Pyuria Is Associated with Dysbiosis of the Urinary Microbiota in Type 2 Diabetes Patients Receiving Sodium–Glucose Cotransporter 2 Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrolment and Information
2.2. DNA Extraction, PCR, and MiSeq Sequencing
2.3. Bioinformatic Analyses
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of All Patients
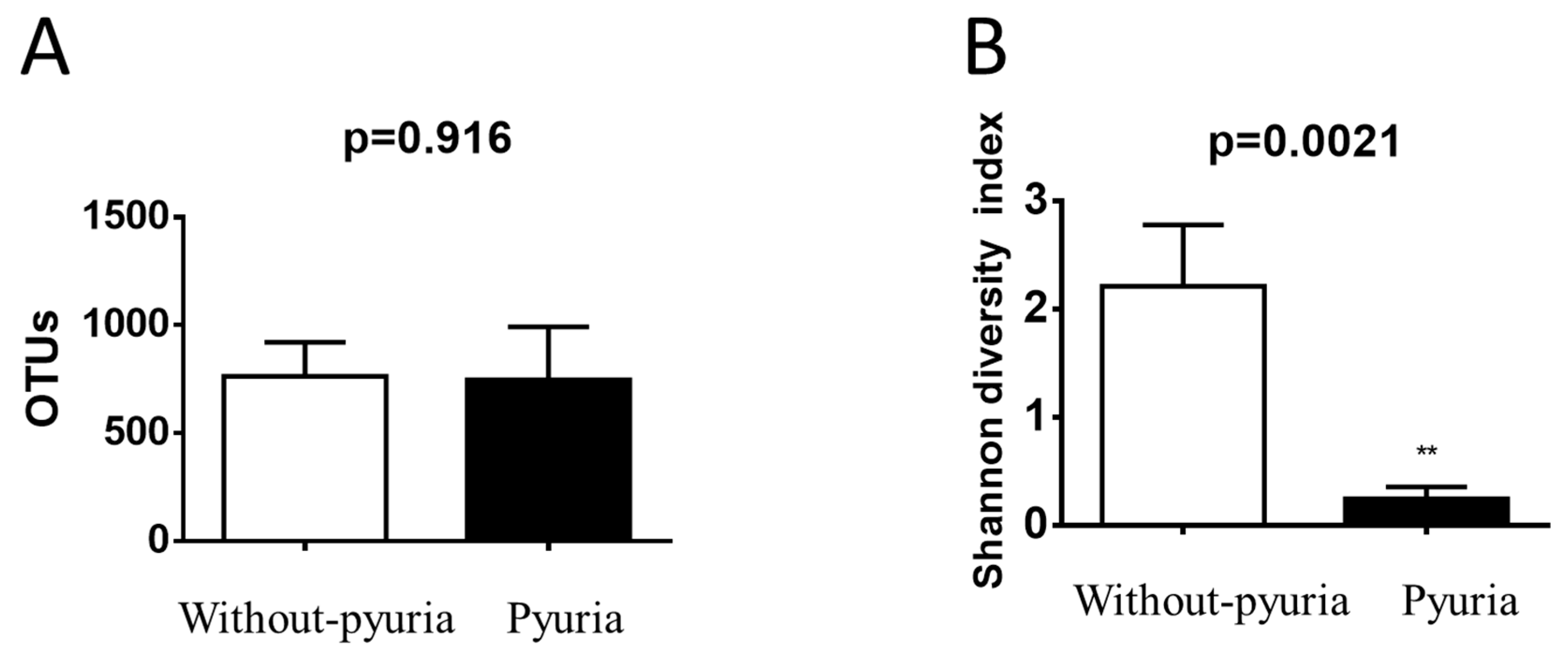
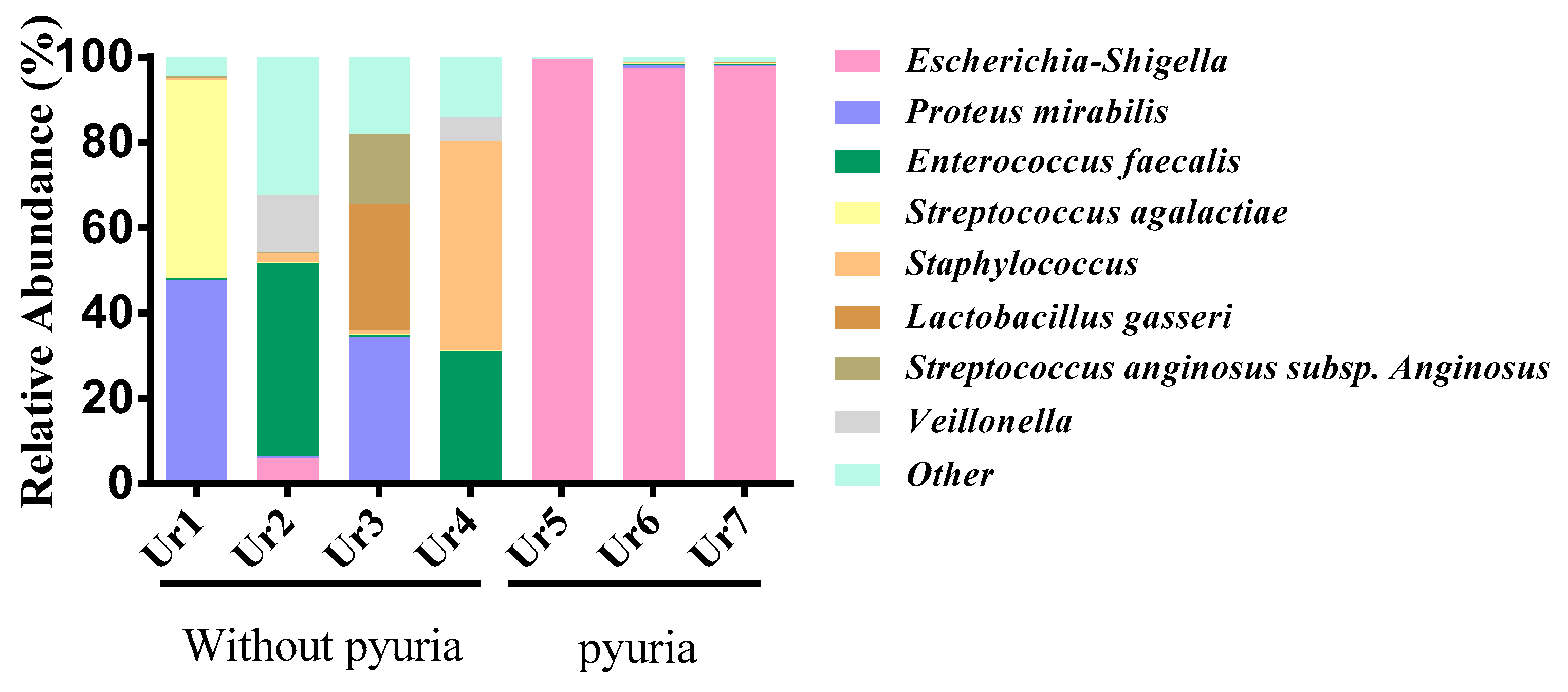
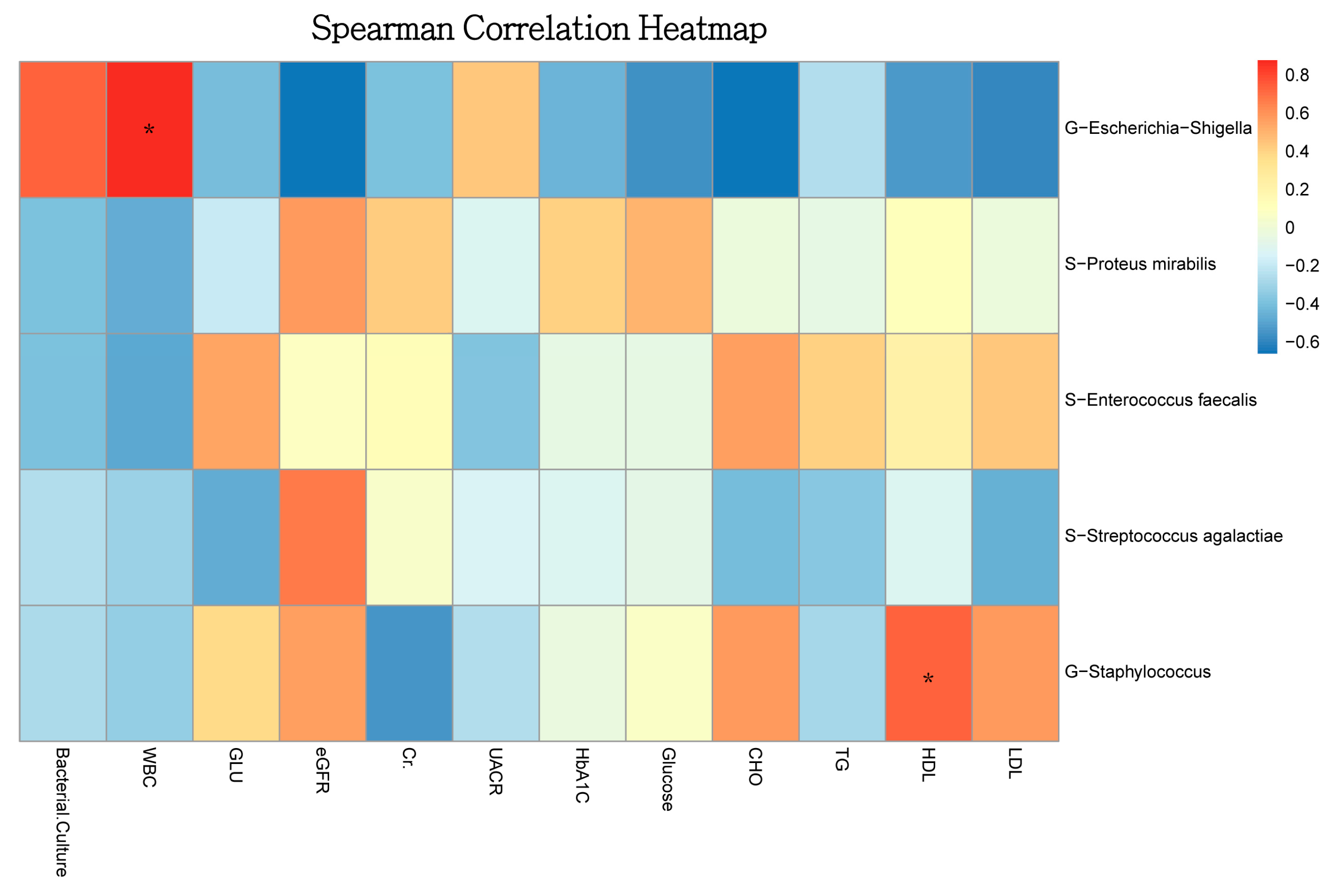
3.2. Urinary Microbiota Differed between the Pyuria and Non-Pyuria Groups Treated with SGLT2 Inhibitors
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronald, A.; Ludwig, E. Urinary tract infections in adults with diabetes. Int. J. Antimicrob. Agents 2001, 17, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Fu, A.Z.; Qiu, Y.; Engel, S.S.; Shankar, R.; Brodovicz, K.G.; Rajpathak, S.; Radican, L. Disease burden of urinary tract infections among type 2 diabetes mellitus patients in the U.S. J. Diabetes Complicat. 2014, 28, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, A. Urinary tract infections in patients with diabetes. Am. J. Med. 2002, 113 (Suppl. 1A), 80s–84s. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, S.; Jia, P.; Deng, K.; Chen, W.; Sun, X. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 2824. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, T.; Shen, S.; Fang, Z.; Dong, Y.; Tang, H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2017, 19, 348–355. [Google Scholar] [CrossRef]
- Donnan, J.R.; Grandy, C.A.; Chibrikov, E.; Marra, C.A.; Aubrey-Bassler, K.; Johnston, K.; Swab, M.; Hache, J.; Curnew, D.; Nguyen, H.; et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: A systematic review and meta-analysis. BMJ Open 2019, 9, e022577. [Google Scholar] [CrossRef]
- Zaidi, S.M.J.; Kaneez, M.; Almas, T.; Fatima, L.; Safian, H.A.; Jamal, A.M.; Satti, M.Z.; Dhillon, R.A.; Zubair, A.B.; Bukhari, S.F. Gauging the risk factors for asymptomatic bacteriuria in Type-2 diabetic women: A case-control study. Cureus 2020, 12, e9069. [Google Scholar] [CrossRef]
- Varshney, N.; Billups, S.J.; Saseen, J.J.; Fixen, C.W. Sodium-glucose cotransporter-2 inhibitors and risk for genitourinary infections in older adults with type 2 diabetes. Ther. Adv. Drug Saf. 2021, 12, 2042098621997703. [Google Scholar] [CrossRef]
- Ueda, P.; Svanstrom, H.; Melbye, M.; Eliasson, B.; Svensson, A.M.; Franzen, S.; Gudbjornsdottir, S.; Hveem, K.; Jonasson, C.; Pasternak, B. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: Nationwide register based cohort study. BMJ 2018, 363, k4365. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Lega, I.C.; Bronskill, S.E.; Campitelli, M.A.; Guan, J.; Stall, N.M.; Lam, K.; McCarthy, L.M.; Gruneir, A.; Rochon, P.A. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: A population-based study of older women and men with diabetes. Diabetes Obes. Metab. 2019, 21, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ling, Z.; Xiao, Y.; Lv, L.; Yang, Q.; Wang, B.; Lu, H.; Zheng, L.; Jiang, P.; Wang, W.; et al. Dysbiosis of urinary microbiota is positively correlated with type 2 diabetes mellitus. Oncotarget 2017, 8, 3798–3810. [Google Scholar] [CrossRef] [PubMed]
- Khasriya, R.; Barcella, W.; De Iorio, M.; Swamy, S.; Gill, K.; Kupelian, A.; Malone-Lee, J. Lower urinary tract symptoms that predict microscopic pyuria. Int. Urogynecology J. 2018, 29, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Ranjan, R.; McGee, H.S.; Andropolis, K.E.; Panchal, D.V.; Hajjiri, Z.; Brennan, D.C.; Finn, P.W.; Perkins, D.L. Urinary microbiome of kidney transplant patients reveals dysbiosis with potential for antibiotic resistance. Transl. Res. J. Lab. Clin. Med. 2017, 181, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Oba, K.; Saito, Y.; Ouchi, M.; Yamashita, N.; Okamura, K.; Takai, E.; Mizuno, S.; Matsumura, N.; Inuzuka, Y.; et al. Asymptomatic pyuria in diabetic women. J. Nippon Med. Sch. 2001, 68, 405–410. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.; Cao, Y.; Zhang, G.; Chen, Y.; Zhong, J.; Huang, W.; Zeng, J.; Wu, P. Relationship between alterations of urinary microbiota and cultured negative lower urinary tract symptoms in female type 2 diabetes patients. BMC Urol. 2019, 19, 78. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Kuo, I.C.; Lee, J.J.; Hwang, D.Y.; Lim, L.M.; Lin, H.Y.; Hwang, S.J.; Chen, H.C.; Hung, C.C. Pyuria, urinary tract infection and renal outcome in patients with chronic kidney disease stage 3–5. Sci. Rep. 2020, 10, 19460. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fak, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4592–4598. [Google Scholar] [CrossRef]
- Tao, S.; Li, L.; Li, L.; Liu, Y.; Ren, Q.; Shi, M.; Liu, J.; Jiang, J.; Ma, H.; Huang, Z.; et al. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: An analysis of the gut microbiota composition. Acta Diabetol. 2019, 56, 581–592. [Google Scholar] [CrossRef]
- Yoo, J.J.; Shin, H.B.; Song, J.S.; Kim, M.; Yun, J.; Kim, Z.; Lee, Y.M.; Lee, S.W.; Lee, K.W.; Kim, W.B.; et al. Urinary microbiome characteristics in female patients with acute uncomplicated cystitis and recurrent cystitis. J. Clin. Med. 2021, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Schmiemann, G.; Kniehl, E.; Gebhardt, K.; Matejczyk, M.M.; Hummers-Pradier, E. The diagnosis of urinary tract infection: A systematic review. Dtsch. Arztebl. Int. 2010, 107, 361–367. [Google Scholar] [CrossRef]
- Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 2015, 66, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, M.; Philip, S.; Nesbitt, A.; Joshi, A.; Perera, M. Sodium glucose-linked transport protein 2 inhibitors: An overview of genitourinary and perioperative implications. Int. J. Urol. 2021, 28, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Whang, A.; Nagpal, R.; Yadav, H. Bi-directional drug-microbiome interactions of anti-diabetics. EBioMedicine 2019, 39, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Battson, M.L.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc. Diabetol. 2018, 17, 62. [Google Scholar] [CrossRef] [PubMed]
| Without Pyuria (n = 4) | Pyuria (n = 3) | p Value | |
|---|---|---|---|
| Duration of diabetes (y) | 9.75 ± 6.12 | 14.33 ± 5.89 | 0.614 |
| Age (y) | 59 ± 5.7 | 59.66 ± 6.5 | 0.942 |
| Gender (female, n) | 1 | 3 | |
| Urine WBC > 1 cell (%) | 0% | 100% | 0.008 ** |
| eGFR (mL/min/1.73 m2) stage II (n) | 25% | 100% | 0.047 * |
| UACR > 30 mg/g (%) | 0% | 33.3% | 0.212 |
| HbA1c (%) | 7.15 ± 0.2 | 6.7 ± 0.3 | 0.336 |
| FPG (mg/dL) | 141 ± 13.1 | 116.33 ± 7.8 | 0.174 |
| TC (mg/dL) | 189.55 ± 24.9 | 130.33 ± 4.4 | 0.097 |
| TG (mg/dL) | 137.25 ± 23.4 | 130.33 ± 4.4 | 0.097 |
| HDL-C (mg/dL) | 49.5 ± 3.42 | 43 ± 3.21 | 0.226 |
| LDL-C (mg/dL) | 122 ± 22.84 | 77 ± 7.37 | 0.142 |
| Hypertension (%) | 50% | 66% | 0.723 |
| Insulin use (%) | 0% | 66% | 0.062 |
| Urinary tract infection history (%) | 0% | 66% | 0.062 |
| Group | Sample ID | Shannon Diversity Index | Number of Species Identified (OTUs) | Top 5 Species | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Ur1 | 1.542 | 718 |
Proteus
mirabilis | Streptococcus agalactiae | Corynebacteriaceae |
Escherichia-
Shigella | Staphylococcus | |
| 47.08% | 46.27% | 1.87% | 0.68% | 0.65% | ||||
| Without pyuria | Ur2 | 2.634 | 688 | Enterococcus faecalis | Corynebacterium | Veillonella | Bifidobacterium dentium | Escherichia-Shigella |
| 45.20% | 16.76% | 13.46% | 8.37% | 6.07% | ||||
| Ur3 | 2.725 | 996 |
Proteus
mirabilis |
Lactobacillus
gasseri |
Streptococcus
anginosus | C. sp. NML 100378 | Lactobacillus | |
| 33.46% | 29.58% | 16.29% | 5.90% | 4.77% | ||||
| Ur4 | 1.947 | 652 | Staphylococcus |
Enterococcus
faecalis | Corynebacteriaceae | Veillonella | kroppenstedtii | |
| 48.98% | 30.39% | 11.01% | 5.49% | 1.52% | ||||
| Ur5 | 0.126 | 1028 |
Escherichia-
Shigella | Escherichia coli | Bifidobacterium dentium | C. pyruviciproducens ATCC BAA-1742 | Staphylococcus | |
| 99.44% | 0.14% | 0.06% | 0.05% | 0.05% | ||||
| Pyuria | Ur6 | 0.339 | 632 |
Escherichia-
Shigella | Proteus mirabilis |
Enterococcus
faecalis | Streptococcus agalactiae | Staphylococcus |
| 97.44% | 0.63% | 0.26% | 0.24% | 0.19% | ||||
| Ur7 | 0.276 | 581 |
Escherichia-
Shigella | Proteus mirabilis | Lactobacillus | Enterococcus faecalis | Streptococcus agalactiae | |
| 97.80% | 0.42% | 0.63% | 0.18% | 0.14% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-J.; Chuang, H.-N.; Jhan, P.-P.; Ye, H.-Y.; Lee, I.-T.; Hsiao, T.-H.; Liu, P.-Y. Pyuria Is Associated with Dysbiosis of the Urinary Microbiota in Type 2 Diabetes Patients Receiving Sodium–Glucose Cotransporter 2 Inhibitors. Microbiol. Res. 2023, 14, 34-41. https://doi.org/10.3390/microbiolres14010003
Lin H-J, Chuang H-N, Jhan P-P, Ye H-Y, Lee I-T, Hsiao T-H, Liu P-Y. Pyuria Is Associated with Dysbiosis of the Urinary Microbiota in Type 2 Diabetes Patients Receiving Sodium–Glucose Cotransporter 2 Inhibitors. Microbiology Research. 2023; 14(1):34-41. https://doi.org/10.3390/microbiolres14010003
Chicago/Turabian StyleLin, Hsueh-Ju, Han-Ni Chuang, Pei-Pei Jhan, Han-Yu Ye, I-Te Lee, Tzu-Hung Hsiao, and Po-Yu Liu. 2023. "Pyuria Is Associated with Dysbiosis of the Urinary Microbiota in Type 2 Diabetes Patients Receiving Sodium–Glucose Cotransporter 2 Inhibitors" Microbiology Research 14, no. 1: 34-41. https://doi.org/10.3390/microbiolres14010003
APA StyleLin, H.-J., Chuang, H.-N., Jhan, P.-P., Ye, H.-Y., Lee, I.-T., Hsiao, T.-H., & Liu, P.-Y. (2023). Pyuria Is Associated with Dysbiosis of the Urinary Microbiota in Type 2 Diabetes Patients Receiving Sodium–Glucose Cotransporter 2 Inhibitors. Microbiology Research, 14(1), 34-41. https://doi.org/10.3390/microbiolres14010003





