Antiviral Potential of Selected N-Methyl-N-phenyl Dithiocarbamate Complexes against Human Immunodeficiency Virus (HIV)
Abstract
1. Introduction
2. Experimental Design
2.1. Materials and Physical Measurements
2.2. Synthesis of N-Methyl-N-phenyl Dithiocarbamate Ligand and Complexes
2.2.1. Synthesis of Ammonium N-Methyl-N-phenyl Dithiocarbamate Ligand
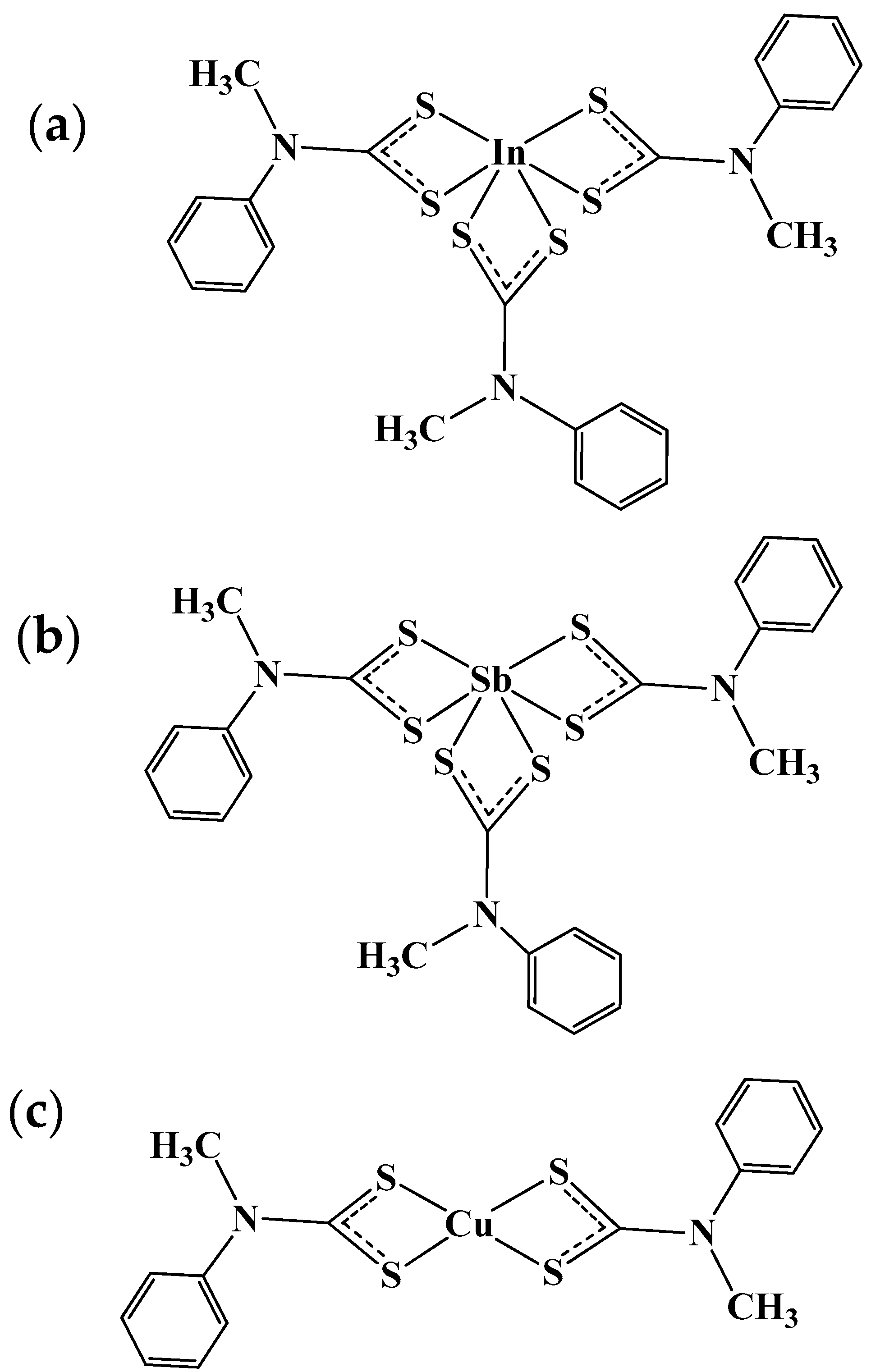
2.2.2. Synthesis of the Complexes
2.3. Cells, Envelope Plasmids, Reagents, and Chemical Inhibitors
2.4. Generation of HIV-1 Env-Pseudoviruses
2.5. Cytotoxicity Assay
2.5.1. MTT Assay
2.5.2. Neutral Red Uptake Assay
2.6. Neutralization Assay
2.7. Statistical Analysis
3. Results and Discussion
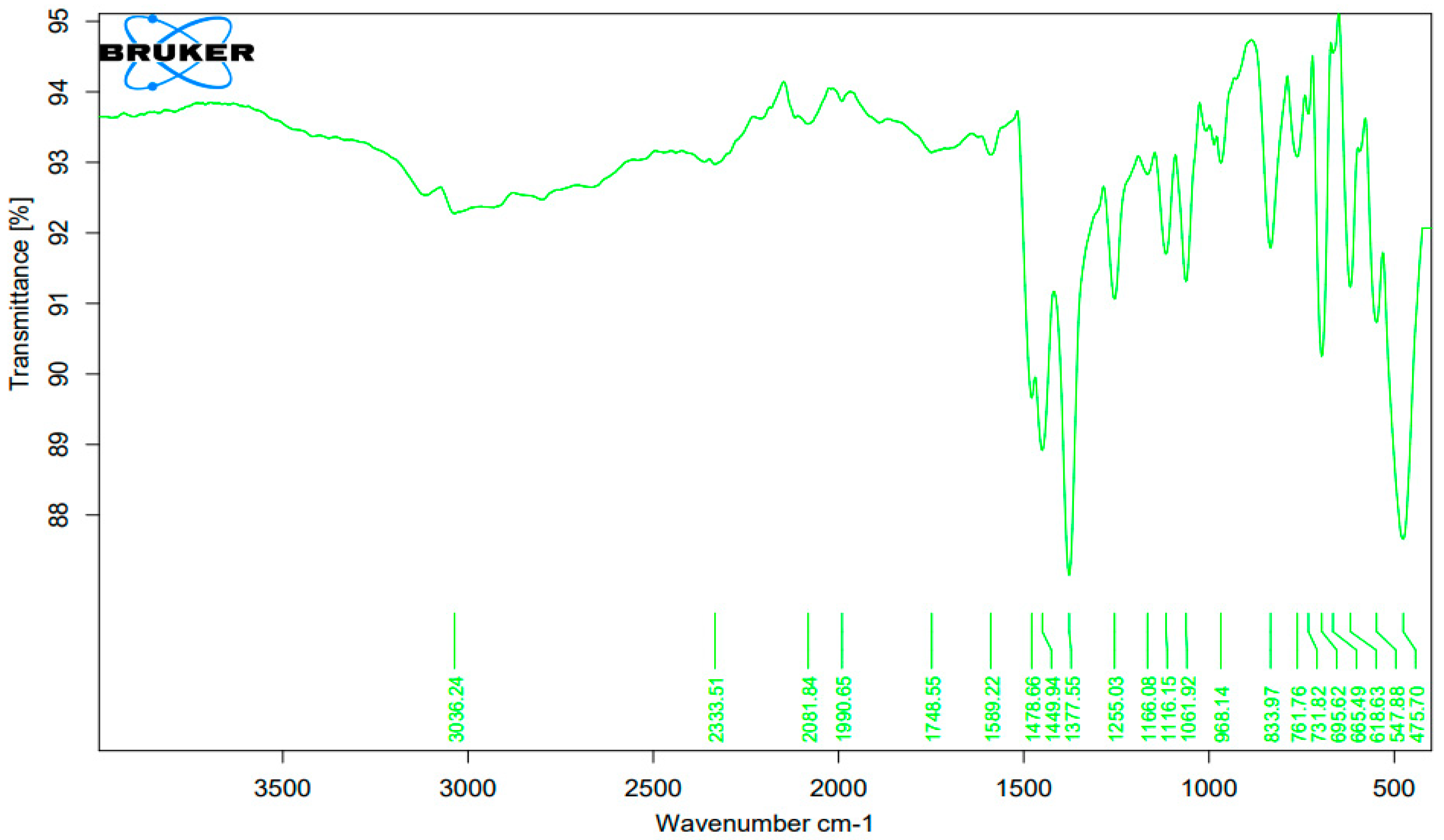
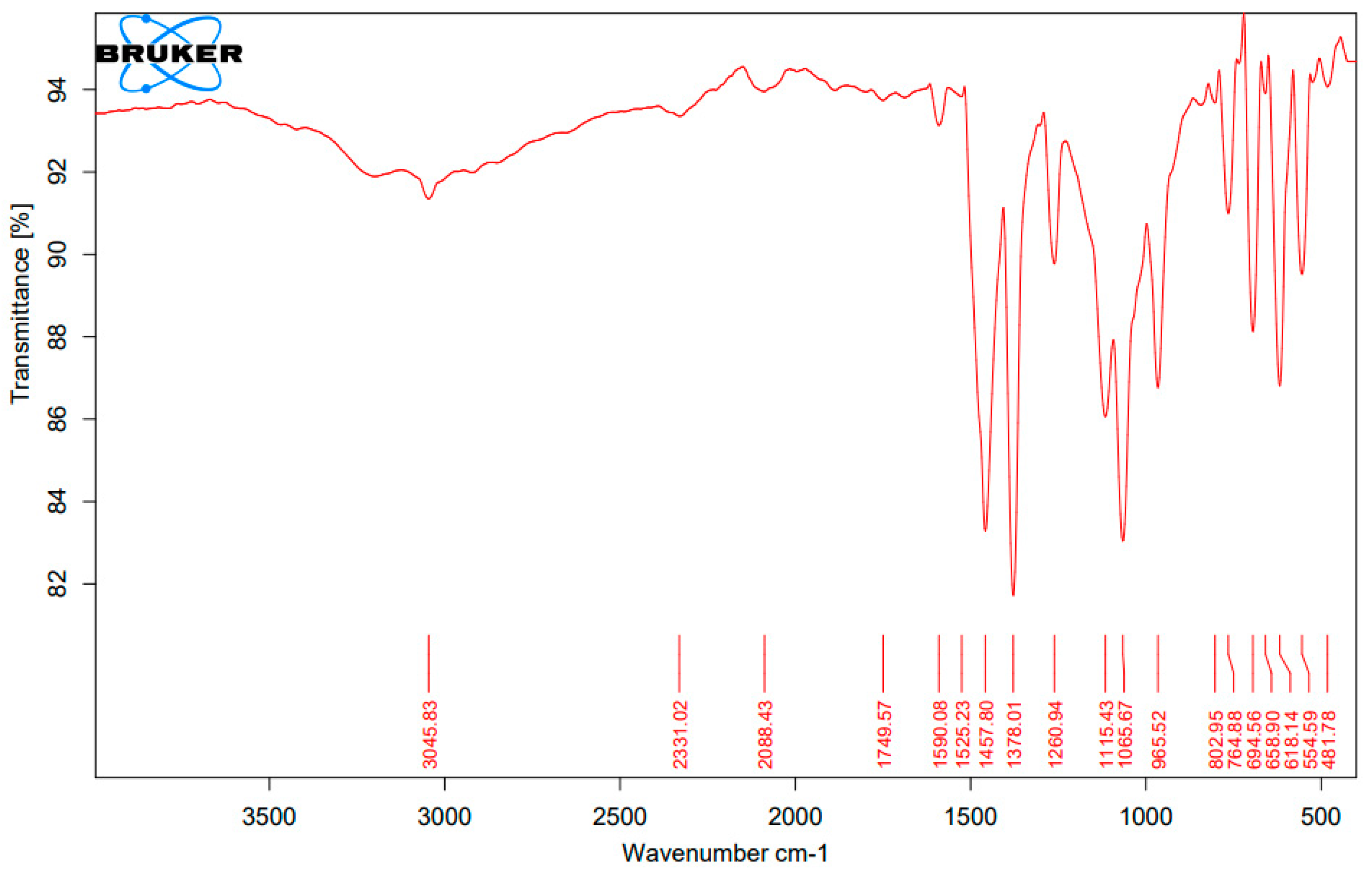
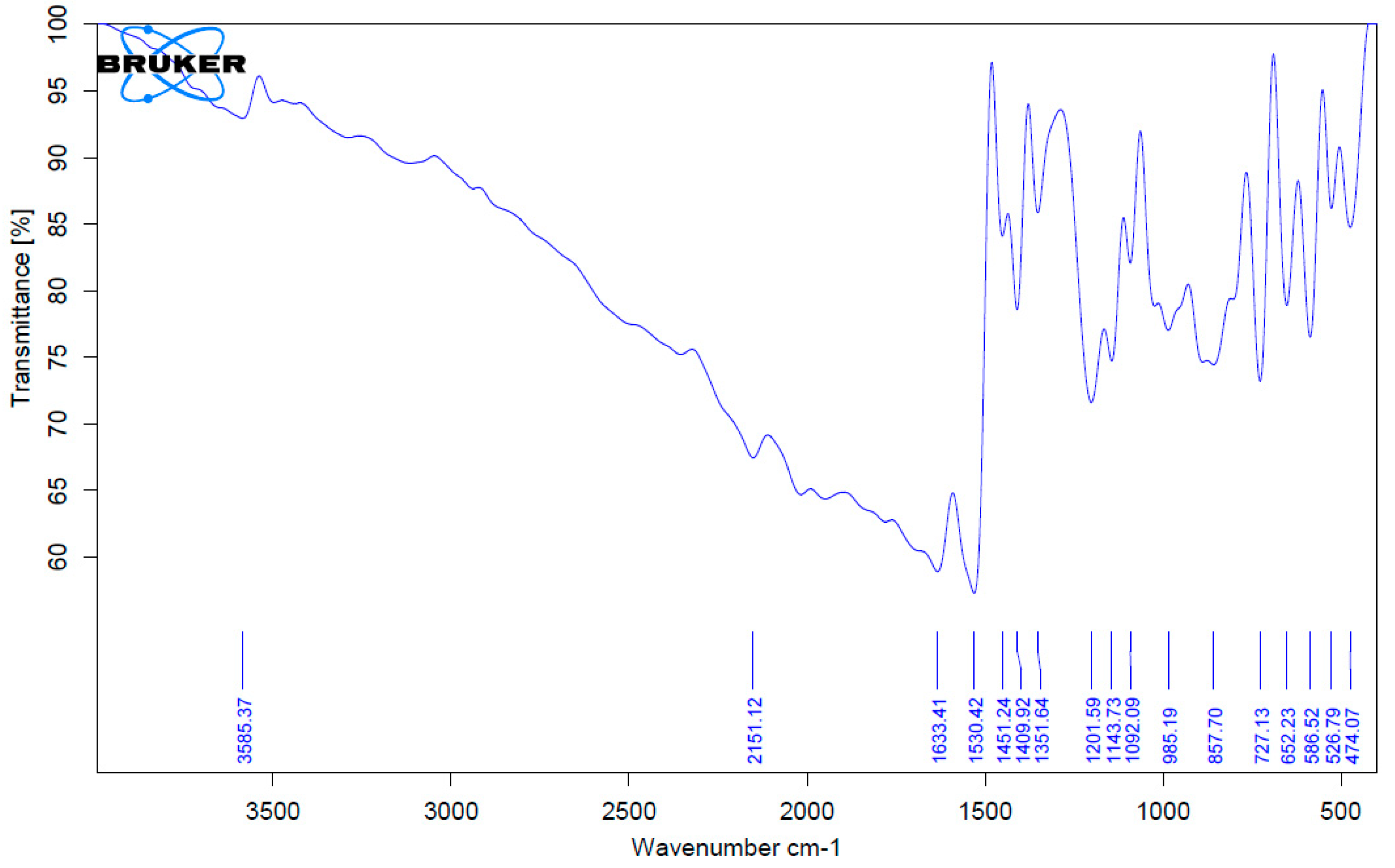
3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of the Complexes
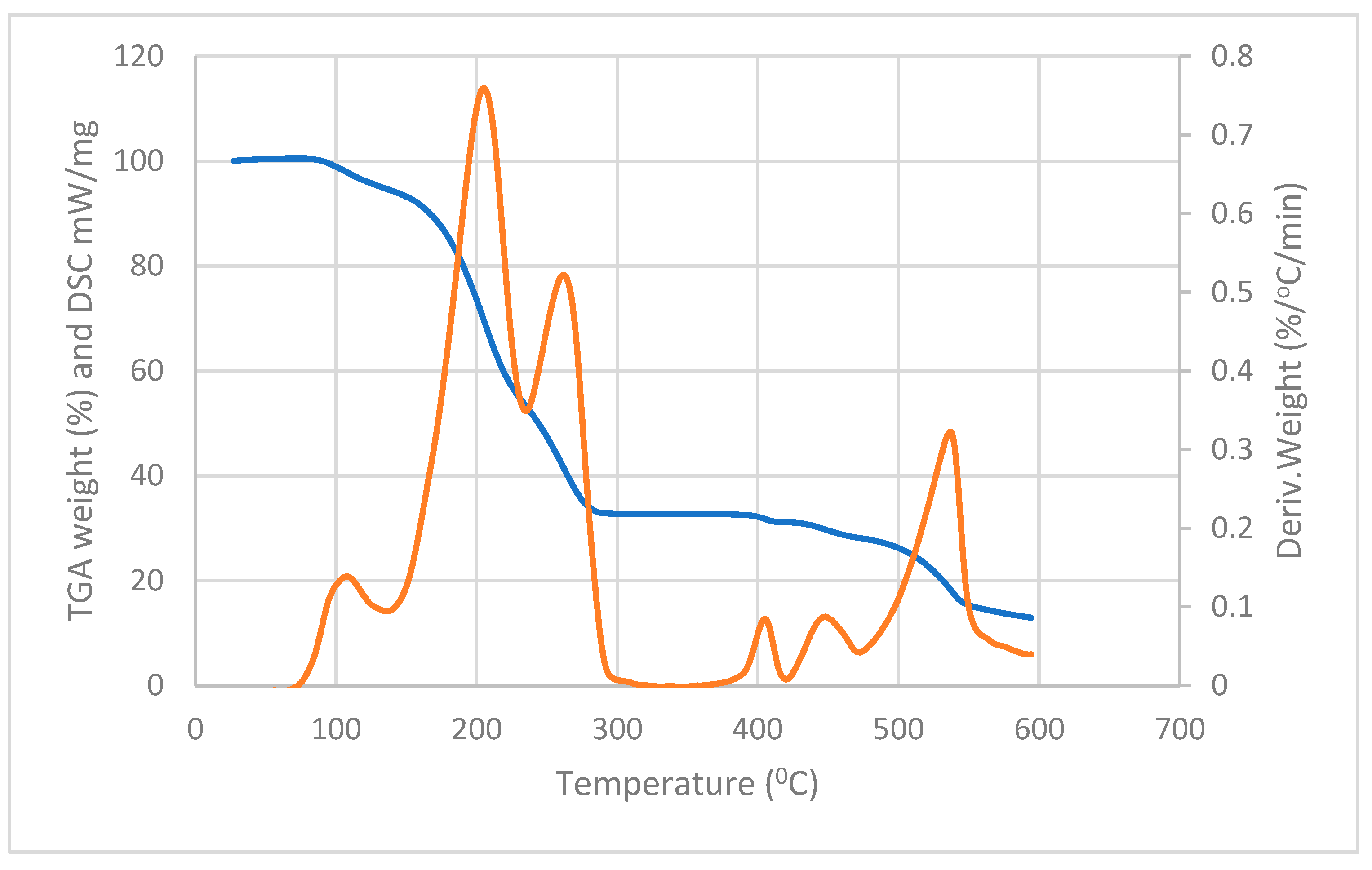
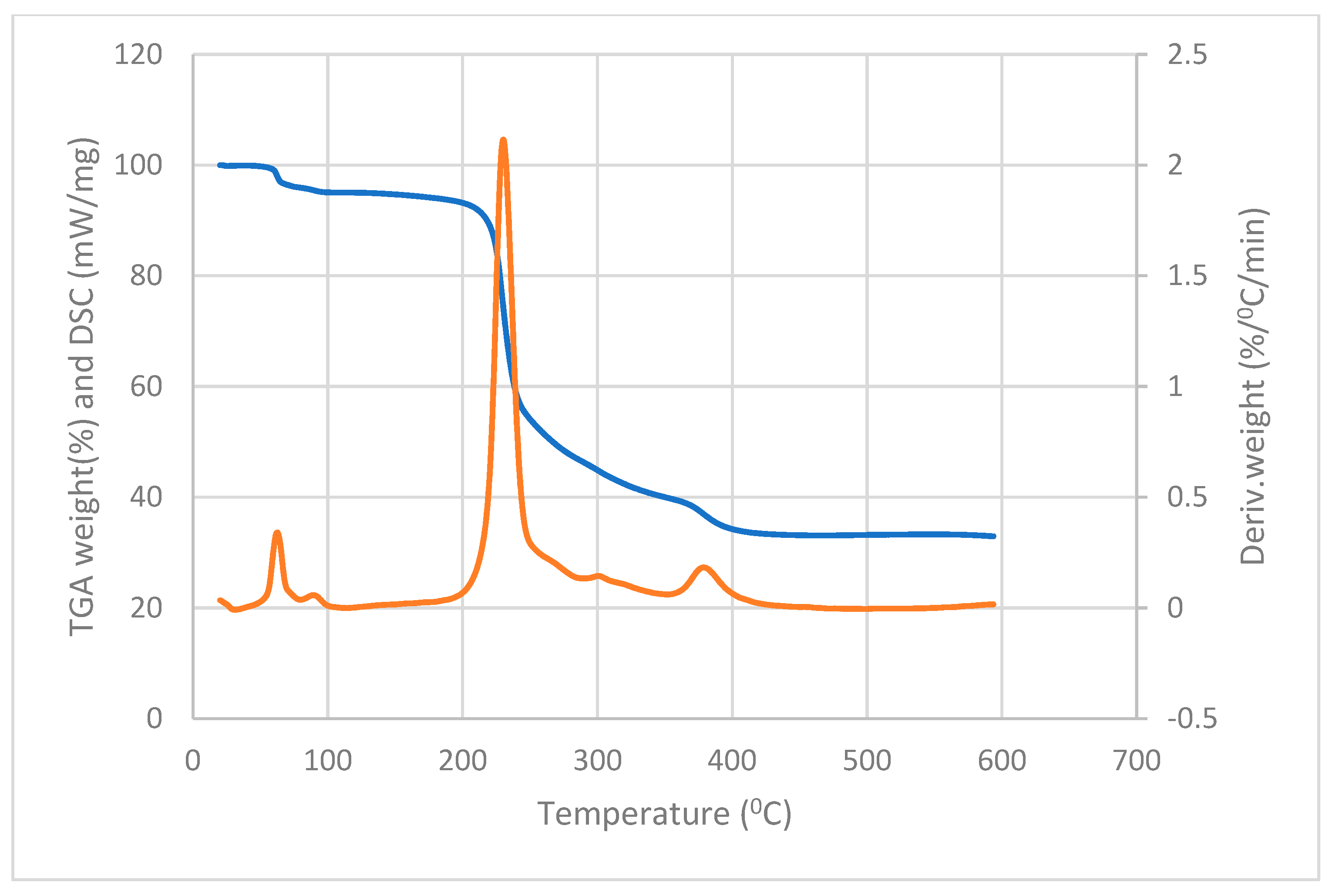
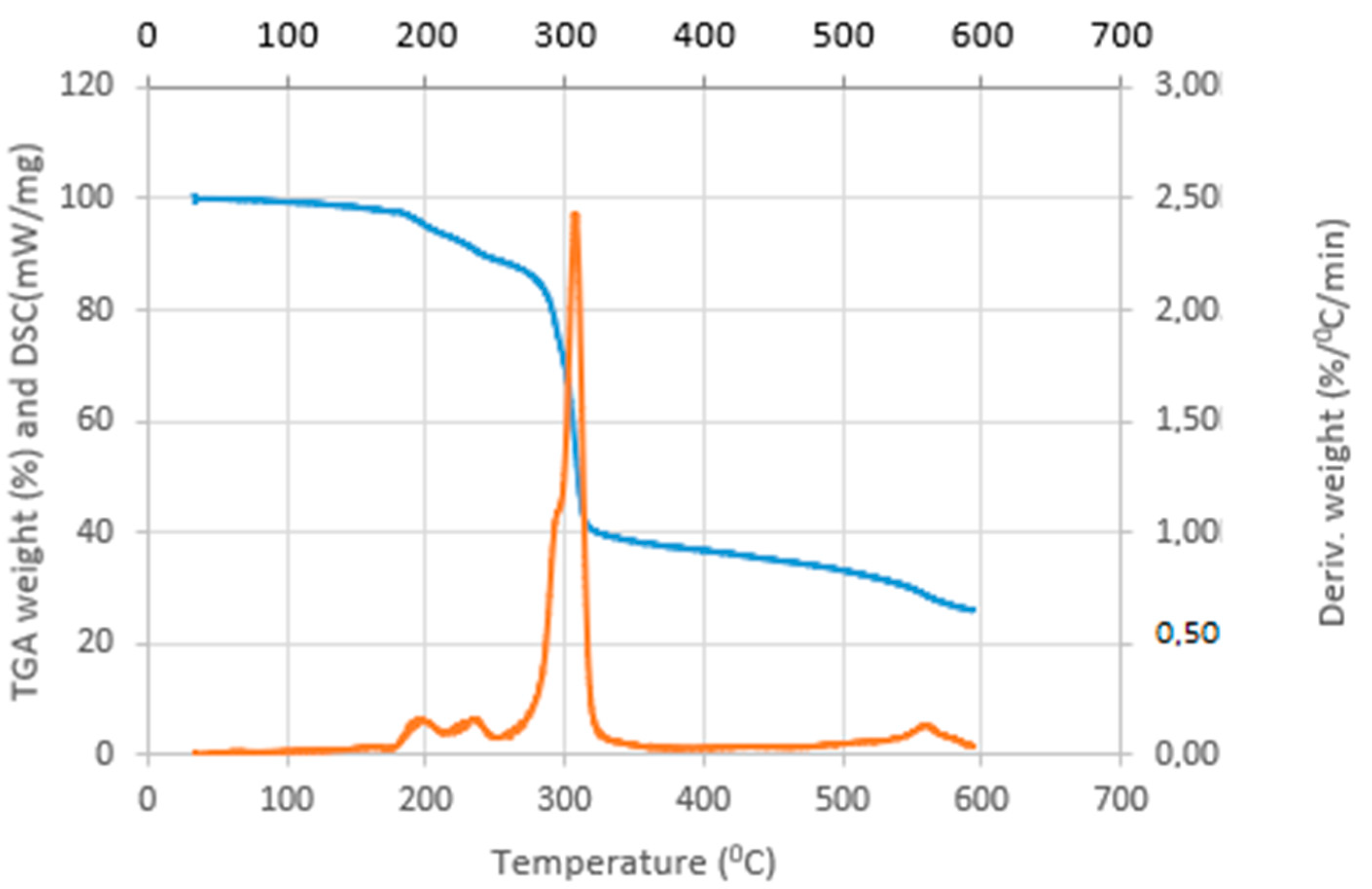
3.2. Thermal Analysis of the Complexes
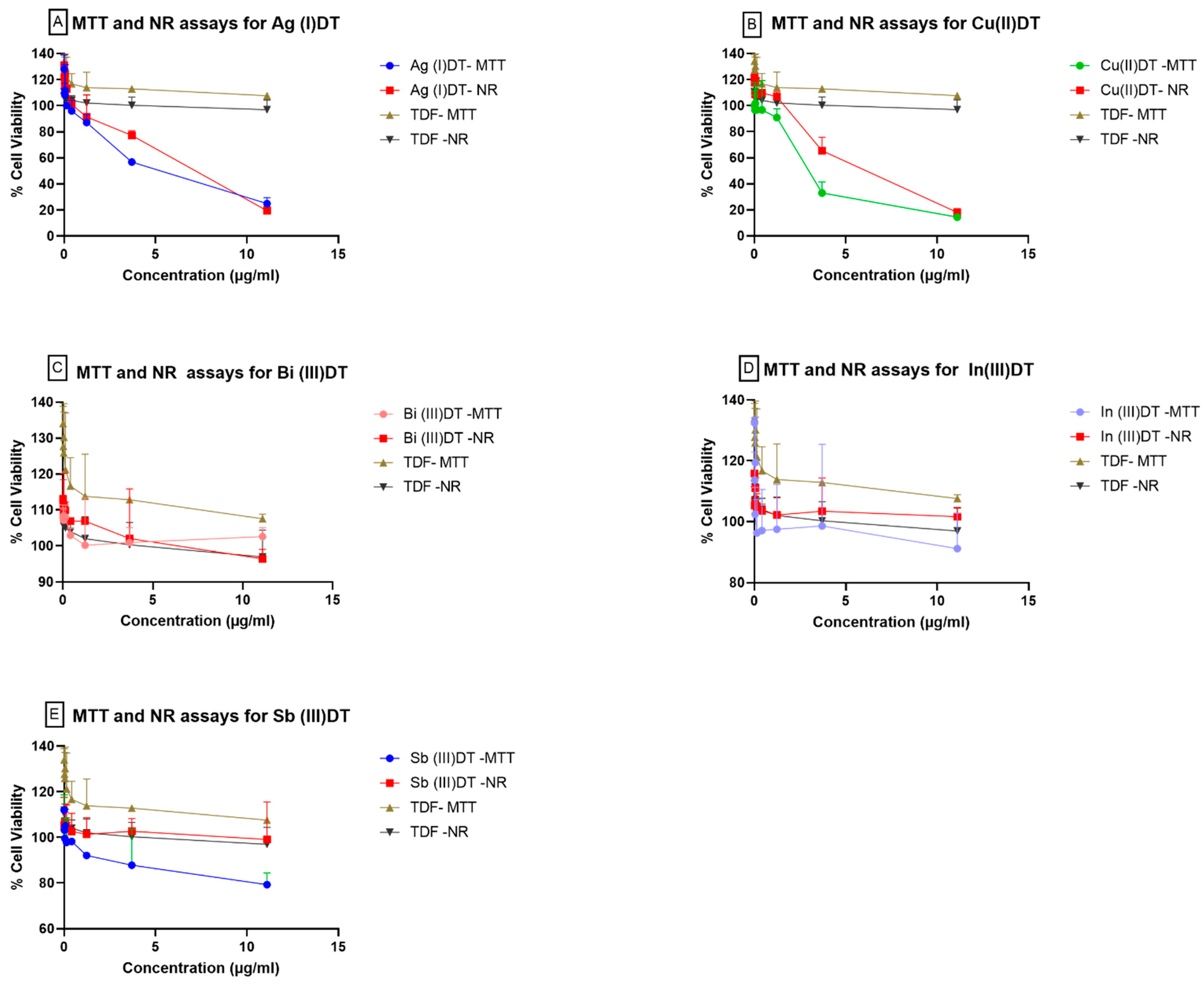
3.3. Effects of Inorganic Dithiocarbamate Complexes on TZM-bl Cells
3.4. Pseudovirus Neutralization Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, Y.-X.; Liu, M.; Zhou, Y.-Q.; Bi, X.-D.; Gao, F. Terpyridyl ruthenium complexes as visible spectral probe for poly(A) RNA and bifunctional TAR RNA binders and HIV-1 reverse transcriptase inhibitors. Inorg. Chim. Acta 2022, 539, 121027. [Google Scholar] [CrossRef]
- Bahare, S.; Kumar, N.V.A.; Şener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.B.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N.; et al. Medicinal Plants Used in the Treatment of Human Immunodeficiency Virus. Int. J. Mol. Sci. 2018, 19, 1459. [Google Scholar]
- Maina, E.; Adan, A.; Mureithi, H.; Muriuki, J.; Lwembe, R. A review of current strategies towards the elimination of latent HIV-1 and subsequent hiv-1 cure. Curr. HIV Res. 2021, 19, 14–26. [Google Scholar] [PubMed]
- Sun, R.W.-Y.; Ma, D.-L.; Wong, E.L.-M.; Che, C.-M. Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Trans. 2007, 43, 4884–4892. [Google Scholar]
- de Los Rios, P.; Okoli, C.; Castellanos, E.; Allan, B.; Young, B.; Brough, G.; Muchenje, M.; Eremin, A.; Corbelli, G.M.; McBritton, M. Physical, emotional, and psychosocial challenges associated with daily dosing of HIV medications and their impact on indicators of quality of life: Findings from the positive perspectives study. AIDS Behav. 2021, 25, 961–972. [Google Scholar] [CrossRef]
- Obisesan, O.; Katata-Seru, L.; Mufamadi, S.; Mufhandu, H. Applications of nanoparticles for herpes simplex virus (HSV) and human immunodeficiency virus (HIV) treatment. J. Biomed. Nanotechnol. 2021, 17, 793–808. [Google Scholar] [CrossRef]
- Behzad, F.; Kalyani, F.N.; Samadi, A.; Adabi, M. A promising treatment for HIV-1 using biosynthesis of metal nanoparticles. J. Ind. Eng. Chem. 2022, 115, 20–25. [Google Scholar] [CrossRef]
- Soundararajan, D.; Ramana, L.N.; Shankaran, P.; Krishnan, U.M. Nanoparticle-based strategies to target HIV-infected cells. Colloids Surf. B Biointerfaces 2022, 213, 112405. [Google Scholar] [CrossRef]
- Nakibuuka, M.M.; Mugab, R. Ethnobotanical study of indigenous nutri-medicinal plants used for the management of HIV/AIDS opportunistic ailments among the local communities of central Uganda. Sci. Afr. 2022, 16, e01245. [Google Scholar] [CrossRef]
- Chawuke, P.; van den Berg, N.; Fouche, G.; Maharaj, V.; Shoko, T.; van der Westhuizen, C.J.; Invernizzi, L.; Alexandre, K.B. Lobostemon trigonus (Thunb.) H. Buek, a medicinal plant from South Africa as a potential natural microbicide against HIV-1. J. Ethnopharmacol. 2021, 277, 114222. [Google Scholar] [CrossRef]
- Nyamukuru, A.; Tabuti, J.R.S.; Lamorde, M.; Kato, B.; Sekagya, Y.; Aduma, P.R. Medicinal plants and traditional treatment practices used in the management of HIV/AIDS clients in Mpigi District, Uganda. J. Herb. Med. 2017, 7, 51–58. [Google Scholar] [CrossRef]
- Anywar, G.; Kakudidi, E.; Byamukama, R.; Mukonzo, J.; Schubert, A.; Oryem-Origa, H.; Jassoy, C. A review of the toxicity and phytochemistry of medicinal plant species used by herbalists in treating people living with HIV/AIDS in Uganda. Front. Pharmacol. 2021, 12, 615147. [Google Scholar] [CrossRef]
- Nyakudya, T.T.; Tshabalala, T.; Dangarembizi, R.; Erlwanger, K.H.; Ndhlala, A.R. The Potential Therapeutic Value of Medicinal Plants in the Management of Metabolic Disorders. Molecules 2020, 25, 2669. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxidative Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef]
- Mujawdiya, P.K.; Kapur, S. Mangiferin: A potential natural molecule for management of metabolic syndrome. Int. J. Pharm. Pharm. Sci. 2015, 7, 9–13. [Google Scholar]
- Fonteh, P.N.; Keter, F.K.; Meyer, D. New bis(thiosemicarbazonate) gold(III) complexes inhibit HIV replication at cytostatic concentrations: Potential for incorporation into virostatic cocktails. J. Inorg. Biochem. 2011, 105, 1173–1180. [Google Scholar] [CrossRef]
- Duca, M.; Malnuit, V.; Barbault, F.; Benhida, R. Design of novel RNA ligands that bind stem–bulge HIV-1 TAR RNA. Chem. Commun. 2010, 46, 6162–6164. [Google Scholar] [CrossRef]
- Joly, J.-P.; Mata, G.; Eldin, P.; Briant, L.; Fontaine-Vive, F.; Duca, M.; Benhida, R. Artificial Nucleobase–Amino Acid Conjugates: A New Class of TAR RNA Binding Agents. Chem.—A Eur. J. 2014, 20, 2071–2079. [Google Scholar] [CrossRef]
- Wang, M.-F.; Li, Y.; Bi, X.-D.; Guo, Y.-X.; Liu, M.; Zhang, H.; Gao, F. Polypyridyl ruthenium complexes as bifunctional TAR RNA binders and HIV-1 reverse transcriptase inhibitors. J. Inorg. Biochem. 2022, 234, 111880. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Ajiboye, T.T.; Marzouki, R.; Onwudiwe, D.C. The Versatility in the Applications of Dithiocarbamates. Int. J. Mol. Sci. 2022, 23, 1317. [Google Scholar] [CrossRef]
- Bala, V.; Jangir, S.; Mandalapu, D.; Gupta, S.; Chhonker, Y.S.; Lal, N.; Kushwaha, B.; Chandasana, H.; Krishna, S.; Rawat, K.; et al. Dithiocarbamate–Thiourea Hybrids Useful as Vaginal Microbicides Also Show Reverse Transcriptase Inhibition: Design, Synthesis, Docking and Pharmacokinetic Studies. Bioorganic Med. Chem. Lett. 2015, 25, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Elliott, J.H.; McMahon, J.; Hartogenesis, W.; Bumpus, N.N.; Lifson, J.D.; Savic, R.M. Population Pharmacokinetics and Pharmacodynamics of Disulfiram on Inducing Latent HIV-1 Transcription in a Phase IIb Trial. Clin. Pharmacol. Ther. 2019, 105, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Chen, D.; Cui, Q.C.; Dou, Q.P. Sodium diethyldithiocarbamate, an AIDS progression inhibitor and a copper-binding compound, has proteasome-inhibitory and apoptosis-inducing activities in cancer cells. Int. J. Mol. Med. 2007, 19, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Takamune, N.; Misumi, S.; Shoji, S. Cyclic Zinc-Dithiocarbamate-S,S′-Dioxide Blocks CXCR4-Mediated HIV-1 Infection1. Biochem. Biophys. Res. Commun. 2000, 272, 351–356. [Google Scholar] [CrossRef]
- Watanabe, K.; Kazakova, I.; Furniss, M.; Miller, S.C. Dual Activity of Pyrrolidine Dithiocarbamate on κB-Dependent Gene Expression in U937 Cells: I. Regulation by the Phorbol Ester TPA. Cell. Signal. 1999, 11, 479–489. [Google Scholar] [CrossRef]
- Schreck, R.; Meier, B.; Männel, D.N.; Dröge, W.; Baeuerle, P.A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 1992, 175, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oluwarinde, B.O.; Montso, P.K.; Ateba, C.N.; Onwudiwe, D.C. Antimicrobial activities of Cu(II), In(III), and Sb(III) complexes of N-methyl-N–phenyl dithiocarbamate complexes. Results Chem. 2021, 3, 100241. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Marzouki, R.; Onwudiwe, D.C. Photocatalytic Reduction of Hexavalent Chromium Using Cu3. 21Bi4. 79S9/g-C3N4 Nanocomposite. Catalysts 2022, 12, 1075. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Song, J.; Kong, L.-J.; Sha, B.-B.; Tian, X.-Y.; Liu, X.-J.; Hu, T.; Chen, P.; Zhang, S.-Y. Design, synthesis and evaluation of novel bis-substituted aromatic amide dithiocarbamate derivatives as colchicine site tubulin polymerization inhibitors with potent anticancer activities. Eur. J. Med. Chem. 2022, 229, 114069. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Venkatesh, R.; Kandasamy, J. Synthesis of functionalized S-benzyl dithiocarbamates from diazo-compounds via multi-component reactions with carbon disulfide and secondary amines. Org. Biomol. Chem. 2022, 20, 6766–6770. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, F.; Mascola, J.; Stamatatos, L.; Polonis, V.; Koutsoukos, M.; Voss, G.; Goepfert, P.; Gilbert, P.; Greene, K. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005, 79, 10108–10125. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the calculation of TCID50 for quantitation of virus infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Vajrabhaya, L.-O.; Korsuwannawong, S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Ates, G.; Vanhaecke, T.; Rogiers, V.; Rodrigues, R. Assaying Cellular Viability Using the Neutral Red Uptake Assay, in Cell Viability Assays; Springer: Berlin/Heidelberg, Germany, 2017; pp. 19–26. [Google Scholar]
- Jia, M.; Liberatore, R.; Guo, Y.; Chan, K.-W.; Pan, R.; Lu, H.; Waltari, E.; Mittler, E.; Chandran, K.; Finzi, A. VSV-displayed HIV-1 envelope identifies broadly neutralizing antibodies class-switched to IgG and IgA. Cell Host Microbe 2020, 27, 963–975.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Svehla, K.; Louder, M.; Wycuff, D.; Phogat, S.; Tang, M.; Migueles, S.; Wu, X.; Phogat, A.; Shaw, G. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 2009, 83, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Onwudiwe, D.C.; Ajibade, P.A. Synthesis, Characterization and Thermal Studies of Zn(II), Cd(II) and Hg(II) Complexes of N-Methyl-N-Phenyldithiocarbamate: The Single Crystal Structure of [(C6H5)(CH3)NCS2]4Hg2. Int. J. Mol. Sci. 2011, 12, 1964–1978. [Google Scholar] [CrossRef]
- Andrew, F.P.; Ajibade, P.A. Synthesis, characterization, and electrochemical studies of Co(II, III) dithiocarbamate complexes. J. Coord. Chem. 2019, 72, 1171–1186. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Onwudiwe, D.C. Synthesis and Antioxidant Investigation of Ag2S Nanoparticles Obtained from Silver (I) Complex of N-Methyl-N-Phenyl-Dithiocarbamate. J. Nano Res. 2022, 76, 131–143. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Nkwe, V.M. Morphological variations in Bi2S3 nanoparticles synthesized by using a single source precursor. Heliyon 2020, 6, e04505. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ajibade, P.A. Synthesis and characterization of metal complexes of N-alkyl-N-phenyl dithiocarbamates. Polyhedron 2010, 29, 1431–1436. [Google Scholar] [CrossRef]
- Singh, R.P.; Maurya, V.K.; Maiti, B.; Siddiqui, K.A.; Prasad, L.B. Synthesis, structure and thermogravimetric analysis of novel dithiocarbamate based Zn(II),Cd(II) and Hg(II) complexes. J. Mol. Struct. 2019, 1198, 126912. [Google Scholar] [CrossRef]
- Chun, U.-K.; Choi, K.; Yang, K.-H.; Park, J.-K.; Song, M.-J. Waste minimization pretreatment via pyrolysis and oxidative pyroylsis of organic ion exchange resin. Waste Manag. 1998, 18, 183–196. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ajibade, P.A. Thermal Studies of Zn(II), Cd(II) and Hg(II) Complexes of Some N-Alkyl-N-Phenyl-Dithiocarbamates. Int. J. Mol. Sci. 2012, 13, 9502–9513. [Google Scholar] [CrossRef] [PubMed]
- Paca, A.M.; Ajibade, P.A. Synthesis and structural studies of iron sulphide nanocomposites prepared from Fe(III) dithiocarbamates single source precursors. Mater. Chem. Phys. 2017, 202, 143–150. [Google Scholar] [CrossRef]
- Nomura, R.; Kanaya, K.; Matsuda, H. Preparation of copper sulfide powders and thin films by thermal decomposition of copper dithiocarbamate complexes. Ind. Eng. Chem. Res. 1989, 28, 877–880. [Google Scholar] [CrossRef]
- Larsson, P.; Engqvist, H.; Biermann, J.; Rönnerman, E.W.; Forssell-Aronsson, E.; Kovács, A.; Karlsson, P.; Helou, K.; Parris, T. Optimization of cell viability assays to improve replicability and reproducibility of cancer drug sensitivity screens. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Kahm, M.; Hasenbrink, G.; Lichtenberg-Fraté, H.; Ludwig, J.; Kschischo, M. Grofit: Fitting biological growth curves. Nat. Preced. 2010, 1. [Google Scholar] [CrossRef]
- Alharbi, S.A.; Mashat, B.; Al-Harbi, N.A.; Wainwright, M.; Aloufi, A.; Alnaimat, S. Bismuth-inhibitory effects on bacteria and stimulation of fungal growth in vitro. Saudi J. Biol. Sci. 2012, 19, 147–150. [Google Scholar] [CrossRef]
- Bahrami, A.; Arabestani, M.R.; Taheri, M.; Farmany, A.; Norozzadeh, F.; Hosseini, S.M.; Nouri, F. Exploring the role of heavy metals and their derivatives on the pathophysiology of COVID-19. Biol. Trace Elem. Res. 2022, 200, 2639–2650. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, R.; Chan, J.F.-W.; Zhang, A.J.; Cheng, T.; Chik, K.K.-H.; Ye, Z.-W.; Wang, S.; Lee, A.C.-Y.; Jin, L. Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 2020, 5, 1439–1448. [Google Scholar] [CrossRef]
- Sajadi, M.M.; Dashti, A.; Tehrani, Z.R.; Tolbert, W.; Seaman, M.; Ouyang, X.; Gohain, N.; Pazgier, M.; Kim, D.; Cavet, G. Identification of near-pan-neutralizing antibodies against HIV-1 by deconvolution of plasma humoral responses. Cell 2018, 173, 1783–1795.e14. [Google Scholar] [CrossRef] [PubMed]
- Hioe, C.E.; Wrin, T.; Seaman, M.; Yu, X.; Wood, B.; Self, S.; Williams, C.; Gorny, M.; Zolla-Pazner, S. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PLoS ONE 2010, 5, e10254. [Google Scholar] [CrossRef] [PubMed]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.R.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral properties of copper and its alloys to inactivate COVID-19 virus: A review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
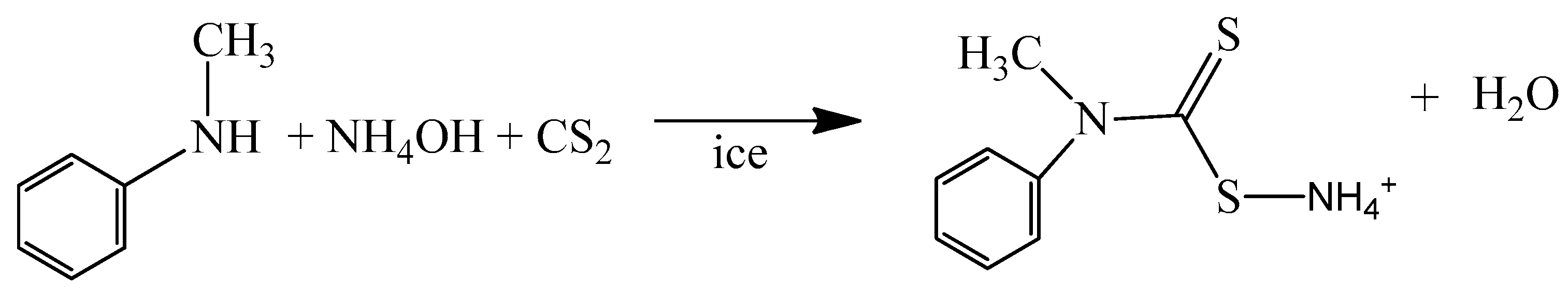


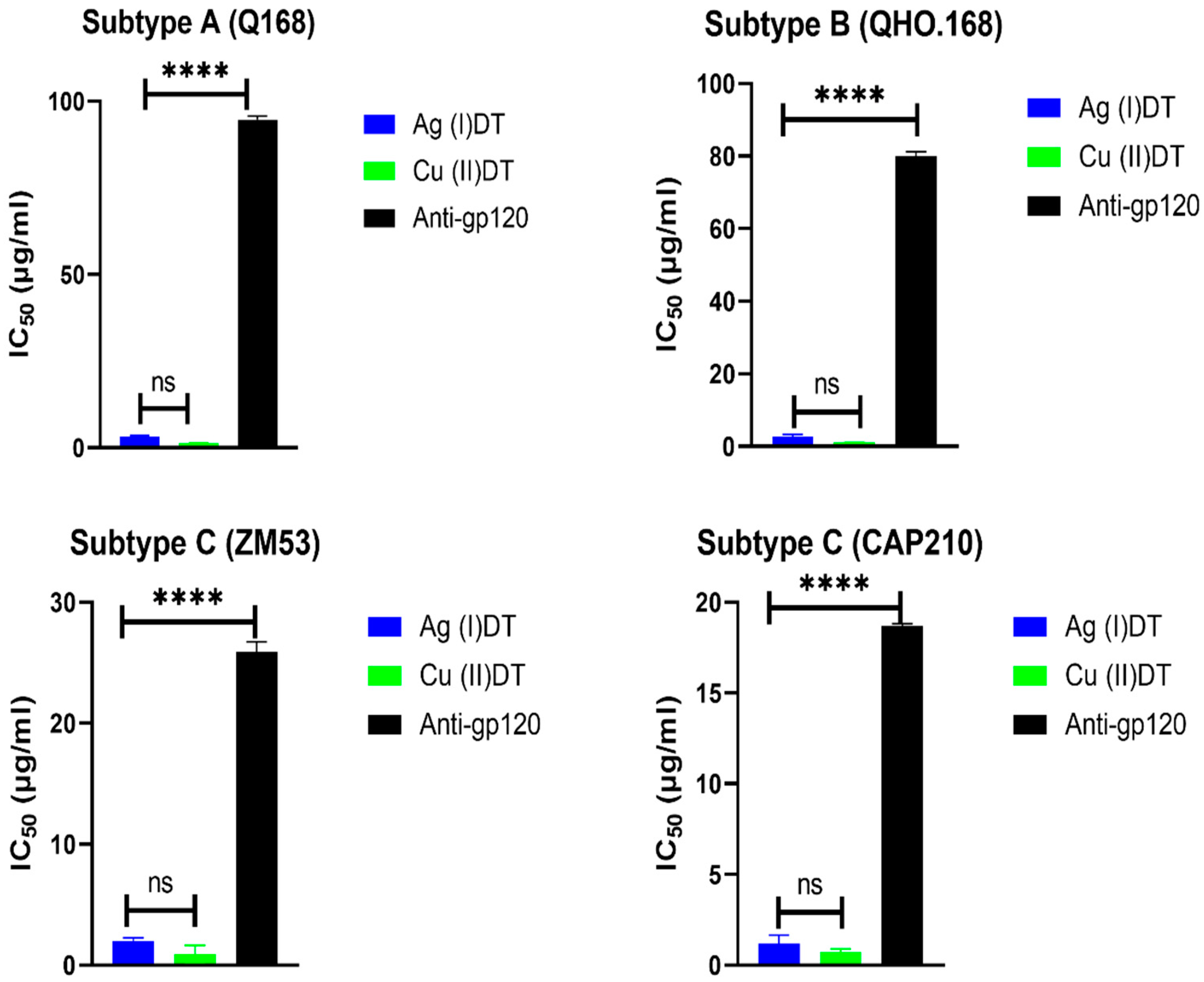
| Compound | Pseudovirus | Clade | Accession Number | IC50 (µg/mL) | CC50 (µg/mL) |
|---|---|---|---|---|---|
| Cu (II)DT | ZM53 | C | AY423984 | 0.93 | 3.44 |
| CAP210 | C | DQ435683 | 0.73 | 3.44 | |
| Q168 | A | AF407148 | 1.31 | 3.44 | |
| QHO.168 | B | AY835439 | 1.06 | 3.44 | |
| Anti-HIV-1 gp120 mAb | ZM53 | C | AY423984 | 25.2 | ND |
| CAP210 | C | DQ435683 | 18.7 | ND | |
| Q168 | A | AF407148 | 94.73 | ND | |
| QHO.168 | B | AY835439 | 79.89 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mufhandu, H.T.; Obisesan, O.S.; Ajiboye, T.O.; Mhlanga, S.D.; Onwudiwe, D.C. Antiviral Potential of Selected N-Methyl-N-phenyl Dithiocarbamate Complexes against Human Immunodeficiency Virus (HIV). Microbiol. Res. 2023, 14, 355-370. https://doi.org/10.3390/microbiolres14010028
Mufhandu HT, Obisesan OS, Ajiboye TO, Mhlanga SD, Onwudiwe DC. Antiviral Potential of Selected N-Methyl-N-phenyl Dithiocarbamate Complexes against Human Immunodeficiency Virus (HIV). Microbiology Research. 2023; 14(1):355-370. https://doi.org/10.3390/microbiolres14010028
Chicago/Turabian StyleMufhandu, Hazel T., Oluwafemi S. Obisesan, Timothy O. Ajiboye, Sabelo D. Mhlanga, and Damian C. Onwudiwe. 2023. "Antiviral Potential of Selected N-Methyl-N-phenyl Dithiocarbamate Complexes against Human Immunodeficiency Virus (HIV)" Microbiology Research 14, no. 1: 355-370. https://doi.org/10.3390/microbiolres14010028
APA StyleMufhandu, H. T., Obisesan, O. S., Ajiboye, T. O., Mhlanga, S. D., & Onwudiwe, D. C. (2023). Antiviral Potential of Selected N-Methyl-N-phenyl Dithiocarbamate Complexes against Human Immunodeficiency Virus (HIV). Microbiology Research, 14(1), 355-370. https://doi.org/10.3390/microbiolres14010028







