1. Introduction
Lymphatic filariasis (LF) is a vector-borne disease caused by parasitic helminths and constitutes a serious public health issue in tropical regions. The filarial nematodes that cause the disease are mostly identified as
Wuchereria bancrofti,
Brugia timori and
Brugia malayi species, depending on the geographical region. The LF disease is categorized as one of the neglected tropical diseases (NTD), commonly present in tropical and sub-tropical countries. LF infection involves asymptomatic, acute and chronic manifestations, where the majority of people who have been infected by the parasite do not show any external symptoms or signs of infection, but still contribute to the transmission chain [
1]. Individuals who suffer from LF have swelling of lymph spots or lymph nodes (lymphadenopathy), leading to permanent disability, although it rarely causes mortality [
2].
Globally, LF affects more than 120 million people in 72 countries, and it is estimated that more than 40 million people become disabled as a direct result of this chronic condition [
3]. A number of studies have demonstrated the impact of LF on quality of life and labor activities [
4,
5,
6]. In addition, it is estimated that around 40 million people suffer from the stigmatizing and disabling clinical manifestations of the disease, including 15 million who have lymphoedema (elephantiasis) [
7]. According to the World Health Organization (WHO), infected cases in Southeast Asia constitute 50% of the estimated 120 million infections globally. Furthermore, people at risk of LF in Southeast Asia are at a higher proportion due to the local population density, placing 63% of 1,34 billion people at risk [
8].
The Indonesian population is vulnerable to such infections by the parasites, since Indonesia is a tropical country where all of the parasites and their vectors are commonly found [
9]. In Indonesia, LF is caused by all filarial species, such as
Wuchereria bancrofti,
Brugia timori and
Brugia malayi. Specifically, in 2018, there were 10,681 LF cases recorded throughout Indonesia, while 70% of all filariasis cases are estimated to be caused by
Brugia malayi [
10]. As a consequence, 236 districts of a total of 514 districts in the entire country were declared as endemic areas [
11]. The direct economic loss due to loss of productivity is significant, estimated at IDR 13,245,807,890,000, or equal to USD 947,333,557, calculations based on the 2014 rates of Upah Minimum Regional (UMR) or Minimum Wage using officially reported cases [
10].
To address the burden at a global level, the WHO established a global action plan and commitment to eliminate the burden of LF by the year 2020; it was launched as the Global Program to Eliminate Lymphatic Filariasis (GPELF) in 2000, and related to the third sustainable development goal for the elimination of NTDs. The program includes two concurrent strategies, which are: (1) to stop the spread of further infections by interrupting transmission; and (2) to reduce the suffering of affected populations by controlling morbidity [
12]. In line with this global movement, and based on the WHO recommendations, Indonesia created the equivalent BELKAGA national program to eliminate the disease, running as a national program since 2005 [
10]. The BELKAGA includes a mass drug administration (MDA) once a year for five consecutive years. The drugs distributed consist of Diethylcarbamazine (DEC) in a 100 mg dose and Albendazole in a 400 mg dose.
The global program to eliminate filariasis has been running for the last 19 years and has been conducted as a full national initiative for the last eight years in Indonesia. To date, however there is a distinct lack of studies describing the relative change(s) of prevalence and distribution of LF over this period of time in Indonesia. Thus, this manuscript aims to address this gap and explore the spatial-temporal trends of LF cases and prevalence in Indonesia for the past 17 years (2001–2017)—during the global and national LF elimination programs—using national registry-based data and subsequent analyses.
2. Materials and Methods
2.1. Study Area
Geographically, Indonesia is in Southeast Asia, lying between the Indian and Pacific Oceans (lat: 5°00′ N, lon: 120°00′ E). It is an archipelagic country comprising five major islands (Sumatera, Kalimantan, Java, Sulawesi and Papua) and thousands of smaller islands. Indonesia is located adjacent to the equator line and is a tropical region with two seasons: the rainy (October–March) and dry seasons (April–September).
Indonesia is administratively divided into 34 provinces and 514 cities and regencies, with independent local governments and parliamentary bodies. It has 10,138 public health centers (PUSKESMAS—primary healthcare facilities) organized by province and district, which have been reporting the chronic LF cases since 2000.
2.2. Lymphatic Filariasis Data
The chronic LF data were obtained under permission by the Indonesian Ministry of Health. Specifically, the use of data, which were anonymized, aggregated, and at the population level, was permitted by the Indonesian Ministry of Health under Regulation Number 45 (2014), Article 3, paragraphs 1 and 2.
The data used in the current manuscript were collected from the smallest reporting units within Indonesia, i.e., reported by the village/district, through case findings from the head of village and/or health care staff. Case findings are carried out by those units on a continuous basis. Each reported case is subsequently surveyed and recorded by trained primary health care workers at the village level. Upon case confirmation, the primary health care worker reports the case to the district/city level and the data are then aggregated at the province and national levels to inform public health policy interventions. Furthermore, confirmed cases are followed up by the designated health office and ministry of health overseeing bodies [
13].
According to the Regulation of the Ministry of Health, No. 94 (2014), diagnostic tests are performed to detect the presence of microfilaria by microscopic investigation, and IgG4 anti-filarial antibody detection by rapid test and ELISA. Finger Prick Bloods (FPB) are collected and prepared into a thick-blood smear slide and subsequently stained using Giemsa or Wright staining, before being examined under the microscope. The rapid test measurement uses recombinant antigens (BmR1 and BmSXP) and the results are characterized by the development of 2–3 strips (bands) indicating positive results or the presence anti-filarial antibodies of IgG4 in the sample serum. The instrument used for detecting the presence of Brugia spp. infection is the Brugia rapid test, while for detecting W. Bancrofti, the ICT bancrofti or Pan LF kits are utilized. In some circumstances, the measurement of IgG4 anti-filarial antibody levels is done using the ELISA technique, though the application of ELISA remains scarce within the dataset. As per the above regulation, individuals who live in endemic areas and present stage 1 symptoms (i.e., swelling in the leg that usually disappears when they wake up in the morning) are tested using the rapid LF test. Individuals who are in stages 2–3 have a rapid test or blood examination to confirm the stage of the disease, and individuals in stages 4–7 are provided with follow-up clinical examination(s).
The data presented in the current manuscript therefore consist of confirmed, cumulative LF cases at both district and province levels across the entire area of Indonesia, as reported between January 2001 and December 2018. The definition of LF cases is people who are infected by parasitic helminths and show chronic symptoms, such as lymphedema, lymph scrotum, chyluria or hydrocele. People who are found showing the symptoms are followed up by a confirmatory clinical examination and interview by a trained primary health care officer [
13].
2.3. Data Analysis
The data were entered into an excel database and analyzed to find temporal changes of the prevalence rate of filariasis each year from 2001 to 2018. The prevalence rate was calculated by dividing the number of cases by the population number in the same year. The population number was obtained by the Indonesian National Statistics Services (BPS-Statistics Indonesia) from 2001 to 2018.
To show the temporal and geo-spatial trend(s) of LF, the 34 provinces were categorized into their respective five main island groupings, including (i) Sumatera, (ii) Java and Bali, (iii) Kalimantan, (iv) Sulawesi and (v) Papua and Maluku. More specifically, the Sumatera Island group consists of Aceh, North Sumatera, West Sumatera, Riau, Riau Island, Jambi, West Sumatera, Bengkulu, Lampung and Bangka Belitung Island; the Java and Bali islands group consists of Jakarta, West Java, Central Java, Di Yogyakarta, East Java, Banten, Bali, West Nusa Tenggara and East Nusa Tenggara; the Kalimantan island group consists of West Kalimantan, Central Kalimantan, South Kalimantan, East Kalimantan and North Kalimantan; the Sulawesi island group consists of North Sulawesi, Central Sulawesi, East Sulawesi, West Sulawesi and Gorontalo; the Papua and Maluku group consist of Maluku, North Maluku, Papua and West Papua.
3. Results
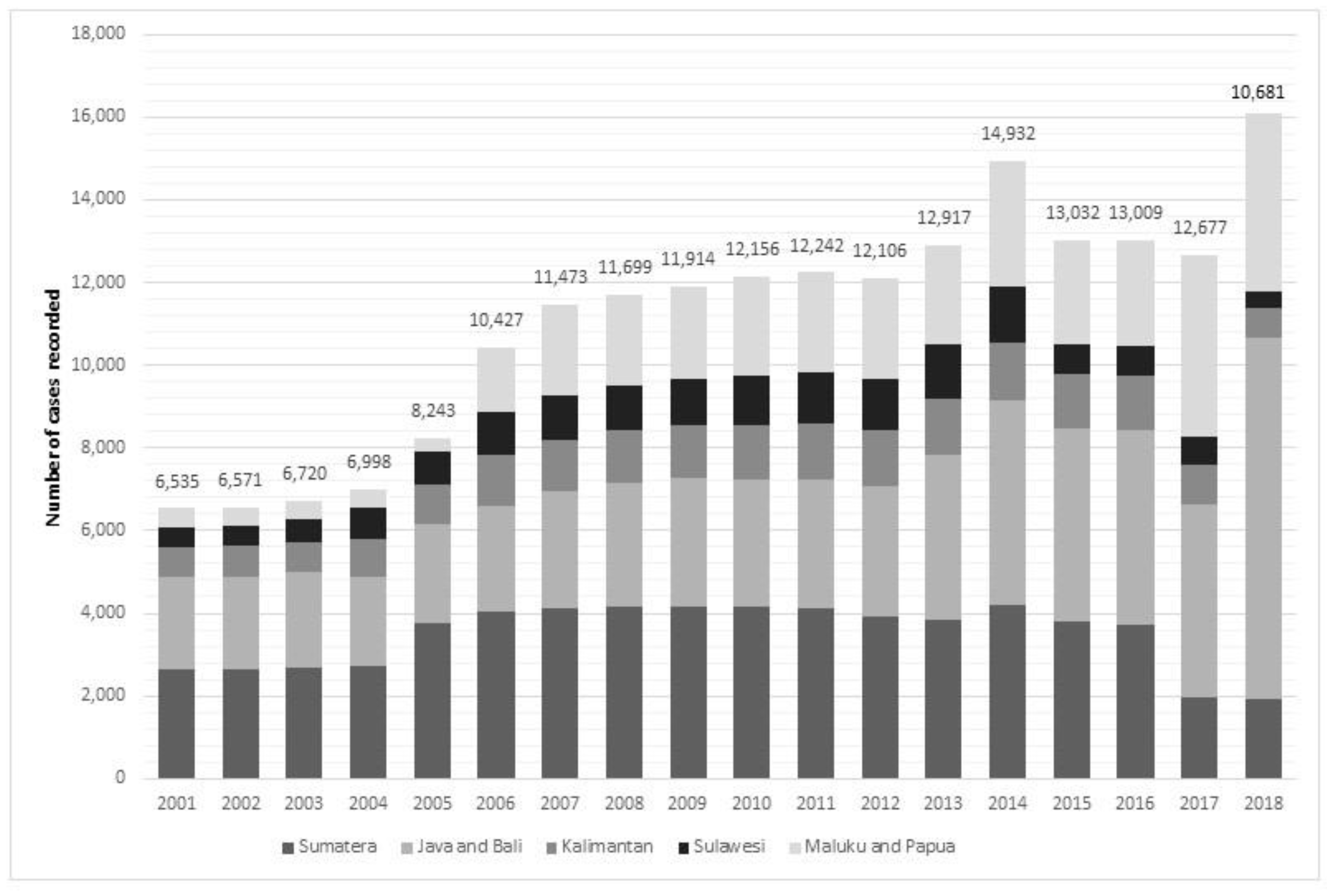
In 2018, the total confirmed cases of LF in Indonesia stood at 10,681. The absolute number has almost doubled within the last 18 years, compared to 6535 confirmed new cases in 2001 (
Figure 1). The highest number of cases occurred in 2014, where 14,932 Indonesian people were confirmed as infected with the disease. It becomes evident that Sumatera and Java and Bali Islands have contributed consistently over 50%—and up to 74%—of the national cases. On the other hand, Sulawesi has contributed steadily the lowest number for national cases.
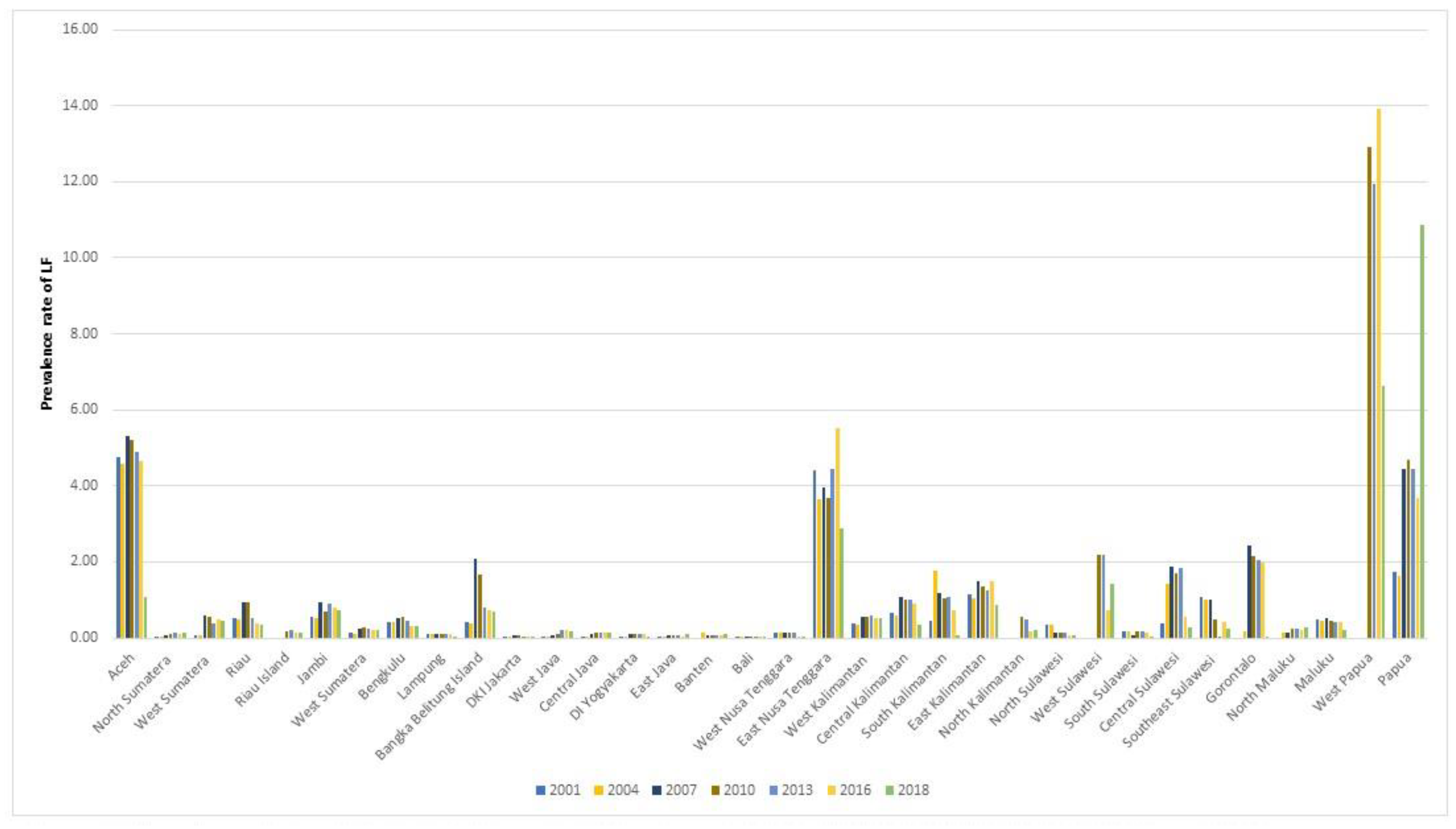
To explore the changing number of LF cases in each province over the 18-year period with a higher geographical granularity, the absolute number of confirmed new cases was presented in triennial intervals (
Figure 2; years shown: 2001, 2004, 2007, 2010, 2013, 2016 and 2018). The highest overall absolute number of LF cases was contributed from Aceh (total of 37,174 cases within 18 years), followed by East Nusa Tenggara (35,552 cases), Papua (21,737 cases) and West Papua (13,638 cases). In 2018, Papua showed the highest number of cases in Indonesia (3615/10,681 cases), contributing 34% of the national cases, followed by East Nusa Tenggara (14%, 1542/10,681 cases) and West Java (7%, 781/10,681 cases).
The LF burden of disease was further expressed using the prevalence rates for the same period. The prevalence rate fluctuates differently in different broad geographical areas. Java and Bali and Sulawesi islands had the lowest rate of LF per 10,000 inhabitants, with 0.17 and 0.33 in 2001 moving to 0.21 and 0.21 in 2018, respectively. Java and Bali presented a slight increase (from 0.17 in 2001 to 0.21 in 2018). On the other hand, though Sumatera and Kalimantan had the second highest rate in 2001, accounting for 0.60 and 0.61 cases per 10,000 (respectively), the prevalence rate continued to decrease until 2018, reporting 0.33 and 0.43 cases. The originally highest LF prevalence rate recorded for Papua and Maluku in 2001 (1.07) began to increase notably from 2005 onwards. At the end of 2018, the prevalence rate was five-times higher than 2001, at 5.93 cases per 10,000 people.
Figure 3 shows the prevalence rate in 34 provinces in 2001, 2004, 2007, 2010, 2013, 2016 and 2018. It explores the prevalence rates in each province in Indonesia. The figure shows that most of the provinces have less than one case per 10,000 people. However, there are four provinces that recorded more than two cases per 10,000 people, including Aceh, East Nusa Tenggara, Papua and West Papua. West Papua constantly contributes the highest prevalence rate in Indonesia, accounting for 12.91 cases in 2010 and the highest number of cases in 2014, accounting for 20.77 cases per 100,000 people.
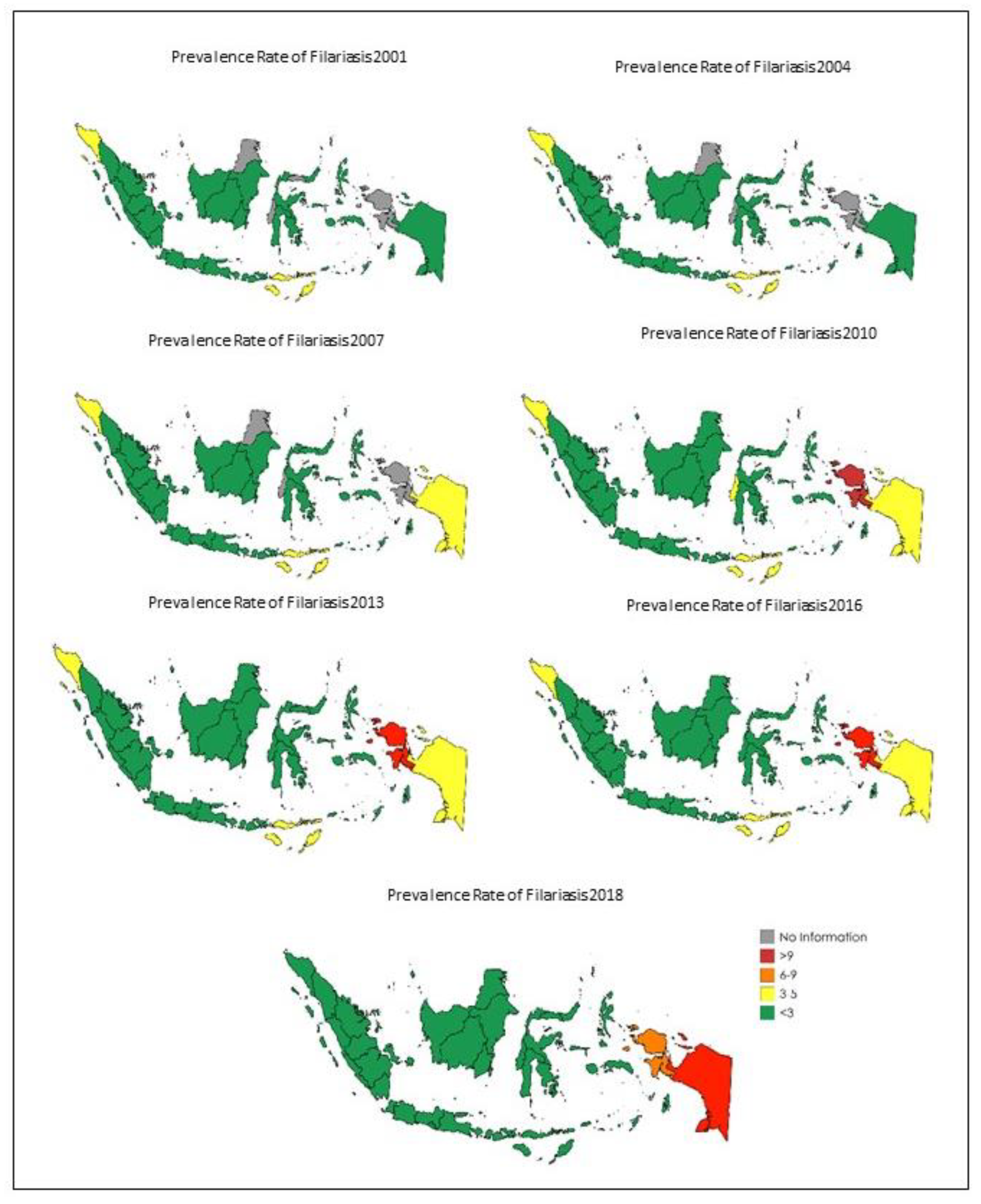
Besides the temporal distribution of LF, we also assessed the spatial distribution of the disease over the 18-year period. In
Figure 4, the distribution of filariasis is assessed using the same three-year intervals, as previously shown per province in Indonesia. It is shown clearly that there are 27 out of 34 provinces in Indonesia with consistently fewer than three cases per 10,000 people for the entire 18-year period of the study. On the contrary, there are specific provinces in the east (e.g., Aceh) and west of the country (e.g., West Papua) which have shown limited demonstrable improvement, or even a deterioration, and as such remain of particular concern. In addition, East Nusa Tenggara is the only province located in central Indonesia with a consistently higher incidence rate than all of its neighboring provinces.
4. Discussion
Overall, our study shows that the prevalence rate of LF in Indonesia has remained a fragmented and fluctuating picture for the 18 years considered between 2001 and 2018. Notably, while most provinces provide a relatively flat (Java and Bali) or even positive trend (Sumatera), Papua and Maluku have experienced the most significant increase for the last 12 years (2005–2017).
The observed increase in the 2006 reported figures likely reflects the expansion of scope and reach of the LF control program (BELKAGA) by the government of Indonesia rather any particular underlying epidemiological factor. Therefore, as the reach of the program increased from 13.24% (in 2005) to 77.48% (in 2018) coverage of the population, new cases were found and reported, contributing to the increase in the absolute numbers [
14,
15]. On the contrary, during the same time, in the provinces in Indonesia where the program coverage was high from the outset, the absolute number of cases has declined, since the program has successfully reached out to the target locations and prevented further cases [
10].
The drug distribution program (MDA) started in 2012 [
10] and the data from the Indonesia Ministry of Health show that the program successfully contributed towards a decline in the number of endemic areas (<1% microfilaria cases) in 25 endemic provinces in Indonesia [
13]. A similar result is shown for Sierra Leone, where a significant reduction of LF prevalence and density was reported in 12 co-endemic districts following five annual LF MDAs [
16].
On the contrary, during the MDA, the prevalence rate of filariasis increased in Papua and Maluku, including West Papua. The results from a recent study show that the risk factors of LF in West Papua are low income and level of knowledge, not using a bed net, minimal clothing and living near swamps [
17]. Additionally, the program to eliminate LF only reached the Papua and Maluku areas in 2004 [
10]. In 2010, the Indonesian Ministry of Health categorized several districts and cities in Papua and Maluku as endemic areas. By definition, districts and cities that have >1% microfilariasis cases are categorized as endemic area [
13].
On the other side of the country, in 2005, Aceh and East Nusa Tenggara contributed the most to the total number of LF cases nationally. This was a likely outcome of the MDA coverage decreasing in 2005 [
18]. The relationship between coverage and LF cases was explained in India by the mathematical modelling of lymphatic filariasis elimination programs, showing that, in high endemicity areas such as Aceh and West Nusa Tenggara, 4–12 rounds are needed, with a minimum coverage of 50% of the total area. In the case of Aceh, where the MDA generally has a lower coverage, as the population ages, one would expect to see less of a reduction in LF cases than in other provinces with high MDA coverage. Therefore, continuity of the programs is required in order to further decrease the cases. In low endemic settings, the number of MDAs needed (2–4 rounds) is fewer than in settings with intermediate (3–7) and high (4–12) baseline endemicity. The required duration doubles or triples with decreasing coverage levels for all settings or increasing endemicity: 2–4 rounds of MDA at 80% coverage to 4–12 rounds with 50% coverage [
19]. According to the Indonesian Ministry of Health, in 2019, 2 of 12 districts in Aceh and 4 of 18 districts/cities in West Nusa Tenggara successfully decreased the endemicity level into non-endemicity. The rest of the districts/cities are going to be evaluated this year (2022).
The collected data clearly indicate that the risk of filarial infection in Indonesia does not remain constant throughout the country, but rather is province-dependent. This aligns with findings from previous studies in India [
20]. Factors such as the environment, landscape and climate, e.g., temperature, annual rainfall, altitude and humidity, had a significant impact to the transmission of filariasis [
21]. A study in Indonesia shows that temperature, precipitation and humidity show a correlation with LF cases [
22]. However, several studies in eastern and western Indonesia showed that human behaviors are equally as important, for example, using insect repellent, outdoor activities during the night, mosquito net usage and existence of water plants all have some level of correlation with LF cases [
22,
23,
24,
25,
26].
The strength of our study is that the data have been collected continuously and uniformly from 2001 to 2018. Therefore, the change in terms of cases, prevalence rates and distribution can be described over the long term. Moreover, it has enough granularity to represent each one of the 34 provinces in Indonesia. As the data were collected from within the province, initiated at the community level, we consider that this study can capture true and representative population-level data, though it should be noted that clinical case counts are likely to be a drastic undercounting of the true LF morbidity, as it has been shown that chronic cases rarely have active infection [
27]. However, our study has some limitations. The program was rolled out nationally gradually, and therefore some provinces did not report data regularly until after 2010 when they were included in the program. There are five provinces, including Riau Island, North Kalimantan, West Sulawesi, Gorontalo and West Papua, which record their own data after the administrative proliferation in 2010, and there might be some limited inconsistencies. A further limitation is that, although the data were collected from primary healthcare centers and/or practitioners, the characteristic of LF that part of the infected population can be asymptomatic can contribute to some systematic underreporting. Furthermore, some cases may be reported twice, as there is a small background of internal migration within Indonesia, and the records cannot identify such cases. In addition, the continuous monitoring of cases is heavily dependent on the smaller healthcare units; however, their relative proportional population coverage differs between islands and this might play a role in the differing LF cases reported.
5. Conclusions
This is the first time that the national picture of LF is presented for Indonesia, one of the largest and most populous nations globally. Additionally, this is done systematically and over a long-term window of observation, spanning almost two decades. In terms of absolute numbers, the LF cases recorded in 2017 in Indonesia were 12,667, almost two-times higher than in 2001, in which there were 6535 cases. Therefore, the prevalence rate is 39 cases per 100,000 people. Java and Bali show the highest number, followed by Papua and Maluku. However, this increase is primarily due to the gradual expansion of the national program to the entire country, and the successful identification of cases in geographical areas which were severely underreported previously.
The granularity of the data allows the observation that there is a widely pronounced, province-dependent variation of LF cases across Indonesia. Some provinces have had the same level of LF cases reported for 17 years, while other provinces have faced a significant increase during specific periods of time. Additionally, the distribution of cases is highest at the two geographical extremes of Indonesia, the eastern and western parts.
These data demonstrate that the national program has been effective by and large in the areas it has been active the longest, while there are provinces lagging behind in the successful suppression of LF. It is important to note that the high geographical fragmentation of the country, with the associated ecological parameters related to LF incidence, likely plays an important role in maintaining the highly varied incidence rate across Indonesia. As such, future public health interventions and strategic decisions need to take into account the local specificities, so that the successful national initiative can reach the WHO and Indonesian goal of controlled LF management and eventual elimination in the near future.