hosseini, H.A.; Hashemzadeh, A.; Darroudi, M. Eco-friendly biosynthesis of nickel oxide nanoparticles mediated by okra plant extract and investigation of their photocatalytic, magnetic, cytotoxicity, and antibacterial properties. J. Clust. Sci. 2019, 30, 1425–1434. [Google Scholar] [CrossRef]
- Akhtar, J.; Siddique, K.M.; Bi, S.; Mujeeb, M. A review on phytochemical and pharmacological investigations of miswak (Salvadora persica Linn). J. Pharm. Bioallied Sci. 2011, 3, 113. [Google Scholar]
- Ohtani, K.; Kasai, R.; Yamasaki, K.; Tanaka, O.; Kamel, M.S.; Assaf, M.H.; El-Shanawani, A.A.; Ali, A.A. Lignan glycosides from stems of Salvadora persica. Phytochemistry 1992, 31, 2469–2471. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahamed, N.; Dar, J.M.; Mohammad, U.J. Ethnobotany, pharmacology and chemistry of Salvadora persica L. A review. Res. Plant Biol. 2012, 2, 2565. [Google Scholar]
- Ibrahim, A.Y.; El-Gengaihi, S.E. Safety profile of meswak root extract on liver, kidney, sexual hormones and hematological parameters of rats. Not. Sci. Biol. 2012, 4, 18–23. [Google Scholar] [CrossRef][Green Version]
- Gupta, A.; Verma, S.; Kushwaha, P.; Srivastava, S.; Rawat, A. Phytochemical and antioxidant studies of Salvadora persica L. stem and twig. Indian Pharm. Educ. Res. 2015, 49, 71–75. [Google Scholar] [CrossRef]
- Iyer, D.; Patil, U.K. Efficacy of stigmast-5-en-3b-ol isolated from Salvadora persica L. as antihyperlipidemic and anti-tumor agent: Evidence from animal studies. Asian Pac. J. Trop. Dis. 2012, 2 (Suppl. S2), S849–S855. [Google Scholar] [CrossRef]
- Iyer, D.; Patil, U. Evaluation of antihyperlipidemic and antitumor activities of isolated coumarins from Salvadora indica. Pharm. Biol. 2014, 52, 78–85. [Google Scholar] [CrossRef]
- Lica, I.C.L.; dos Santos Soares, A.M.; de Mesquita, L.S.S.; Malik, S. Biological Properties and pharmacological potential of plant exudates. Food Res. Int. 2018, 105, 1039–1053. [Google Scholar] [CrossRef]
- Uddin, S.; Safdar, L.B.; Iqbal, J.; Yaseen, T.; Laila, S.; Anwar, S.; Abbasi, B.A.; Saif, M.S.; Quraishi, U.M. Green synthesis of nickel oxide nanoparticles using leaf extract of Berberis balochistanica: Characterization, and diverse biological applications. Microsc. Res. Technol. 2021, 84, 2004–2016. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Ding, H.; Liu, S.; Han, X.; Gui, J.; Liu, D. Subcritical ethanol extraction of flavonoids from Moringa oleifera leaf and evaluation of antioxidant activity. Food Chem. 2017, 218, 152–158. [Google Scholar] [CrossRef]
- Mohan, R.; Birari, R.; Karmase, A.; Jagtap, S.; Bhutani, K.K. Antioxidant activity of a new phenolic glycoside from Lagenaria siceraria Stand. fruits. Food Chem. 2012, 132, 244–251. [Google Scholar] [CrossRef]
- Al-Dbass, A.M.; Daihan, S.A.; Al-Nasser, A.A.; Al-Suhaibani, L.S.; Almusallam, J.; Alnwisser, B.I.; Saloum, S.; Alotaibi, R.S.; Alessa, L.A.; Bhat, R.S. Biogenic Silver Nanoparticles from Two Varieties of Agaricus bisporus and Their Antibacterial Activity. Molecules 2022, 27, 7656. [Google Scholar] [CrossRef]
- Srihasam, S.; Thyagarajan, K.; Korivi, M.; Lebaka, V.R.; Mallem, S.P.R. Phytogenic generation of NiO nanoparticles using Stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules 2020, 10, 89. [Google Scholar] [CrossRef]
- Mandal, B.K.; Mandal, R.; Sikdar, S.; Sarma, S.; Srinivasan, A.; Chowdhury, S.R.; Das, B.; Das, R. Green synthesis of NiO nanoparticle using Punica granatum peel extract and its characterization for methyl orange degradation. Mater. Today Commun. 2023, 34, 105302. [Google Scholar] [CrossRef]
- Hong, J.; Kim, C.; In, C.M.; Kim, S.; Cho, W.J.; Pecunia, V.; Lee, M.J.; Hwang, I. Serendipitous Doping in Nickel Oxide upon Microwave-Induced Low-Temperature Crystallization Enhances Efficiency of Perovskite Solar Cells. Solar RRL 2022, 6, 2100992. [Google Scholar] [CrossRef]
- Bhat, R.S.; Alghamdi, J.M.; Aldbass, A.M.; Aljebrin, N.A.; Alangery, A.B.; Soliman, D.A.; Al-Daihan, S. Biochemical and FT-IR profiling of Tritium aestivum L. seedling in response to sodium fluoride treatment. Fluoride 2022, 55, 81–89. [Google Scholar]
- Bhat, R.S.; Aldbass, A.M.; Alghamdi, J.M.; Alonazia, M.A.; Al-Daihana, S. Trigonella foenum-graecum L. seed germination under sodium halide salts exposure. Fluoride 2023, 56, 168–179. [Google Scholar]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green synthesis of zinc oxide nanoparticles using pomegranate fruit peel and solid coffee grounds vs. chemical method of synthesis, with their biocompatibility and antibacterial properties investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Vishwakarma, J.; Rai, S.; Alomar, T.S.; AlMasoud, N.; Bhattarai, A. Green route synthesis and characterization techniques of silver nanoparticles and their biological adeptness. ACS Omega 2022, 7, 27004–27020. [Google Scholar] [CrossRef]
- Khan, N.A.; Saeed, K.; Khan, I.; Gul, T.; Sadiq, M.; Uddin, A.; Zekker, I. Efficient photodegradation of orange II dye by nickel oxide nanoparticles and nanoclay supported nickel oxide nanocomposite. Appl. Water Sci. 2022, 12, 131. [Google Scholar] [CrossRef]
- Dehno Khalaji, A. Nickel Oxide (NiO) nanoparticles prepared by solid-state thermal decomposition of Nickel (II) schiff base precursor. J. Ultrafine Grained Nanostruct. Mater. 2015, 48, 1–4. [Google Scholar]
- Uddin, S.; Safdar, L.B.; Anwar, S.; Iqbal, J.; Laila, S.; Abbasi, B.A.; Saif, M.S.; Ali, M.; Rehman, A.; Basit, A.; et al. Green synthesis of nickel oxide nanoparticles from Berberis balochistanica stem for investigating bioactivities. Molecules 2021, 26, 1548. [Google Scholar] [CrossRef]
- Mervat, E.H.; Ali, H.M.; Ashmawy, N.A.; Salem, M.Z.M. Chemical composition and bioactivity of Salvadora persica extracts against some potato bacterial pathogens. BioResources 2017, 12, 1835–1849. [Google Scholar]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. A Review of commonly used methodologies for assessing the antibacterial activity of honey and honey products. Antibiotics 2022, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.T. Benzylamides from Salvadora persica. Arch. Pharmacal Res. 2006, 29, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.K.; Gustafsson, A.; Pütsep, K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar] [CrossRef]
- Allandale, H.; Almesaileikh, E.; Bhardwaj, R.G.; Al Khabbaz, A.; Karched, M. The Effect of Benzyl Isothiocyanate on the Expression of Genes Encoding NADH Oxidase and Fibronectin-Binding Protein in Oral Streptococcal Biofilms. Front. Oral. Health 2022, 3, 863723. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Mokeem, L.; Melo, M.A.S.; Gregory, R.L. Antibacterial Activities of Methanol and Aqueous Extracts of Salvadora persica against Streptococcus mutans Biofilms: An In Vitro Study. Dent. J. 2021, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Shnawa, B.H.; Jalil, P.J.; Hamad, S.M.; Ahmed, M.H. Antioxidant, protoscolicidal, hemocompatibility, and antibacterial activity of nickel oxide nanoparticles synthesized by ziziphus spina-christi. BioNanoSci 2022, 12, 1264–1278. [Google Scholar] [CrossRef]
- Angel Ezhilarasi, A.; Judith Vijaya, J.; Kaviyarasu, K.; John Kennedy, L.; Ramalingam, R.; Al-Lohedan, H.A. Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity antibacterial photocatalytic properties. J. Photochem. Photobiol. B Biol. 2018, 180, 39–50. [Google Scholar] [CrossRef]
- Prabhu, S.; Thangadurai, T.D.; Bharathy, P.V.; Kalugasalam, P. Synthesis and characterization of nickel oxide nanoparticles using Clitoria ternatea flower extract: Photocatalytic dye degradation under sunlight and antibacterial activity applications. Results Chem. 2022, 4, 100285. [Google Scholar] [CrossRef]
- Rajith Kumar, C.R.; Betageri, V.S.; Nagaraj, G.; Pujar, G.H.; Suma, B.P.; Latha, M.S. Photocatalytic nitrite sensing antibacterial studies of facile bio-synthesized nickel oxide nanoparticles. J. Sci. Adv. Mater. Dev. 2020, 5, 48–55. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Behera, N.; Arakha, M.; Priyadarshinee, M.; Pattanayak, B.S.; Soren, S.; Jha, S.; Mallick, B.C. Oxidative stress generated at nickel oxide nanoparticle interface results in bacterial membrane damage leading to cell death. RSC Adv. 2019, 9, 24888–24894. [Google Scholar] [CrossRef] [PubMed]
- Jubu, P.R.; Yam, F.K.; Igba, V.M.; Beh, K.P. Tauc-plot scale and extrapolation effect on bandgap estimation from UV–vis–NIR data—A case study of β-Ga2O3. J. Solid-State Chem. 2020, 290, 121576. [Google Scholar] [CrossRef]
- Juan, C.A.; Perez de la Lastra, J.M.; Plou, F.J.; Perez-Lebena, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).