Abstract
In the proposed review, the pharmacological profile of unique, rare, and unusual fatty acids derived from natural amides is considered. These amides are produced by various microorganisms, lichens, and fungi. The biological activity of some natural fatty acid amides has been determined by their isolation from natural sources, but the biological activity of fatty acids has not been practically studied. According to QSAR data, the biological activity of fatty acids is shown, which demonstrated strong antifungal, antibacterial, antiviral, antineoplastic, anti-inflammatory activities. Moreover, some fatty acids have shown rare activities such as antidiabetic, anti-infective, anti-eczematic, antimutagenic, and anti-psoriatic activities. For some fatty acids that have pronounced biological properties, 3D graphs are shown that show a graphical representation of unique activities. These data are undoubtedly of both theoretical and practical interest for chemists, pharmacologists, as well as for the pharmaceutical industry, which is engaged in the synthesis of biologically active drugs.
1. Introduction
The amide bond is one of the most prevalent and widespread linkages in nature [1]. Natural fatty acid amides (R-COO-NR1R2) have many potential pharmacological uses because they have different biological activities or enzyme inhibitors [2,3,4]. Fatty acid amides are a diverse family of underappreciated biologically active lipids [1,2,3,4,5,6].
This article is a review of rare and unusual fatty acids (FA) derived from naturally occurring amides that are produced by fungal endophytes, lichenized ascomycetes, basidiomycetes, actinomycetes, and related microorganisms. The natural compounds presented in this review are of great scientific interest, and many of them demonstrate a wide range of biological activities and have strong antimicrobial, antifungal, phototoxic, HIV-inhibiting, antitumor, immunosuppressive, and other pharmacologically useful properties [7,8,9,10,11,12,13], which is of great interest, especially for medicinal chemistry, pharmacology, and the pharmaceutical industry [14,15,16,17]. This review is devoted to the biological activity of fatty acids included in amides.
2. Fatty Acids Derived from Microorganisms and Fungi
Two polycyclopropane FA amides—U-106305 and FR-900848 (1, structure FA see Figure 1)—were isolated from microbiological sources. Thus, antibiotic U-106305 was produced by Actinomycete Streptomyces sp. [18], and Streptoverticillium fervens (syn: Streptomyces fervens) produced antibiotic, FR-900848, which has strong antifungal activity against filamentous fungi: Aspergillus niger, Mucor rouxianus, Aureobasidium pullulans, Penicillium chrysogenum, Trichophyton metagrophytes, T. astervides, T. rubrum, Fusarium oxysporum, and Sclerotinia arachidis [19].
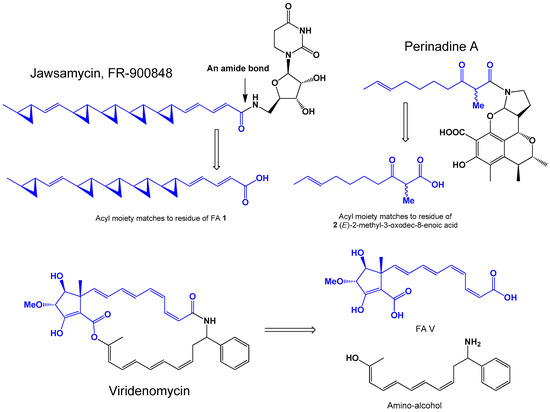
Figure 1.
Examples of FA amides derived from Actinomycetes: Streptomyces and Penicillium.
Perinadine A (for structure, see Figure 1), a tetracyclic alkaloid isolated from from marine-derived fungus Penicillium citrinum (see Figure 2), contains FA (2). Perinadine A demonstrated insignificant cytotoxicity against murine leukemia L1210 cells, and antibacterial activity against Micrococcus luteus and Bacilius subtilis [20]. The 3D graph that shows the predicted and calculated antifungal, antiviral, and antineoplastic activity of FR-900848 and FA (1) is shown in Figure 3. FA (2) was found in scalusamide A, and scalusamide B and C contain a similly FA (3, for structure see Figure 4) and (4), respectively. The fungus Penicillium citrinum produces scalusamides that have been isolated from the broth during mycelium cultivation [21]. Two epimeric alkaloids, namely tumonoic acids K (with (S,E)-2-methyl-3-oxodec-8-enoic acid (5) and L (with (R,E)-2-methyl-3-oxodec-8-enoic acid (6), were isolated from the marine-derived fungus Penicillium citrinum and showed cytotoxic activity against A-375 cell lines [22].
Figure 2.
Examples of some fungal endophytes and fungi that synthesize the FA amides presented in this article. See text for details on their metabolites. In the pictures: (a)—Penicillium citrinum, (b)—Streptomyces viridochromogenes, (c)—Pestalotiopsis theae, and (d)—Isaria tenuipes. Pictures of fungal endophytes, lichenized ascomycetes, and fungi are from sites that allow the use of pictures for non-commercial use. In addition, all pictures are adapted by the author.
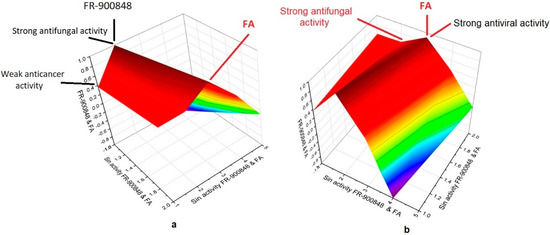
Figure 3.
The 3D graph ((a), X and (b), Y views) shows the predicted and calculated antifungal, antiviral, and antineoplastic activities of FR-900848 and FA (1) showing the highest degree of confidence. The presented 3D graph demonstrates the comparative characteristics of the biological activities and the pharmacological profile of the individual fragments of the pseudopeptide referred to as FR-900848. In particular, the red zone of the 3D graph indicates the strong biological properties of both FR-900848 and its fatty acid. To build 3D graphs of the biological activity of fatty acids from natural amides, a proprietary computer program OriginPro 2021 was used, into which data were entered from another computer program PASS, which calculates the degree of reliability of biological activity.
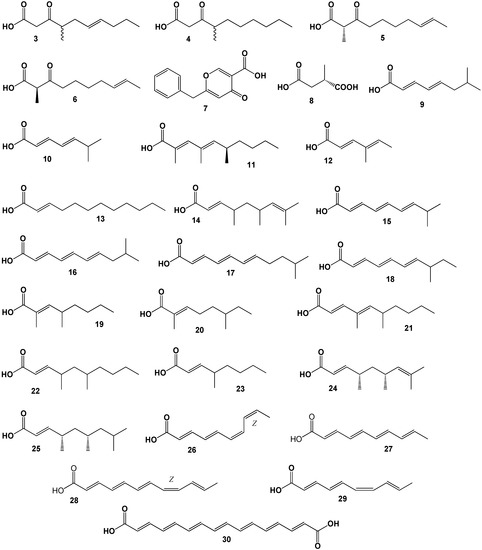
Figure 4.
Rare and unusual FA derived from marine and soil microorganisms.
Antibiotic viridenomycin (for structure, see Figure 1) with fatty acid (FA V) was isolated from the culture broth of Streptomyces viridochromogenes strain No T-24146 and shows strong activity against Trichomonas vaginalis and gram-positive bacteria [23].
Figure 1 shows samples of natural FA amides isolated from actinomycetes Streptomyces and Penicillium and shows fatty acid amide bonds. Table 1 demonstrates the biological activity of both whole molecules of FA amides.

Table 1.
Pharmacological profile of some amides and their FA derived from microorganisms.
A unique nucleoside polycyclopropane antibiotic named FR-900848 is known to exhibit strong antifungal activity as shown in numerous experimental studies [24,25,26]. The software PASS has also shown that this antibiotic exhibits strong antifungal activity with 92% confidence (see Table 1). Another interesting property of this antibiotic is the anticancer activity, which was also found in experimental work [27,28,29], and PASS confirms these data. However, unique (2E,4E)-5-((1R,1′R,1″R,1‴R,2S,2′R,2″R,2‴S)-2‴-((E)-2-((1R,2R)-2-methylcyclopropyl)vinyl)-[1,1′:2′,1″:2″,1‴-quartercyclopropan]-2-yl)penta-2,4-dienoic acid (1) exhibits antiviral, antifungal, and anti-inflammatory properties to a greater extent.
Another pseudopeptide named perinadine A, which is synthesized by marine-derived fungus Penicillium citrinum (see Figure 2), showed strong cytotoxicity against murine leukemia L1210 cells, and these data are confirmed by PASS. The (E)-2-methyl-3-oxodec-8-enoic acid (2) of this pseudopeptide also shows antineoplastic activity.
Viridenomycin, which also belongs to the class of amides, is represented by an acid (FA V) and an amino alcohol, demonstrates antineoplastic activity against sarcoma as well as its FA, although the amino alcohol shows antiviral activity against Arbovirus (data are shown in Table 1).
The phytopathogenic fungus Pestalotiopsis theae, which was isolated from branches of Camellia sinensis, afforded amides, pestalaminde A, which contain unusual 6-benzyl-4-oxo-4H-pyran-3-carboxylic acid (7), and both pestalamindes, A and B contain (S)-2-methylsuccinic acid (8) [30]. Pestalaminde B inhibited HIV-1 replication in C8166 cells and exhibited potent antifungal activity against A. fumigatus.
A marine-derived Streptomyces sp. CNQ-085 produces antitumor antibiotics designated as daryamides A, B, and C [31]. Both daryamides contain (2E,4E)-7-methylocta-2,4-dienoic acid (9), and daryamide C contains (2E,4E)-6-methylhepta-2,4-dienoic acid (10).
The chlorine containing manumycin derivatives named chinikomycins A and B have been found in extracts of Streptomyces sp. M045, which is obtained from sediment (Jiaozhou Bay, China). Both compounds displayed anti-tumour activity against several human cancer cell lines, and these metabolites contain (R,2E,4E)-2,4,6-trimethyldeca-2,4-dienoic acid (11) [32].
Jomthonic acids A, B, and C were found in the culture fluid of actinomycetes of the genus Streptomyces, and only jomthonic acid A induced the differentiation of preadipocytes into mature adipocytes [33]. (2E,4E)-4-methylhexa-2,4-dienoic acid (12) was found in jomthonic acid A and C.
Spirocyclic and bicyclic hemiacetals such as isariotins E, F, and TK-57-164A were detected in lipid extracts in the entomopathogenic fungus Isaria tenuipes BCC 12625. It is known from published sources that isariotin F exhibited activity against the malaria parasite Plasmodium falciparum K1, and cytotoxic activities against cancer cell lines (KB, BC, and NCI-H187) and non-malignant (Vero) cells. All isolated compounds contain (E)-dodec-2-enoic acid (13) [34].
Manumycin A contain (R,2E,4E)-2,4,6-trimethyldeca-2,4-dienoic acid (11), which was found in chinikomycin A and B. Antibiotic U-56407 was isolated from fermentations of Streptomyces hagronensis (strain 360) with (E)-4,6,8-trimethyl-nona-2,7-dienoic acid (14) was active in vitro against gram-positive bacteria [35].
Asukamycins A-II, B-II, C-II, D-II, and E-II are polyketides that are members of the manumycin family of antibiotics and exhibit potent antineoplastic, antifungal, and antibacterial activities that have been found and identified from lipid extracts of the actinomycete bacterium Streptomyces nodosus subsp. asukaensis [36]. Asukamycin B and asukamycin B-II contain (2E,4E,6E)-8-methylnona-2,4,6-trienoic acid (15). Asukamycin C and asukamycin C-II contain (2E,4E,6E)-9-methyldeca-2,4,6-trienoic acid (16). Asukamycin D and asukamycin D-II contain (2E,4E,6E)-8-methyldeca-2,4,6-trienoic acid (17), and asukamycin E and asukamycin E-II contain (2E,4E,6E)-10-methylundeca-2,4,6-trienoic acid (18).
Antitumor antibiotics named TMC-1 A, B, C, and D were obtained from a fermentation broth of Streptomyces sp. A-230. These antibiotics showed strong cytotoxic activities against various tumor cell lines in vitro: HCT-1 16 (human colon carcinoma), SW480 (human colon adenocarcinoma), Saos-2 (humanosteogenic sarcoma), WiDr (human colon adenocarcinoma), OVCAR-3 (human ovarian adenocarcinoma), HL-60 (human promyelocytic leukemia), HeLa S3 (human epitheloid carcinoma), and P388D1 (murine lymphoid neoplasm), and manumycin A exhibited antibacterial activity against gram-positive bacteria: Staphylococcus aureus, Enterococcus faecalis, Bacillus subtilis [37]. (E)-2,4-dimethyloct-2-enoic acid (18) TMC-1A, (E)-2,6-dimethyloct-2-enoic acid (19) TMC-1B, (2E,4E)-4,6-dimethyldeca-2,4-dienoic acid (20) TMC-1C, and (E)-4,6-dimethyldec-2-enoic acid (21) TMC-1D.
It is known that inhibitors of the enzyme, EI-1511-3, -5, EI-1625-2, U-56, 407, manumycins A, B, and G, converting interleukin-1β were found in the culture broths of Streptomyces sp. selectively inhibited the activity of recombinant human ICE [38]. EI-1511-5 contains FA (15), EI-1625-3 contains (2E,4E)-7-methylocta-2,4-dienoic acid (9), and (E)-4-methyloct-2-enoic acid (23) was found in EI-1625-2.
A halophilic strain of Streptomyces isolated from a salt pan on Shinui Island (Korea) is a producer of salternamides A–D. Salternamide A, which is the first chlorinated compound in the manumycin family, is an inhibitor of a human colon cancer cell line (HCT116) and a gastric cancer cell line (SNU638). Salternamides A and D have been found to be inhibitors of Na+/K+ ATPase [39]. Salternamides A and C contain (4S,6R,E)-4,6,8-trimethylnona-2,7-dienoic acid (24), and salternamide E contains (4S,6S,E)-4,6,8-trimethylnon-2-enoic acid (25).
Colabomycin A and D [40] were isolated from Streptomyces griseoflavus TU 2880 [41,42], and antimicrobial antibiotic U-62162 was found in Streptomyces verdensis UC-8157 [43]. Colabomycin A and D consist of (2E,4E,6Z,8E)-deca-2,4,6,8-tetraenoic acid (26) and (2E,4E,6E,8E)-deca-2,4,6,8-tetraenoic acid (27), respectively. It is known that colabomycin E inhibited IL-1β release from THP-1 cells and might thus potentially act as an anti-inflammatory agent [44], and it is produced by a strain of Streptomyces aureus (see Figure 5). Several FA with different properties has been found in isolated antibiotics. Thus, colabomycin E has a (2E,4E,6E,8Z,10E)-dodeca-2,4,6,8,10-pentaenoic acid (28), colabomycin F has an FA (14), colabomycin G—FA (15), dinocolabomycin E has an FA (15), and dinocolabomycin A has an FA (29), respectively.
Figure 5.
Examples of fungal endophyte, Streptomyces aureus (a), pathogenic fungus Bipolaris spp (b), and discomycete Trichopeziza mollissima (c), which produce bioactive FA amides. Among the microbial species cited, of great interest are Discomycetes, which include cup fungi, spongy fungi, and brain fungi, as well as some club-shaped fungi. In recent years, interest in these fungi has grown as they are a source of biologically active metabolites, including amides. Among the Discomycetes, saprobionts predominate, which grow in conditions of high soil moisture, humus, and on dead wood. They are typical cups or discs, but other forms such as sponges, saddles, tongues, and bells are not uncommon. The pictures (c) show varieties of Trichopeziza mollissima.
From the culture solution of Streptomyces limosus was obtained a yellow crystalline, nitrogenous dye stuff that limocrocin was isolated [45]. Rare polyenoic (2E,4E,6E,8E,10E,12E,14E)-hexadeca-2,4,6,8,10,12,14-heptaenedioic acid (30) is present in limocrocin. Figure 6 demonstrates in a 3D graph the predicted and calculated antiviral activity of a rare FA (30), and activity is presented in Table 2.
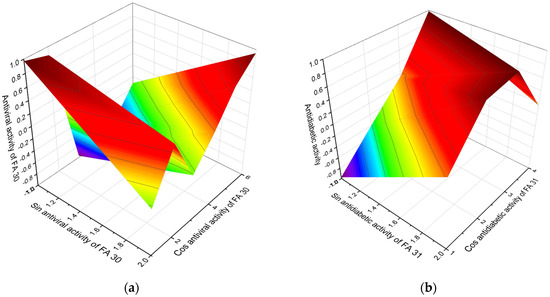
Figure 6.
The 3D graph shows the predicted and calculated antiviral activity of FA (30, a). Limocrocin with this rare polyenoic FA, which contains seven conjugated carbon-carbon double bonds shows maximum UV absorption at 260, 420, and 440 nm, and exhibits strong antiviral activity against Arbovirus, and it is also a reverse transcriptase inhibitor. The 3D graph shows the predicted and calculated antidiabetic activity of FA (31, b). Complex molecules of antimycin and closely related antibiotics that contain (S)-2-((1S,2R)-1,2-dihydroxypropyl)-octanoic acid (31) exhibit antidiabetic properties.

Table 2.
Pharmacological profile of FA derived from microorganisms.
Antimycin is a mixture of closely related antibiotics produced by Streptomyces sp. [46,47]. Many known antibiotics belonging to the antimycin A family are produced by Streptomyces species [48,49,50,51], and these compounds have significant antifungal activity and act by blocking the electron transport chain through inhibition of the cytochrome bc1 complex [48,49]. Several FA were contain in antimycin derivatives: (S)-2-((1S,2R)-1,2-dihydroxypropyl)octanoic acid (31, 3D graph see Figure 6, right side) in A1a, A1b, A2a, A2b, A15, and A16 (S)-2-((1S,2R)-1,2-dihydroxypropyl)hexanoic acid A3a, A3b, A4a, A4b, A9, A11, A13 (32), (2S,3S,4R)-2-ethyl-3,4-dihydroxypentanoic acid in A5, A6 (33), and (2S,3S,4R)-3,4-dihydroxy-2-isobutylpentanoic acid has been found in A7a, A7b, A8, A12, and A14 (34).
All antibiotics belonging to the antimycin family were active against Caenorhabditis elegans and Artemia salina. Antimycin A9 demonstrated antimicrobial activity against Aspergillus niger KF 103, Bacillus subtilis ATCC 6633, Candida albicans KF1, Escherichia coli NIHJ, Mucor racemosus IFO 4581, Penicillium chrysogenum KF 270, Pseudomonas aeruginosa IFO3080, Saccharomyces cerevisiae KF26, Shizosaccharomyces pombe IFO 0347, Staphylococcus aureus ATCC 6538P, and Trichophyton mentagrophytes KF 331 [52,53]. Moreover, antimycins A1, A2, A3, A4, A10, A11, A12, A13, A14, A15, and A16 were obtained from the fermentation broth of strains of Streptomyces spp. SPA-10191 and SPA-8893 [53]. These compounds exhibited antifungal activity against Candida utilis [54]. (2S)-2-((1S,2R)-1,2-dihydroxypropyl)-6-methyloctanoic acid (35) was present in antimycin antibiotics A10a and A10b. Kitamycins A and B acted as plant growth inhibitors produced by Streptomyces sp. K385 [55]. Urauchimycins A and B were isolated from a fermentation broth of a Streptomyces sp. Ni-80, which was detected in an unidentified sponge [56]. These antibiotics contain (32) acid, and (S)-2-((1R,2R)-1,2-dihydroxypropyl)-6-methylheptanoic acid (36), and kitamycin B contains fatty acid (36).
Antibiotics named splenocins A–J which are the cytokine inhibitors have been found in extracts of marine-derived Streptomyces sp., and another strain of Streptomyces CNQ431. Studies of these amides have shown that splenocin B is a potent inhibitor of the pro-inflammatory cytokine, splenocins A–I display suppression of cytokine production by OVA stimulated splenocytes at low nanomolar concentrations, and splenocin J exhibits low micromolar activity in the splenocyte assay [52,54,57,58,59]. Splenocins A, B, C, I, and J contain in molecules: (2S,3R,4R)-2-benzyl-3,4-dihydroxypentanoic acid (37), splenocin D, (2S,3R,4R)-2-ethyl-3,4-dihydroxypentanoic acid (38), splenocin E, (S)-2-((1R,2R)-1,2-dihydroxy-propyl)hexanoic acid (39), splenocin F, (S)-2-((1R,2R)-1,2-dihydroxy-propyl)-heptanoic acid (40), splenocin G, (S)-2-((1R,2R)-1,2-dihydro-xypropyl)-octanoic acid (41), splenocin H, (2S)-2-((1R,2R)-1,2-dihydroxypropyl)-4-methyloctanoic acid (42, for structure see Figure 7, and for activity see Table 3).
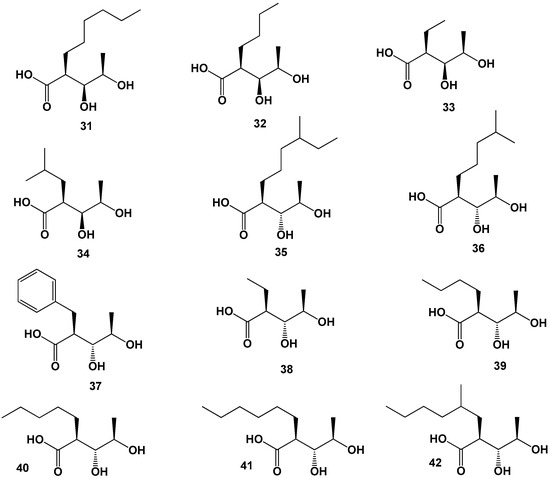
Figure 7.
Rare FA derived from marine and soil fungal endophytes.

Table 3.
Pharmacological profile of FA derived from microorganisms.
Carbapenem compounds, to which the OA-6129 group of antibiotics belong, had a relatively strong antimicrobial activity against gram-positive and gram-negative bacteria [60,61]. Antibiotic OA 6129A, B1, B2, and C contains (R)-2,4-dihydroxy-3,3-dimethylbutanoic acid (42, for structure see Figure 8, and for activity see Table 4).
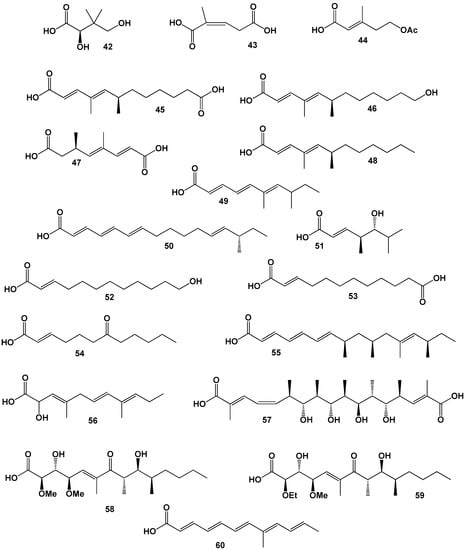
Figure 8.
Unusual FA derived from fungal endophytes, and soil fungi.

Table 4.
Pharmacological profile of FA derived from microorganisms.
An endophytic Streptomyces sp. isolated from the mangrove tree Bruguiera gymnorrhiza is the source of biologically active compounds named divergolides A–D, which were active against B. subtilis and Mycobacterium vaccae [62,63]. Divergolides A and B contains (Z)-2-methylpent-2-enedioic acid (43).
Metabolites produced by endophytic fungus Bipolaris sp. MU34 from Thai medicinal plants, bipolamides A and B, and pathogenic fungus Pestalotiopsis oenotherae isolated from leaves, Rhizophora mucronata, (Hainan Is., China) yielded pestalotiopamide E and D with (E)-5-acetoxy-3-methylpent-2-enoic acid (44) [64]. Pestalotiopen A showed moderate antimicrobial activity against Enterococcus faecalis [65]. The plant pathogen endophytic fungus Pestalotiopsis sp. was obtained from the leaves of the Chinese mangrove Rhizophora mucronata yielded bioactive compounds named pestalotiopamide E and D with FA (44) [64].
The fungus Gymnascella dankaliensis found in soil in the vicinity of the Giza pyramids (Egypt) produced bioactive compounds that exhibit cytotoxicity against the murine lymphoma cell line L5178Y [66]. 11′-Carboxy-gymnastatin N contains (R,2E,4E)-4,6-dimethyldodeca-2,4-dienedioic acid (45), 12′-hydroxy-gymnastatin N, and dankamide—(R,2E,4E)-12-hydroxy-4,6-dimethyldodeca-2,4-dienoic acid (46), gymnastatin S—(R,2E,4E)-4,6-dimethylocta-2,4-dienedioic acid (47), gymnastatin A, B, and N, aranorosinol A, aranorosin, aranorosin-2-methylether, and other metabolites contain (R,2E,4E)-4,6-dimethyldodeca-2,4-dienoic acid (48).
Metabolites produced by endophytic fungus Bipolaris sp. MU34 from Thai medicinal plants bipolamides A and B were discovered. Bipolamide B showed antifungal activity against Aspergillus niger ATCC 6275, Cladosporium cladosporioides FERMS-9, C. cucumerinum NBRC 6370, Rhisopus oryzae ATCC 10404, and Saccharomyces cerevisiae ATCC 9804 [67]. (2E,4E,6E)-6,8-dimethyldeca-2,4,6-trienoic acid (49) was present in both metabolites.
The fungus Penicillium variabile HXQ-H-1 cultivated with the DNA methyltransferase inhibitor 5-azacytidine is a producer of the antibiotic varitatin A. This compound with (S,2E,4E,6E,12E)-14-methylhexadeca-2,4,6,12-tetraenoic acid (50) demonstrated cytotoxicity against HCT-116 cells and inhibition of protein tyrosine kinase [68].
The soil fungus Streptomyces ostreogriseus is a producer of the cyclopeptide antibiotic ostreogrycin A with (4S,5R,E)-5-hydroxy-4,6-dimethylhept-2-enoic acid (51) and is highly active against gram-positive bacteria, especially methicillin-resistant S. aureus [69].
Fungi belonging to the genus Isaria are known to be pathogenic for insects belonging to Homoptera, Lepidoptera, and Coleoptera and are producers of unique compounds called isariotins A–D, which possess a unique bicyclo [3.3.1]nonane ring. These amides were found in lipid extracts of the insect pathogenic fungus Isaria tenuipes BCC 7831 [70]. Three FA, (E)-12-hydroxydodec-2-enoic (52), (E)-dodec-2-enedioic (53), and (E)-7-oxododec-2-enoic (54, 3D graph see Figure 9) were found in isariotins A, B, and C, respectively.
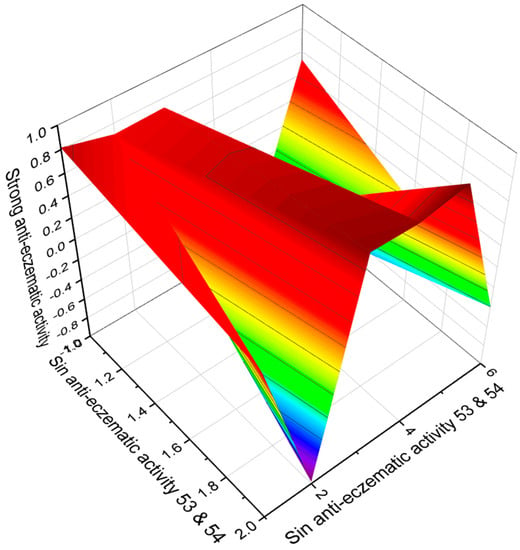
Figure 9.
The 3D graph shows the predicted and calculated anti-eczematic activity of FA (53 and 54). Both acids have been found in the insect pathogenic fungus Isaria tenuipes. It is known that this fungus is one of the potent species of the Isaria genus, which is known to have many biologically active substances of therapeutic value. In addition, the crude methanol extract showed potent antioxidant and antiproliferative activity, which is indicative of natural antioxidant and antiproliferative agents.
Scyphostatin, a neutral sphingomyelinase inhibitor, with (2E,4E,6E,8R,10S,12E,14R)-8,10,12,14-tetramethylhexadeca-2,4,6,12-tetraenoic acid (55) was obtained from a discomycete, Trichopeziza mollissima SANK 13,892 exhibited potent inhibitory activity [71].
Bioactive compound JBIR-66 with (3E,6E,8E)-2-hydroxy-4,8-dimethylundeca-3,6,8-trienoic acid (56) was obtained from the culture broth of the tunicate-derived fungus Saccharopolyspora sp. SS081219JE-28 [72]. Two β-hydroxy acetamides, BE-52211, BE-52211 B, and BE-52211 C, as structural analogues of JBIR-66, were obtained from Streptomyces sp. They inhibited cell division of starfish embryos at a concentration of 2.5 μg/mL and contain acid (56) [73,74].
Antibiotic streptovaricin U with FA (57) produced by Streptontvices spectabilis [75], and chondrochlorens A and B were obtained from Chondromyces crocatus Cmc5 [76] with FA (58) and (59), respectively.
A microbial metabolite Sch 725,424 with (2E,4E,6E,8E,10E)-8-methyldodeca-2,4,6,8,10-pentaenoic acid (60) was detected in the culture of Kitasatospora sp., and it demonstrated inhibitory activity against Staphylococcus aureus and Saccharomyces cerevisiae [77].
An actinomycete Streptomyces cavourensis YY01-17 from the Antarctic area is a producer of pseudopeptide with (E)-3-hydroxy-2,4-dimethylhept-4-enoic acid (61, for structure see Figure 10, and activity in Table 5) [78]. Streptomyces versipellis 4083-SVS6 is a producer of JBIR-07 and JBIR-08 with (6E,8Z)-3-hydroxy-8-(hydroxymethyl)-2,6-dimethyldeca-6,8-dienoic acid (62), and (6Z,8E)-3-hydroxy-6-(hydroxymethyl)-2,8-dimethyldeca-6,8-dienoic acid (63) [79].
Figure 10.
Unusual and rare FA-derived from marine and parasitic fungal endophytes.

Table 5.
Pharmacological profile of FA derived from microorganisms.
An entomopathogenic fungus Metarhizium acridum is a producer of 17-membered macrocycles named metacridamides A and B. Metacridamide A showed cytotoxicity to three cancer lines against Caco-2 (epithelial colorectal adenocarcinoma), MCF-7 (breast cancer), and HepG2/C3A (hepatoma) cell lines, and metacridamide B was active against HepG2/C3A [80]. Both compounds related to FA (64) and (65), respectively.
Unusual (8E,14E)-7,13-dihydroxy-4,10,14-trimethyl-3-oxoheptadeca-8,14,16-trienoic acid (66) was detected in macrolide antibiotic angiolam A, which was produced by Angiococcus disciformis [81].
An endophytic fungus Sporormiella minimoides (Sporormiaceae) isolated from bark Hintonia latiflora is a producer of an antibiotic with (2E,4E,6E)-3-methyloctadeca-2,4,6-trienoic acid (67) that had antifungal properties [82].
Neutrophic agent named as farinosone C with (2E,4E)-4,6-dimethylocta-2,4-dienedioic acid (68) produced by Paecilomyces farinosus RCEF 0101 [83]. The fungus Gymnasella dankaliensis from the sponge Halichondria japonica has supplied the several gymnastatins A-H, most of which are halogenated compounds. All isolated pseudo-dipeptides contain the (R,2E,4E)-5,7-dimethyltrideca-2,4-dienoic acid (69). The same FA was detected in aranochlors A and B [84].
A highly unsaturated macrolide lactam named mirabilin with (6S,7S,E)-7-hydroxy-4,4,6,8-tetramethyl-5-oxonon-2-enoic acid (70) was found in an unidentified fungus extract that was associated with the marine sponge Siliquariaspongia mirabilis. An isolated compound inhibits the growth of the tumor cell line HCT-116 [85].
Streptomyces sp. K04-0144 is a producer of nosokomycins A, B, C, and D, which belong to the moenomycin family, consisting of an oligosaccharide moiety, a 2,3-dihydroxypropionic acid, and an unusual sesterterpenoid moiety. All isolated nosokomycins contain FA (71) [86]. The marine actinomycete B-1758 from collection of the Alfred Wegener Institute for Polar and Marine Research in Bremerhafen (Germany) was isolated diamide, which contains (2Z,9Z)-4,8-dihydroxyundeca-2,9-dienedioic acid (72) [87].
The culture supernatant extract of the strain Myxococcus xanthus Mx X12 contained the polyene antibiotic compounds named myxalamid A, B, C, and D [88]. Isolated antibiotics contained FA (73), (74), (75), and (76, 3D graph, see Figure 11), respectively.
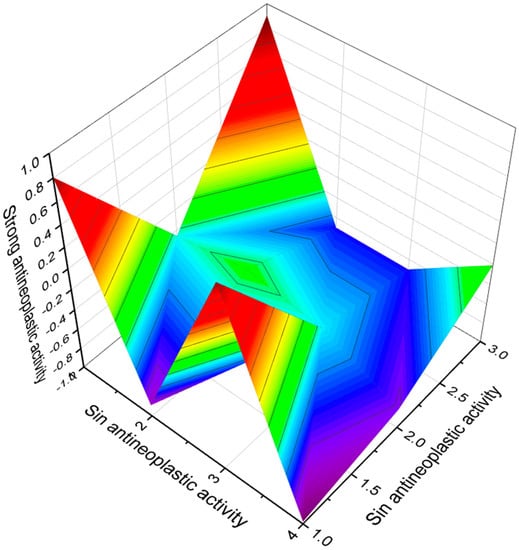
Figure 11.
The 3D graph shows the predicted and calculated antineoplastic activity of FA (73, 74, 75, and 76). These FA contain amides that are synthesized by the gram-negative rod-shaped myxobacteria, Myxococcus xanthus. With an excess of nutrition in cultivated conditions, this bacterium exists in the form of a predatory, saprophytic single-species biofilm called a swarm.
The polyene antifungal antibiotics, 6E,2′-O-methylmyxalamide D, 6E,10Z-2′-O-methyl-myxalamide D, 2′-O-methyl-myxalamide D, and acetate derivative of (77) have been obtained from myxobacterium Cystobacter fuscus AJ-13278, contained FA (78), (79), and (80), respectively [89]. All compounds showed antifungal activities of against the phythopathogenic fungus Phythopthora capsici.
Eliamid with (6E,10E)-2,4,6,8,10-pentamethyl-9-oxododeca-6,10-dienoic acid (81) is a secondary metabolite isolated from Sorangium cellulosum (see Figure 12) [90]. An actinomycete Saccharothrix longispora DSM 43,749 (T) from a Saharan soil in Ghardaïa (Algeria) is a producer of D-(-)-threo chloramphenicol with 2,2-dichloroacetic acid (82, for structure see Figure 13, and activity see Table 6) [91].
Figure 12.
Examples of myxobacteria Sorangium cellulosum (a), lichenized ascomycete Ramalina terebarata (b), marine bacteria Pseudoalteromonas sp. (c), and the subterranean fungus Melanogaster broomeianus (d) whose extracts contain bioactive FA amides.
Figure 13.
Unusual and rare FA derived from marine and soil microorganisms.

Table 6.
Pharmacological profile of FA derived from microorganisms.
Microsphaerone A with FA (83) is γ-pyrone derivative derived from the sponge-derived fungus Microsphaeropsis sp. [92], and a ubiquitin-activating enzyme inhibitor named himeic acid A with FA (84) was detected in a culture of marine-derived fungus Aspergillus sp. [93].
Liquid culture broth of Pseudomonas sp. MF381-IODS yielded two antimicrobial agents named pseudotrienic acids A and B with (3E,5E)-7-hydroxy-4-methylhexadeca-3,5-dienoic (85) and (3E,5E)-7-hydroxy-4-methyltetradeca-3,5-dienoic (86) acids, respectively. Both compounds are growth inhibitors of Staphylococcus aureus and Pseudomonas syringae pv. syringae [94]. Citrate-hydroxamate siderophores named nannochelins A, B, and C with 3-hydroxypentane-1,3,5-tricarboxylic acid (87) has been obtained from the Nannocystis exedens strain Na e485 [95].
An antibiotic named korormicin with (3R,4Z,6E,9S,10R)-10-bromo-9-hydroxy-3-methyldodeca-4,6-dienoic acid (88) was obtained from an extract of the marine actinomycete Pseudoalteromonas sp. F-420 and had a specific inhibitory activity against marine gram-negative bacteria [96].
A plant growth regulator, amidenin with (2E,4Z)-deca-2,4-dienoic acid (89) was obtained from an extract of the fermentation broth of an Amycolatopsis sp. [97]. In addition, actinonin with 2-pentylsuccinic acid (90) has been shown to be an inhibitor of CD13/aminopeptidase and is cytotoxic to some tumor cell lines in vitro [98].
Two aromatic compounds named citrinamides A and B were found and isolated from the culture broth of Penicillium sp. FKI-1938. Both citrinamides A and B containing 2,2-dimethylbut-3-enoic acid (91) showed moderate potentiation of miconazole activity against Candida albicans [99].
Bacillus laterosporus isolate PNG 276 was a producer of compounds named basiliskamide A and B with (2Z,4E,7R,8R,9S,10R)-7,9-dihydroxy-8,10-dimethyldodeca-2,4-dienoic acid (92). Both compounds show potent in vitro anti-Candida activity [100]. The sub-acute toxicity of an antimicrobial metabolite with (Z)-5-oxoundec-7-enoic acid (93) was isolated from a Streptomyces sp. [101].
An endophytic fungus Aspergillus niger EN-13 isolated from the brown seaweed Colpomenia sinuosa was the source of asperamides A and B. Asperamide A displayed moderate activity against Candida albicans [102], and asperamide B contained (R,3E,5E,7E,9E)-2-hydroxytrideca-3,5,7,9-tetraenoic acid (94).
Gliding bacterium Polyangium brachysporum sp. nov. no. K481-B101 is a producer of antitumor antibiotics named glidobactins A, B, and C, and glidobactin B contained (2E,4E)-dodeca-2,4-dienoic acid (95, 3D graph see Figure 14) [103].
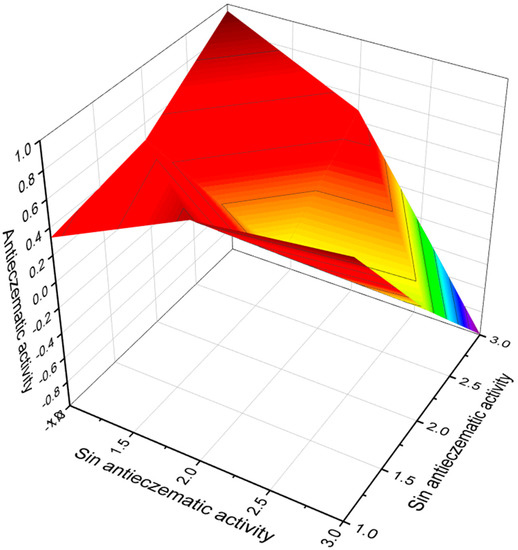
Figure 14.
The 3D graph shows the predicted and calculated anti-eczematic activity of FA (93, 94, and 95). These FA are produced by endophytic microorganisms, Streptomyces sp. (93), Aspergillus niger (94), and a gliding bacterium, Polyangium brachysporum (95), respectively.
Stereocalpin A with (2R,4R,5R)-5-hydroxy-2,4-dimethyl-3-oxooctanoic acid (96) was found in the Antarctic lichens Stereocaulon alpinum (see Figure 15) and Ramalina terebarata [104].

Figure 15.
Examples of Japanese mushroom Boletus laetissimus (a), mushroom Catathelasma ventricosum (b), Antarctic lichen Stereocaulon alpinum (c), and a mesophilic proteobacterium Chondromyces sp. (d) which contain bioactive FA amides.
The FA amides named calcaripeptides A, B, and C were obtained from MeOH/CHCl3 extracts of the fungus Calcarisporium sp. KF525, which found in sediments of the German Wadden Sea [105]. All compounds contained (2S,6R,9R,E)-9-hydroxy-2,4,6-trimethyl-3-oxodec-4-enoic (97), (6R,9R,E)-9-hydroxy-4,6-dimethyl-3-oxodec-4-enoic (98) and (2S,4R,7R)-7-hydroxy-2,4-dimethyl-3-oxooctanoic (99) acids, respectively [105].
Streptovaricins are a group of structurally related macrolide antibiotics. They belong to the larger class of antibiotics known as ansamycins. Streptovaricin U is acyclic antibiotic with FA (100) [106].
Fusaridione A acid form containing (4E,6E,8E,10E,12E,14E)-2,6,12,14-tetramethyl-3-oxohexadeca-4,6,8,10,12,14-hexaenoic acid (101) [107] was produced by the filamentous fungus Fusarium heterosporum ATCC 74349. Two polyene pigments, boletocrocin A and B with common (2E,4E,6E,8E,10E,12E,14E)-hexadeca-2,4,6,8,10,12,14-heptaenedioic acid (102, 3D graph see Figure 16), were isolated from the fruiting bodies of the Japanese mushroom Boletus laetissimus [108]. The same compound named calostomal was detected in the gasteromycete Calostoma cinnabarinum [109].
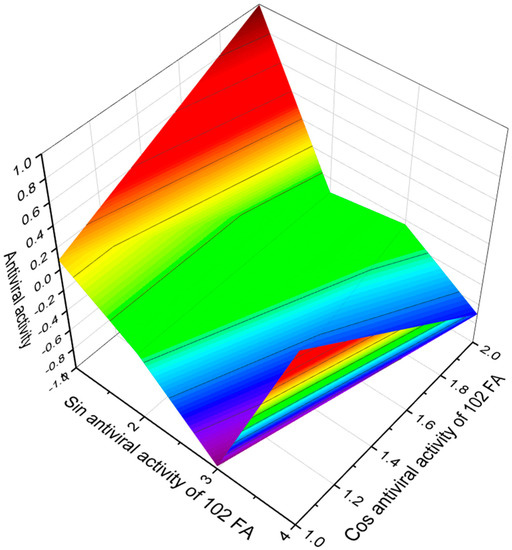
Figure 16.
The 3D graph shows the predicted and calculated antiviral activity of FA (102). Interestingly, this FA is synthesized in various FA amides by the fruiting bodies of the Japanese mushroom Boletus laetissimus or the gasteroid fungus Calostoma cinnabarinum.
A polyene pigment named melanocrocin with FA (103) was obtained from lipid extracts of fruit bodies and mycelial cultures of the subterranean fungus Melanogaster broomeianus. Melanocrocin is the N-acyl derivative of L-phenylalanine methyl ester with a polyolefinic carboxylic acid [110]. Three glycosphingolipids with a cis-17-fatty acyl moiety (104, for structure see Figure 17, and activity see Table 7)), namely, catacerebrosides A-C, were obtained from the mushroom Catathelasma ventricosum [111].
Figure 17.
Unique, rare, and unusual FA derived from microorganisms.

Table 7.
Pharmacological profile of FA derived from microorganisms.
Streptomyces nodosus NPS007994 found in marine sediment near Scripps Canyon (La Jolla, California) is a producer of the lajollamycin family, a nitro-tetraene spiro-β-lactone-γ-lactam antibiotics. Lajollamycin A contains FA (105), and B and C contained (106) and D (107), and it has shown antimicrobial activity against gram-positive bacteria [112].
Unusual peptide-polyketide hybrid compounds containing a unique spiro-linked β-lactone/γ-lactam, a 5-substituted oxazole ring named oxazolomycins, which exhibits a wide range of biological activities, including antitumor and antibacterial activity, and activity against human immunodeficiency virus [113,114,115].
Oxazolomycins B and C demonstrated potent inhibitory activity against crown gall formation [116]. 16-Methyl-oxazolomycin showed antibacterial and antialgal activities against Bacillus subtilis 1069 (MIC, 5.0/μg/mL) and Chlorella vulgaris IFO 15,941 (MIC, 10 μg/mL), respectively, and cytotoxicities (IC50 = 0.23 μg/mL against P388 leukemia cells; 4.6 μg/mL against A-549 human lung adenocarcinoma cells) [117]. Oxazolomycin A and 16-methyloxazolomycin contain FA (108), B (109), C (110), and curromycin A and B contain a fatty acid (111). Antibiotic curromycin A produced by Streptomyces hygroscopicus and Streptomyces sp. [118,119]. Both curromycins have shown an inhibitory effect on human immunodeficiency virus replication [120]. Antibioics triedimycins A and B, which are closely related to curromycin, have been found in the culture of Streptomyces sp. MJ213-62F4 resembling Streptomyces melanosporofaciens. Both triedimycins contain FA (112) and exhibited weak antimicrobial activity against Micrococcus luteus FDA16 (MIC, 25 μg/mL) and Pseudomonas aeruginosa A3 (50 μg/mL) and had potent cytotoxicity to murine leukemia P388 cells (IC50 0.06 and 0.19 μg/mL) [121].
Submerged cultures of the thermophilc fungus Talaromyces thermophilus YM cultivated at 45 °C, yielding macrocyclic amides named thermolides A (113) and D (114, for structure see Figure 18, and activity see Table 8). Thermolide A exhibited strong nematicidal activities against Meloidogyne incognita, Bursaphelenchus xylophilus, and Panagrellus redivivus [122,123].
Figure 18.
Unique, rare, and unusual FA derived from fungi and bacteria.

Table 8.
Pharmacological profile of FA derived from microorganisms.
Le Goff and co-workers [124,125] reported the structural characterization of two alkylhydrazides produced by the bacterial strain Streptomyces sp. LMA-545. Geralcins A, B, and D contain 3-(2-oxo-2,5-dihydrofuran-3-yl)-propanoic acid (115), and geralcin C contains (Z)-2-(1-carboxy-2-hydroxyethyl)-1-hexyldiazene oxide (116) [126].
(2E,4E)-5-Cyclohexylpenta-2,4-dienoic acid (117) was found in alisamycin and nisamycin, which was detected in lipid extracts of the culture broth of Streptomyces sp. K106 [127]. Nisamycin showed cytotoxic activity, as well as antibacterial and antifungal activities against gram-positive bacteria and fungi [128,129]. Antibiotic asukamycin was obtained from of Streptomyces nodosus subsp. asukaensis. This antibiotic showed activity against the growth of gram-positive bacteria including Nocardia asteroides [130]. (2E,4E,6E)-7-cyclohexylhepta-2,4,6-trienoic acid (118) was found in asukamycin and asukamycin A-II.
Neoantimycin and analogues with a rare and unusual ring-extended member was produced by a Streptomyces species (Streptoverticillium orinoci, Streptomyces sp. MST-AS4461) [131,132,133], and all compounds contained (3S,4S)-3,4-dihydroxy-2,2-dimethyl-5-phenyl-pentanoic acid (119). Streptomyces violaceoniger 4521-SVS3 is a producer of prunustatin A with (R)-4-hydroxy-2,2-dimethyl-3-oxo-5-phenylpentanoic acid (120), and this compound exhibits inhibitory activity against GRP78 expression [134]. Unantimycin A, a neoantimycin analog, contains FA (120) [135].
The marine sponge-derived Streptomyces sp. strain RM72 is a producer of trichostatin analogues such as JBIR-109, JBIR-110, and JBIR-111 [136]. All components contain an unusual acid (R,2E,4E)-7-(4-(dimethylamino)-phenyl)-4,6-dimethyl-7-oxohepta-2,4-dienoic acid (121).
Efrotomycin including 6-deoxy-4-O-(6-deoxy-2,4-di-O-methyl-α-L-mannopyranosyl)-3-O-methyl-β-D-allopyranose have been detected in the culture of Nocardia lactamdurans [137,138]. This antibiotic contains FA (122), and aurodox (syn. antibiotic X 5108; goldinodox; goldinomycin; NSC 233989) isolated from Streptomyces sp. K06-0806 and contains FA (123). The antibiotic aurodox was first described by Berger and co-authors [139,140], and it was produced by Streptomyces goldiniensis ATCC 21386. The antibiotic is mainly active against gram-positive bacteria and is an effective poultry growth promotant.
An anamorph, mesophilic fungus Penicillium citrinum is a producer of cysteine protease inhibitors, which have been named cathestatin A–C [74]. Cathestatins A–C and estatins A and B have also been found in Aspergilus terricola and fungus Microascus longirostris, isolated from sponge [141]. All metabolites contain (2S,3S)-oxirane-2,3-dicarboxylic acid (124, 3D graph, see Figure 19).
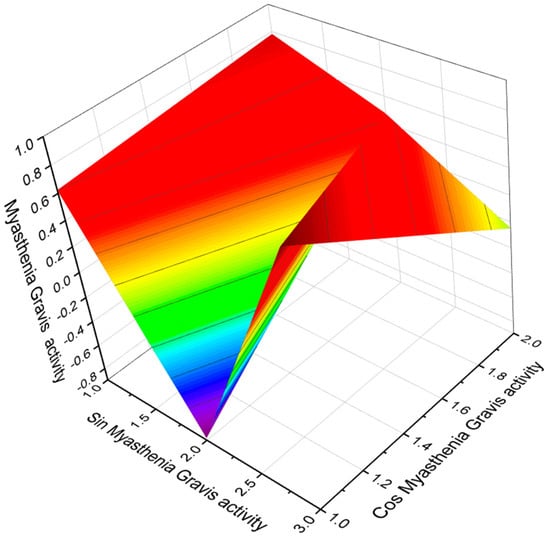
Figure 19.
The 3D graph shows the predicted and calculated Myasthenia Gravis activity of FA (124). An unusual dicarboxylic acid was found in the FA amides of extracts of two fungi, Aspergilus terricola and Microascus longirostris.
Cerulenin, with (2R,3S)-3-((4E,7E)-nona-4,7-dienoyl)-oxirane-2-carboxylic acid (125) was isolated from lipid extracts of an endophytic fungus Phomopsis sp. This compound is an inhibitor of FA and polyketide synthase [142,143].
Unusual bicyclic enol-carbamates named brabantamides A–C, although formally known as SB-253514, SB-253517, and SB-253518 were first isolated from the culture extracts of Pseudomonas fluorescens [144,145]. Brabantamides A and B contains FA (126) and (127), respectively.
The filamentous fungus Alternaria alternata, which produce various toxins and cause disease in various plants such as Japanese pear, strawberry, and mandarin produced AK-toxin, AF-toxin, and ACT-toxin, respectively, and contained (R,2E,4Z,6E)-8-hydroxy-8-((R)-2-methyloxiran-2-yl)-octa-2,4,6-trienoic acid (128) and (R,2E,4E,6E)-8-hydroxy-8-((R)-2-methyloxiran-2-yl)-octa-2,4,6-trienoic acid (129) [146,147,148]. Methyl phenatate A with FA (130) was detected in the organic extract of a fermentation culture of Streptomyces sp. H7372 [149].
Crocacins A–D were produced by Chondromyces pediculatus strain Cm p17. Crocacin A showed activity, an MIC of 0.625 μg/mL, against both Ustilago maydis and Saccharomyces cerevisiae, while crocacin D was more potent against Saccharomyces cerevisiae, showing an MIC of 1.4 ng/mL [150]. All compounds having (2E,4E,6S,7S,8R,9S,10E)-7,9-dimethoxy-3,6,8-trimethyl-11-phenylundeca-2,4,10-trienoic acid (131). A marine actinomycete (strain MST-MA190), which was detected and isolated from a sample of beach sand collected near Lorne on the southwest coast of Victoria (Australia) contained aromatic amides, lorneamide A and lorneamide B [151]. Both compounds contained (Z)-4-(2-((E)-3-hydroxyhex-1-en-1-yl)-4-methylphenyl)-but-3-enoic acid (132) and (Z)-4-(4-methyl-2-(3-oxohexyl)phenyl)-but-3-enoic acid (133), respectively. The antibiotic meroparamycin with 4-(3-oxo-6-propylnonyl)-benzoic acid (134) was produced in the free culture system of Streptomyces sp. strain MAR01 [152]. Streptomyces sp. MJ995-OF5 is a producer of epostatin which has property as an inhibitor dipeptidyl peptidase II (DPP-II, EC 3.4.14.2) with FA (135) [153].
The cultivated strain of Streptomyces LZ35 produces cuevaenes A and C, cuevaenes D and E, and cuevaene B. Cuevaenes A–C displayed moderate activity against Bacillus subtilis and against fungi Fusarium verticillioides, and Rhizoctonia solani [154]. Cuevaenes A, C and cuevaene B contained unusual FA (136–138), respectively.
Ansamycins designated thiazinotrienomycins A–D were obtained from culture broth of Streptomyces sp. MJ672-m3 [155], and contained FA (139), and FA (140). Cytotrienins A–D with FA (139 and 140) are also found in Streptomyces sp. RK95-74.12 [156].
A cytotoxic (human A375-S2 and HELA cell lines) isocoumarin with acid (141), named Sg17-1-4 were obtained from a marine fungus Alternaria tenuis Sg17-1 isolated from an alga (Zhoushan Island, China). The cytotoxicities of these compounds were evaluated in vitro [157].
TPU-0031-A and B antibiotics have been detected in the culture broth of Streptomyces sp. TP-A0556 [158]. TPU-0031-A, B and novobiocin contained 4-hydroxy-3-(3-methylbut-2-en-1-yl)-benzoic acid (142, for structure see Figure 20, and activity see Table 9).
Figure 20.
Unique and unusual FA derived from fungal endophytes.

Table 9.
Pharmacological profile of FA derived from microorganisms.
Nucleoside antibiotics, named streptcytosines A–E, have been detected in a culture broth of Streptomyces sp. TPU1236A (Okinawa, Japan) [159]. Streptcytosine B and D have contained (E)-3-(methylthio)-acrylic (143) and 3-methylbut-2-enoic (81) acids, respectively.
An endophytic Streptomyces sp. YIM65484 isolated from the vine used in traditional Chinese medicine (Tripterygium wilfordii) is a producer of the antimicrobial compound with (2E,4E)-5-(3-hydroxyphenyl)-penta-2,4-dienoic acid (144) [160].
The cultured broth of the marine actinomycete Salinispora arenicola contained a rifamycin antibiotic called salinisporamycin [161], and saliniketals A and B, bicyclic polyketides, were from the same S. arenicola [162]. Salinisporamycin and saliniketal A contained FA (145), and saliniketal B—FA (146, 3D graph, see Figure 21). Korormicin with (4Z,6E)-3-hydroxy-8-(3-nonyloxiran-2-yl)-octa-4,6-dienoic acid (147) had specific inhibitory activity against marine gram-negative bacteria [95].
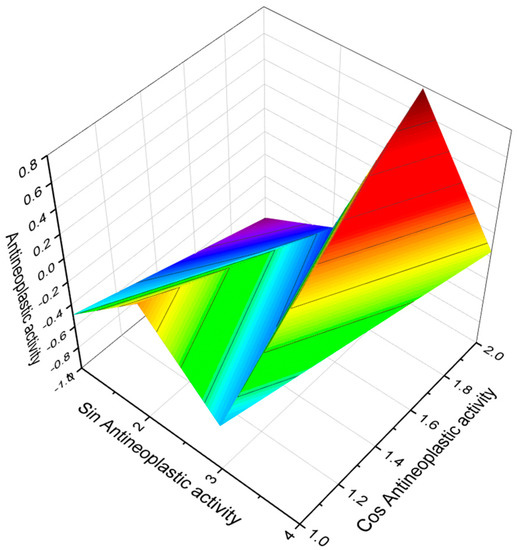
Figure 21.
The 3D graph shows the predicted and calculated antineoplastic activity of FA (145 and 146). Both FA in the amides is produced by the marine actinomycete Salinispora arenicola, and these acids demonstrate anticancer activity with more than 90% confidence.
Myxalamide PI, related to phenalamides, containing FA (148, for structure see Figure 22, and activity see Table 10) was isolated from actinomycete Cystobacter velutus [163]. Actinomycete Myxococcus stipitatus (AJ-12587) from a soil sample from Izu Peninsula, Japan produced antibiotic stipiamide (phenalamide A1) with FA (149) [164]. Phenalamide A1 was found to suppress HIV-1 replication in cell cultures and has been detected in Polyangium sp. and Myxococcus stipitatus. Phenalamide A1 could prevent the HIV-1 infection of MT-4 cells even at concentrations of 1.02 nM, and thiangazole at 4.7 pM [164].
Figure 22.
Unusual FA derived from marine actinomycetes.

Table 10.
Pharmacological profile of FA derived from microorganisms.
In 1992, Trowitzsch-Kienast and co-workers [165] reported the isolation and characterisation of five new compounds, phenalamides A1, B, A2, A3, and C from Myxococcus stipitatus Mx s40. Phenalamide A1 proved to be the same compound as the previously isolated stipiamide. Phenalamide B is a methylated variant of stipiamide, and phenalamide A3 has one less double bond. Phenalamide A2 possesses a cis-alkene, and phenalamide C is an epoxidized derivative. Phenalamides A1, B, A2, and A3 contained (149), (150), (151), (152), and (153, 3D graph, see Figure 23), respectively.
Figure 23.
The 3D graph shows the predicted and calculated antineoplastic activity of FA (150 and 153). Both FA have been found in amide that is synthesized by a mesophilic Proteobacterium Myxococcus stipitatus.
A marine-derived actinomycete Nocardiopsis sp. CMB-M0232 obtained from a sediment sample near Brisbane, Australia is a producer of nocardiopsins A–D [166,167]. Nocardiopsins A and C contained FA (154), and nocardiopsins B and D contained FA (155). Two polyketide metabolites, thailandamide A and thailandamide lactone with (E)-7-hydroxy-8-(4-hydroxyphenyl)-2-methyloct-4-enoic acid (156) have been isolated from gram-negative bacillus Burkholderia thailandensis [168,169].
Antitumor antibiotics named oximidines I and II were obtained from Pseudomonas sp. Q52002. Oximidines I, II and III with (2E)-4-(methoxyimino)-but-2-enoic acid (157) selectively inhibited the growth of rat 3Y1 cells [170]. Oximidine III, an antitumor antibiotic was isolated from Pseudomonas sp. QN05727 [171].
Streptomyces tsukubaenis fermentation broth no. 9993 contained an immunosuppressant, FK-506 [172], and the marine Streptomyces sp. CNH189 and Streptomyces sp. KCTC 11604BP produced of unnatural 36-methyl-FK506 [173]. Both compounds contained fatty acid (158, for structure see Figure 24, activity see Table 10, and 3D graph see Figure 25) and (159), respectively.
Figure 24.
Unusual FA derived from marine fungal endophytes.
Figure 25.
The 3D graph shows the predicted and calculated immunosuppressant activity of FA (158 and 159). Both fatty acids were found in a pseudopeptide synthesized by marine fungal endophytes with rare biological properties.
Actinomycete Amycolatopsis orientalis (see Figure 26), deposited as a vancomycin producer, produced, a glycosidic polyketide ECO-0501, which contained fatty acid (160) [174]. Streptomyces aizunensis NRRL B-11277 is producer of a unique compound, ECO-02301, with antifungal activity contained amino acid, (161, for structure see Figure 27, and for 3D graph see Figure 28) [175]. The fermentation broth of the actinomycete strain Streptomyces hygroscopicus TP-A0623 contained clethramycin with FA (162) and demonstrated in vitro antifungal activity against Candida albicans and C. glabrata [176,177,178,179].

Figure 26.
Examples of fungus Alternaria alternata (a), fungus Microsphaeropsis sp. (b), actinomycete Amycolatopsis orientalis (previously known as Streptomyces orientalis) (c), and bacteria Nocardiopsis sp. (d), which are sources of bioactive amides. Pictures adapted by author.
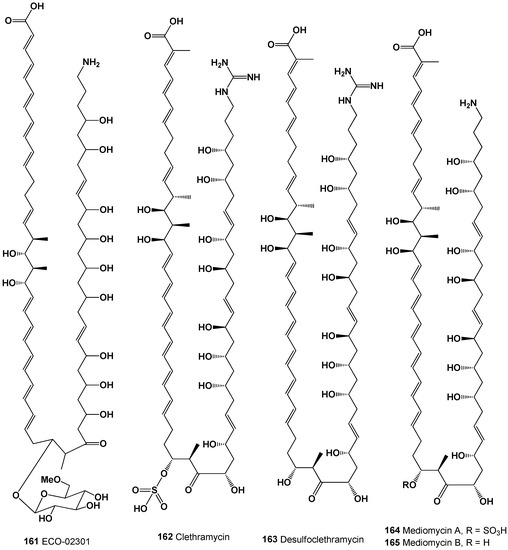
Figure 27.
Unique and unusual FA derived from actinomycetes.
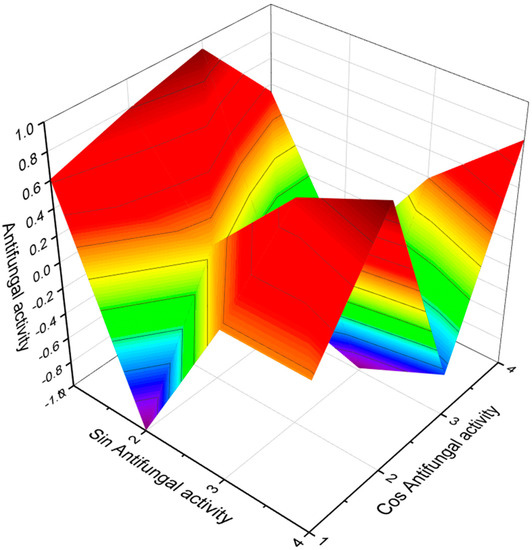
Figure 28.
The 3D graph shows the predicted and calculated antifungal activity of FA (161 and 162). Both FA were found in the pseudopeptide that synthesizes actinomycete Streptomyces, and both acids show antifungal activity with more than 90% confidence.
Streptomyces sp. are producers of zwitterionic polyketides with FA (163) and their biosynthesis is well described [180]. Three polyene antibiotics, mediomycins A (164), B (165), and clethramycin (163), were detected in extracts of Streptomyces mediocidicus ATCC23936 [181,182].
3. Structure–Activity Relationships and Biological Activities of Natural FA Amides
Numerous works in the field of pharmacology have shown that the chemical structure of natural chemical molecules predetermines their biological activity, and their mutual relationships can be described as the structure-activity relationships (SAR). Historical studies have shown that the idea of dependence of activity on the structure of a chemical molecule was first proposed by Brown and Fraser more than 150 years ago, in 1868 [183]. However, according to other data, this was done by Kross in 1863, who established a relationship between the toxicity of primary aliphatic alcohols and their solubility in water [184]. It was established by historians that more than 30 years later Richet in 1893 [185], Meyer in 1899 [186], and Overton in 1901 [187] independently discovered a linear correlation between lipophilicity and biological effects. Additionally, in 1935, Hammett [188,189] described a method for considering the influence of substituents on reaction mechanisms using an equation in which two parameters were considered, namely, the substituent constant and the reaction constant. Moreover, in 1956, Taft made an addition to the Hammett model and proposed an approach to separating the polar, steric, and resonant effects of substituents in aliphatic compounds [190]. Hansch and Fujita, developing these ideas, combined all previous developments and laid the mechanistic basis for the development of the QSAR method [191], and the Hansch linear equation and Hammett electronic constants are described in detail in the book by Hansch and Leo, published in 1995 [192].
At present, well-known computer programs make it possible to evaluate the pharmacological activity of chemical molecules with respect to various biological models with a certain degree of certainty [193,194,195,196]. It is known that classical SAR methods are based on the analysis of (quantitative) structure–activity relationships for one or more types of biological activity using organic compounds belonging to the same chemical series as the training set [196].
The software called PASS used to calculate biological activity has been constantly updated and improved over the past thirty years [197] and is based on the analysis of a heterogeneous training set that includes information on more than 1.3 million known biologically active compounds with data on approx. 10,000 types of biological activity [197,198,199]. Chemical descriptors implemented in PASS, reflecting the features of the ligand-target interaction and the original implementation of the Bayesian approach to elucidation of structure–activity relationships, provide an average accuracy and predictability for several thousand biological activities, equal to approximately 96% [198].
Several comparative studies have shown that PASS is more predictive than some other recently developed methods for assessing biological activity profiles [197,198], although this program is not sufficiently effective for complex molecules or optical isomers. To calculate the pharmacological potential profile of natural substances, we have successfully used PASS for the past fifteen years [200,201,202,203,204,205].
4. Conclusions
The biological activity of natural FA amides has attracted the attention of pharmacologists for a long time. It is known that this class of compounds is found in many living organisms, including plants, algae, and marine and freshwater invertebrates, but microorganisms attract the greatest interest. This is because various bacteria or fungal endophytes can be isolated from natural sources and cultivated to produce bioactive drugs that are increasingly being used in medicine to fight various diseases. The review offered to the reader covers a small number of natural amides as an example for their further study. This review presents natural FA amides found in extracts from the marine-derived fungi, as well as bacteria isolated from various natural sources. Some fatty acids demonstrated strong antifungal, antibacterial, antiviral, antineoplastic, and anti-inflammatory activities, and other fatty acids have shown rare activities such as antidiabetic, anti-infective, anti-eczematic, antimutagenic, and anti-psoriatic activities. As a rule, the indicated biological activities of fatty acids have a certainty of more than 90%. These data are undoubtedly of interest to chemists and pharmacologists, both from a theoretical and practical point of view.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Not applicable.
Acknowledgments
The author is grateful to Tatyana A. Gloriozova (Institute of Biomedical Chemistry, Moscow, No. 122030100170-5) for prompt help in determining the biological activity of natural FA derived from natural amides presented in this article.
Conflicts of Interest
The author declares that he has no known competing financial interests or personal relationships that could affect the work described in this article.
References
- Pitzer, J.; Steiner, K. Amides in nature and biocatalysis. Tetrahedron 1998, 54, 7229–7271. [Google Scholar] [CrossRef] [PubMed]
- Bezuglov, V.V.; Bobrov, M.Y.; Archakov, A.V. Bioactive amides of fatty acids. Biochemistry 1998, 63, 27–37. [Google Scholar]
- Dembitsky, V.M.; Shkrob, I.; Rozentsvet, O.A. Fatty acid amides from freshwater green alga Rhizoclonium hieroglyphicum. Phytochemistry 2000, 54, 965–967. [Google Scholar] [CrossRef]
- Bradshaw, H.B.; Leishman, E. Lipidomics: A corrective lens of enzyme Mopia. Methods Enzymol. 2017, 593, 123–141. [Google Scholar] [PubMed]
- Bode, J.W. Emerging methods in amide- and peptide-bond formation. Curr. Opin. Drug Discov. Develop. 2006, 9, 765–775. [Google Scholar] [CrossRef]
- Divito, E.B.; Cascio, M. Metabolism, physiology, and analyses of primary fatty acid amides. Chem. Rev. 2013, 113, 7343–7353. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive cyclobutane-containing alkaloids. J. Nat. Med. 2008, 62, 1–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.; Ming, Q.; Wu, L. Alkaloids produced by endophytic fungi: A review. Nat. Prod. Commun. 2012, 7, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Naturally occurring bioactive cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomedicine 2014, 21, 1559–1581. [Google Scholar] [CrossRef]
- Ismail, F.M.D.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef]
- Torres, A.; Hochberg, M.; Pergament, I.; Smoum, R.; Niddam, V.; Dembitsky, V.M. A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. Eur. J. Biochem. 2004, 271, 780–784. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T. Metabolites produced by nitrogen fixing Nostoc species. Folia Microbiol. 2005, 50, 363–391. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, A.; Dembitsky, V. Acetylenic anticancer agents. Anti-Cancer Agents Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef]
- Archana, O.; Nagadesi, P.K. Endophytic, non-endophytic fungal alkaloids and its applications. Saudi J. Pathol. Microbiol 2022, 7, 4–19. [Google Scholar]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef]
- Mohan, S.; Krishna, A.; Chandramouli, M.S. Antibacterial natural products from microbial and fungal sources: A decade of advances. Mol. Divers. 2022, 26, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.J.; Li, J.J.; Liang, Z.Z.; Zhao, C.Q. Novel natural products from extremophilic fungi. Mar. Drugs 2018, 16, 194. [Google Scholar] [CrossRef]
- Kuo, M.S.; Zielinski, R.J.; Cialdella, J.I.; Marschke, C.K.; Dupuis, M.J. Discovery, isolation, structure elucidation, and biosynthesis of U-106305, a cholesteryl ester transfer protein inhibitor from UC 11136. J. Am. Chem. Soc. 1995, 117, 10629–10634. [Google Scholar] [CrossRef]
- Yoshida, M.; Ezaki, M.; Hashimoto, M.; Yamashita, M.; Shigematsu, N. A novel antifungal antibiotic, FR-900848. I. Production, isolation, physico-chemical and biological properties. J. Antibiot. 1990, 43, 748–754. [Google Scholar] [CrossRef]
- Sasaki, M.; Tsuda, M.; Sekiguchi, M.; Mikami, Y.; Kobayashi, J. Perinadine A, a novel tetracyclic alkaloid from marine-derived fungus Penicillium citrinum. Org. Lett. 2005, 7, 4261–4264. [Google Scholar] [CrossRef]
- Tsuda, M.; Sasaki, M.; Mugishima, T.; Komatsu, K.; Sone, T.; Tanaka, M. Scalusamides A-C, new pyrrolidine alkaloids from the marine-derived fungus Penicillium citrinum. J. Nat. Prod. 2005, 68, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, K.; Zhong, P.; Hu, X.; Fang, Z.X.; Wu, J.L.; Zhang, Q.Q. Tumonoic acids K and L, novel metabolites from the marine-derived fungus Penicillium citrinum. Heterocycles 2012, 85, 413–419. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kamiya, T.; Henmi, T.; Iwasaki, H.; Yamatodani, S. Viridenomycin, a new antibiotic. J. Antibiot. 1975, 28, 167–175. [Google Scholar] [CrossRef] [PubMed]
- McErlean, M.; Liu, X.; Cui, Z.; Gust, B. Identification and characterization of enzymes involved in the biosynthesis of pyrimidine nucleoside antibiotics. Nat. Prod. Rep. 2021, 38, 1362–1407. [Google Scholar] [CrossRef]
- Barrett, A.G.M.; Kasdorf, K. Total synthesis of the pentacyclopropane antifungal agent FR-900848. J. Am. Chem. Soc. 1996, 118, 11030–11037. [Google Scholar] [CrossRef]
- Barrett, A.G.M.; Doubleday, W.W.; Hamprecht, D. Recent advances in the synthesis of antifungal agents. Pure Appl. Chem. 1997, 69, 383–388. [Google Scholar] [CrossRef]
- Jin, W.B.; Wu, S.; Xu, Y.F.; Yuan, H.; Tang, G.L. Recent advances in HemN-like radical S-adenosyl-l-methionine enzyme-catalyzed reactions. Nat. Prod. Rep. 2020, 37, 17–28. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, W.Q.; Zhu, X.; Zhang, Q. Functional diversity of HemN-like proteins. ACS Bio. Med. Chem. 2022, 2, 109–119. [Google Scholar] [CrossRef]
- Østby, R.B. Syntheses of 3-, 4-and 5-Membered Carbocycles: New Methodology on Old Methods. Ph.D. Thesis, Norwegian University of Life Sciences, Akershus, Norway, 2015. [Google Scholar]
- Ding, G.; Liu, S.C.; Guo, L.D.; Zhou, Y.G.; Che, Y.S. Antifungal metabolites from the plant endophytic fungus Pestalotiopsis foedan. J. Nat. Prod. 2008, 71, 615–618. [Google Scholar] [CrossRef]
- Asolkar, R.N.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Daryamides A−C, weakly cytotoxic polyketides from a marine-derived actinomycete of the genus Streptomyces strain CNQ-085. J. Nat. Prod. 2006, 69, 1756–1759. [Google Scholar] [CrossRef]
- Li, F.; Maskey, R.P.; Qin, S.; Sattler, I.; Fiebig, H.H.; Maier, A.; Zeeck, A.; Laatsch, H. Chinikomycins A and B: Isolation, structure elucidation, and biological activity of novel antibiotics from a marine Streptomyces sp. isolate M045. J. Nat. Prod. 2005, 68, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Yu, L.; Ikeda, M.; Oikawa, T. Jomthonic acid A, a modified amino acid from a soil-derived Streptomyces. J. Nat. Prod. 2012, 75, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Bunyapaiboonsri, T.; Yoiprommarat, S.; Intereya, K.; Rachtawee, P.; Hywel-Jones, N.L.; Isaka, M. Isariotins E and F, spirocyclic and bicyclic hemiacetals from the entomopathogenic fungus Isaria tenuipes BCC 12625. J. Nat. Prod. 2009, 72, 756–759. [Google Scholar] [CrossRef]
- Brodasky, T.F.; Stroman, D.W.; Dietz, A.; Mizsak, S. U-56,407, a new antibiotic related to asukamycin: Isolation and characterization. J. Antibiot. 1983, 36, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Floss, H.G. New type II manumycins produced by Streptomyces nodosus ssp. asukaensis and their biosynthesis. J. Antibiot. 2001, 54, 340–348. [Google Scholar]
- Kohno, J.; Nishio, M.; Kawano, K.; Nakanishi, N.; Suzuki, S.; Uchida, T.; Komatsubara, S. TMC-1 A, B, C and D, new antibiotics of the manumycin group produced by Streptomyces sp. Taxonomy, production, isolation, physico-chemical properties, structure elucidation and biological properties. J. Antibiot. 1996, 49, 1212–1220. [Google Scholar] [CrossRef]
- Tanaka, T.; Tsukuda, E.; Uosaki, Y.; Matsuda, Y. EI-1511-3, -5 and EI-1625-2, novel interleukin-1 beta converting enzyme inhibitors produced by Streptomyces sp. E-1511 and E-1625. III. Biochemical properties of EI-1511-3, -5 and EI-1625-2. J. Antibiot. 1996, 49, 1085–1090. [Google Scholar] [CrossRef][Green Version]
- Kim, S.H.; Shin, Y.; Lee, S.H.; Oh, K.B.; Lee, S.K.; Shin, J.; Oh, D.C. Salternamides A–D from a halophilic Streptomyces sp. Actinobacterium. J. Nat. Prod. 2015, 78, 836–843. [Google Scholar] [CrossRef]
- Sattler, I.; Thiericke, R.; Zeeck, A. The manumycin-group metabolites. Nat. Prod. Rep. 1998, 15, 221–240. [Google Scholar] [CrossRef]
- Grote, R.; Zeeck, A.; Beale, J.M., Jr. Metabolic products of microorganisms. 245. Colabomycins, new antibiotics of the manumycin group from Streptomyces griseoflavus. II. Structure of colabomycin A. J. Antibiot. 1988, 41, 1186–1195. [Google Scholar] [CrossRef]
- Grote, R.; Zeeck, A.; Drautz, H.; Zähner, H. Metabolic products of microorganisms. 244. Colabomycins, new antibiotics of the manumycin group from Streptomyces griseoflavus. I. Isolation, characterization and biological properties. J. Antibiot. 1988, 41, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Slechta, L.; Cialdella, J.I.; Mizsak, S.A.; Hoeksema, H. Isolation and characterization of a new antibiotic U-62162. J. Antibiot. 1982, 35, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Petříčková, K.; Pospíšil, S.; Kuzma, M.; Tylová, T.; Jágr, M.; Tomek, P. Biosynthesis of colabomycin E, a new manumycin-family metabolite, involves an unusual chain-length factor. ChemBioChem 2014, 15, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, H.; Grothe, G. Über Actinomycetenfarbstoffe, II. Mitteil: Limocrocin, ein gelber Actinomycetenfarbstoff. Chem. Berich. 1953, 86, 1110–1115. [Google Scholar] [CrossRef]
- Kido, G.S.; Spyhalski, E. Antimycin A, an antibiotic with insecticidal and miticidal properties. Science 1950, 112, 172–173. [Google Scholar] [CrossRef]
- Nakayama, K.; Okamoto, F.; Harada, Y. Antimycin A: Isolation from a new Streptomyces and activity against rice plant blast fungi. J. Antibiot. 1956, 9, 63–66. [Google Scholar]
- Lennon, R.E. Antimycin A, a piscicidal antibiotic. Adv. Appl. Microbiol. 1973, 16, 55–96. [Google Scholar]
- Slater, E.C. The mechanism of action of the respiratory inhibitor, antimycin. Biochim. Biophys. Acta. 1973, 301, 129–154. [Google Scholar] [CrossRef]
- Cramer, W.A.; Hasan, S.S.; Yamashita, E. The Q cycle of cytochrome bc complexes: A structure perspective. Biochim. Biophys. Acta 2011, 1807, 788–802. [Google Scholar] [CrossRef]
- Seipke, R.F.; Hutchings, M.I. The regulation and biosynthesis of antimycins. Beilstein J. Org. Chem. 2013, 9, 2556–2563. [Google Scholar] [CrossRef]
- Shiomi, K.; Hatae, K.; Hatano, H.; Matsumoto, A.; Takahashi, Y.; Jiang, C.; Tomoda, H.; Kobayashi, S.; Tanaka, H.; Omura, S. A new antibiotic, antimycin a(9), produced by Streptomyces sp. k01–0031. J. Antibiot. 2005, 58, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Viegelmann, C.; Margassery, L.M.; Kennedy, J.; Zhang, T. Metabolomic profiling and genomic study of a marine sponge-associated Streptomyces sp. Mar. Drugs 2014, 12, 3323–3351. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, N.; Kumagai, K.; Nakagawa, H.; Shimatani, T.; Saji, I. Antimycins A10 approximately A16, seven new antimycin antibiotics produced by Streptomyces spp. SPA-10191 and SPA-8893. J. Antibiot. 2005, 58, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nozaki, H. Kitamycins, new antimycin antibiotics produced by Streptomyces sp. J. Antibiot. 1999, 52, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Imamura, N.; Nishijima, M.; Adachi, K.; Sano, H. Novel antimycin antibiotics, urauchimycins A and B, produced by marine actinomycete. J. Antibiot. 1993, 46, 241–246. [Google Scholar] [CrossRef]
- Barrow, C.J.; Oleynek, J.J.; Marinelli, V.; Sun, H.H.; Kaplita, P.; Sedlock, D.M. Antimycins, inhibitors of ATP-citrate lyase, from a Streptomyces sp. J. Antibiot. 1997, 50, 729–733. [Google Scholar] [CrossRef]
- Fondja Yao, C.B.; Schiebel, M.; Helmke, E.; Anke, H.; Laatsch, H. Prefluostatin and new urauchimycin derivatives produced by Streptomycete isolates. Z. Naturforsch. 2006, 61B, 320–325. [Google Scholar]
- Ishiyama, T.; Endo, T.; Otake, N.; Yonehara, H. Deisovalerylblastmycin produced by Streptomyces sp. J. Antibiot. 1976, 29, 804–808. [Google Scholar] [CrossRef]
- Sakamoto, M.; Kojima, I.; Okabe, M.; Fukagawa, Y.; Ishikura, T. Studies on the OA-6129 group of antibiotics, new carbapenem compounds. II. In vitro evaluation. J. Antibiot. 1982, 35, 1264–1270. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kojima, I.; Isshiki, K.; Watanabe, A.; Shimauchi, Y.; Okabe, M. Structures of OA-6129A, B1, B2 and C, new carbapenem antibiotics produced by Streptomyces sp. OA-6129. J. Antibiot. 1983, 36, 1473–1482. [Google Scholar] [CrossRef]
- Ding, L.; Maier, A.; Fiebig, H.H.; Görls, H.; Lin, W.H.; Peschel, G.; Hertweck, C. Divergolides A-D from a mangrove endophyte reveal an unparalleled plasticity in ansa-macrolide biosynthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 1630–1634. [Google Scholar] [CrossRef]
- Ding, L.; Franke, J.; Hertweck, C. Divergolide congeners illuminate alternative reaction channels for ansamycin diversification. Org. Biomol. Chem. 2015, 13, 1618–1623. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Wang, B.; Wray, V.; Lin, W.H.; Proksch, P. Pestalotiopamide E, a new amide from the endophytic fungus Pestalotiopsis sp. J. Asian Nat. Prod. Res. 2011, 13, 373–376. [Google Scholar] [CrossRef]
- Hemberger, Y.; Xu, J.; Wray, V.; Proksch, P.; Wu, J.; Bringmann, G. Pestalotiopens A and B: Stereochemically challenging flexible sesquiterpene-cyclopaldic acid hybrids from Pestalotiopsis sp. Chemistry 2013, 19, 15556–155564. [Google Scholar] [CrossRef]
- Hammerschmidt, L.; Aly, A.H.; Abdel-Aziz, M.; Müller, W.E.; Lin, W.; Daletos, G.; Proksch, P. Cytotoxic acyl amides from the soil fungus Gymnascella dankaliensis. Bioorg. Med. Chem. 2015, 23, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Siriwach, R.; Kinoshita, H.; Kitani, S.; Igarashi, Y. Bipolamides A and B, triene amides isolated from the endophytic fungus Bipolaris sp. MU34. J. Antibiot. 2014, 67, 167–170. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Z.; Chen, Y.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Varitatin A, a highly modified fatty acid amide from Penicillium variabile cultured with a DNA methyltransferase inhibitor. J. Nat. Prod. 2015, 78, 2841–2845. [Google Scholar] [CrossRef] [PubMed]
- Delpierre, G.R.; Eastwood, F.W.; Gream, G.E.; Kingston, D.G.; Sarin, P.S.; Todd, L.; Williams, D.H. Antibiotics of the ostreogrycin complex. II. Structure of ostreogrycin A. J. Chem. Soc. Perkin I 1966, 19, 1653–1669. [Google Scholar] [CrossRef]
- Haritakun, R.; Srikitikulchai, P.; Khoyaiklang, P.; Isaka, M. Isariotins A-D, alkaloids from the insect pathogenic fungus Isaria tenuipes BCC 7831. J. Nat. Prod. 2007, 70, 1478–1480. [Google Scholar] [CrossRef]
- Nara, F.; Tanaka, M.; Hosoya, T.; Suzuki-Konagai, K.; Ogita, T. Scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima: Taxonomy of the producing organism, fermentation, isolation, and physico-chemical properties. J. Antibiot. 1999, 52, 525–530. [Google Scholar] [CrossRef]
- Takagi, M.; Motohashi, K.; Izumikawa, M.; Khan, S.T.; Hwang, J.H.; Shin-Ya, K. JBIR-66, a new metabolite isolated from tunicate-derived Saccharopolyspora sp. SS081219JE-28. Biosci. Biotechnol. Biochem. 2010, 74, 2355–2357. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.K.; Ohta, S.; Ohta, E.; Koizumi, F.; Suzuki, M.; Ichimura, M.; Rahayu, E.S.; Ikegami, S. Two new analogues of the cytotoxic substance BE-52211 from Streptomyces sp. J. Nat. Prod. 2004, 67, 85–87. [Google Scholar] [CrossRef]
- Woo, J.T.; Ono, H.; Tsuji, T. Cathestatins, new cysteine protease inhibitors produced by Penicillium citrinum. Biosci. Biotechnol. Biochem. 1995, 59, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Knöll, W.M.; Rinehart, K.L., Jr.; Wiley, P.F.; Li, L.H. Streptovaricin U, an acyclic ansamycin. J. Antibiot. 1980, 33, 249–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rachid, S.; Scharfe, M.; Blöcker, H.; Weissman, K.J.; Müller, R. Unusual chemistry in the biosynthesis of the antibiotic chondrochlorens. Chem. Biol. 2009, 16, 70–81. [Google Scholar] [CrossRef]
- Yang, S.W.; Chan, T.M.; Terracciano, J.; Patel, R.; Loebenberg, D.; Chen, G.; Patel, M.; Gullo, V.; Pramanik, B.; Chu, M. New antibiotic Sch 725424 and its dehydration product Sch 725428 from Kitasatospora sp. J. Antibiot. 2005, 58, 192–195. [Google Scholar] [CrossRef]
- Su, S.S.; Tian, L.; Chen, G.; Li, Z.Q.; Xu, W.F.; Pei, Y.H. Two new compounds from the metabolites of a marine-derived actinomycete Streptomyces cavourensis YY01-17. J. Asian Nat. Prod. Res. 2013, 15, 265–269. [Google Scholar] [CrossRef]
- Ueda, J.Y.; Nagai, A.; Izumikawa, M.; Chijiwa, S.; Takagi, M.; Shin-ya, K. A novel antimycin-like compound, JBIR-06, from Streptomyces sp. ML55. J. Antibiot. 2008, 61, 241–244. [Google Scholar] [CrossRef]
- Krasnoff, S.B.; Englich, U.; Miller, P.G.; Shuler, M.L.; Glahn, R.P.; Donzelli, B.G.G.; Gibson, D.M. Metacridamides A and B, macrocycles from conidia of the entomopathogenic fungus Metarhizium acridum. J. Nat. Prod. 2012, 75, 175–180. [Google Scholar] [CrossRef]
- Kunze, B.; Kohl, W.; Hofle, G.; Reichenbach, H. Production, isolation, physico-chemical and biological properties of angiolam A, a new antibiotic from Angiococcus disciformis (Myxobacterales). J. Antibiot. 1985, 38, 1649–1654. [Google Scholar] [CrossRef]
- Cruz, J.S.; da Silva, C.A.; Hamerski, L. Natural products from endophytic fungi associated with Rubiaceae species. J. Fungi 2020, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Schneider, B.; Riese, U.; Schubert, B.; Li, Z.; Hamburger, M. Farinosones A-C, Neurotrophic alkaloidal metabolites from the entomogenous deuteromycete Paecilomyces farinosus. J. Nat. Prod. 2004, 67, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Numata, A.; Amagata, T.; Minoura, K.; Itoa, T. Gymnastatins, novel cytotoxic metabolites produced by a fungal strain from a sponge. Tetrahedron Lett. 1997, 38, 5675–5678. [Google Scholar] [CrossRef]
- Plaza, A.; Baker, H.L.; Bewley, C.A. Mirabilin, an antitumor macrolide lactam from the marine sponge Siliquariaspongia mirabilis. J. Nat. Prod. 2008, 71, 473–477. [Google Scholar] [CrossRef]
- Uchida, R.; Iwatsuki, M.; Kim, Y.P.; Ohte, S.; Ōmura, S. Nosokomycins, new antibiotics discovered in an in vivo-mimic infection model using silkworm larvae. I: Fermentation, isolation and biological properties. J. Antibiot. 2010, 63, 151–155. [Google Scholar] [CrossRef]
- Šmelcerović, A.; Đorđević, S.; Palić, R. A new metabolite from marine bacteria. Hemijska industrija. Chem. Ind. 2001, 55, 399–401. [Google Scholar]
- Gerth, K.; Jansen, R.; Reifenstahl, G.; Höfle, G. The myxalamids, new antibiotics from Myxococcus xanthus (Myxobacterales) I. production, physico-chemical and biological properties, and mechanism of action. J. Antibiot. 1983, 36, 1150–1156. [Google Scholar] [CrossRef]
- Bangi, A.; Itoua, Y.; Sakagamia, Y.; Fudoub, R.; Yamanakac, S.; Ojika, M. Novel antifungal polyene amides from the myxobacterium Cystobacter fuscus: Isolation, antifungal activity and absolute structure determination. Tetrahedron 2004, 60, 10217–10221. [Google Scholar]
- Höfle, G.; Gerth, K.; Reichenbach, H.; Kunze, B.; Sasse, F.; Forche, E.; Prusov, E.V. Isolation, biological activity evaluation, structure elucidation, and total synthesis of eliamid: A novel complex I inhibitor. Chemistry 2012, 18, 11362–11370. [Google Scholar] [CrossRef]
- Aouiche, A.; Sabaou, N.; Meklat, A.; Zitouni, A.; Bijani, C.; Mathieu, F.; Lebrihi, A. Saccharothrix sp. PAL54, a new chloramphenicol-producing strain isolated from a Saharan soil. World J. Microbiol. Biotechnol. 2012, 28, 943–951. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, B.G.; Brauers, G.; Guan, H.S.; Proksch, P.; Ebel, R. Microsphaerones A and B, two novel γ-pyrone derivatives from the sponge-derived fungus Microsphaeropsis sp. J. Nat. Prod. 2002, 65, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Hirota, H.; Imachi, M.; Fujimuro, M.; Onuki, H. Himeic acid A: A new ubiquitin-activating enzyme inhibitor isolated from a marine-derived fungus, Aspergillus sp. Bioorg. Med. Chem. Lett. 2005, 15, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, A.; Broberg, A.; Johansson, M.; Kenne, L.; Levenfors, J. Pseudotrienic acids A and B, two bioactive metabolites from Pseudomonas sp. MF381-IODS. J. Nat. Prod. 2005, 68, 1380–1385. [Google Scholar] [CrossRef]
- Kunze, B.; Trowitzsch-Kienast, W.; Höfle, G.; Reichenbach, H. Nannochelins A, B and C, new iron-chelating compounds from Nannocystis exedens (myxobacteria). Production, isolation, physico-chemical and biological properties. J. Antibiot. 1992, 45, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Takadera, T.; Adachi, K.; Nishijima, M.; Sano, H. Korormicin, a novel antibiotic specifically active against marine gram-negative bacteria, produced by a marine bacterium. J. Antibiot. 1997, 50, 949–953. [Google Scholar] [CrossRef]
- Kanbe, K.; Naganawa, H.; Okamura, M. Amidenin, a new plant growth-regulating substance isolated from Amycolatopsis sp. Biosci. Biotechnol. Biochem. 1993, 57, 1261–1263. [Google Scholar] [CrossRef]
- López-Bucio, J.; Acevedo-Hernández, G. Novel signals for plant development. Current Opin. Plant Biol. 2006, 9, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Hasegawa, Y.; Sakabe, Y.; Tomoda, H. Citrinamides, New potentiators of antifungal miconazole activity, produced by Penicillium sp. FKI-1938. J. Antibiot. 2008, 61, 550–555. [Google Scholar] [CrossRef]
- Barsby, T.; Kelly, M.T.; Andersen, R.J. Tupuseleiamides and basiliskamides, new acyldipeptides and antifungal polyketides produced in culture by a Bacilluslaterosporus isolate obtained from a tropical marine habitat. J. Nat. Prod. 2002, 65, 1447–1451. [Google Scholar] [CrossRef]
- Akhand, M.; Al-Bari, M.A.A.; Islam, M.A.; Khondkar, P. Characterization and antimicrobial activities of a metabolite from a new Streptomyces species from Bangladeshi soil. J. Sci. Res. 2010, 2, 178–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, X.M.; Cui, C.M.; Feng, C.; Wang, B.G. New sphingolipids with a previously unreported 9-methyl-C20-sphingosine moiety from a marine algous endophytic fungus Aspergillus niger EN-13. Lipids 2007, 42, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Nishiyama, Y.; Ohta, S.; Kamei, H. Glidobactins A, B and C, new antitumor antibiotics I. Production, isolation, chemical properties and biological activity. J. Antibiot. 1988, 41, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Yim, J.H.; Lee, H.K.; Park, S.M.; Sohn, J.H.; Oh, H. Stereocalpin A, a bioactive cyclic depsipeptide from the Antarctic lichen Stereocaulon alpinum. Tetrahedron Lett. 2008, 49, 29–31. [Google Scholar] [CrossRef]
- Silber, J.; Ohlendorf, B.; Labes, A.; Näther, C. Calcaripeptides A–C, cyclodepsipeptides from a Calcarisporium strain. J. Nat. Prod. 2013, 76, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Shen, Y.; Bai, L. Biosynthesis of 3,5-AHBA-derived natural products. Nat. Prod. Rep. 2012, 29, 243–263. [Google Scholar] [CrossRef]
- Kakule, T.B.; Sardar, D.; Lin, Z.; Schmidt, E.W. Two related pyrrolidinedione synthetase loci in Fusarium heterosporum ATCC 74349 produce divergent metabolites. ACS Chem. Biol. 2013, 8, 1549–1557. [Google Scholar] [CrossRef]
- Kahner, L.; Dasenbrock, J.; Spiteller, P.; Steglich, W. Polyene pigments from fruit-bodies of Boletus laetissimus and B. rufo-aureus (basidiomycetes). Phytochemistry 1998, 49, 1693–1697. [Google Scholar] [CrossRef]
- Gruber, G.; Steglich, W. Calostomal, a polyene pigment from the gasteromycete Calostoma cinnabarinum (Boletales). Zeitsch. Naturforsch. 2007, 62B, 129–131. [Google Scholar] [CrossRef]
- Aulinger, K.; Besl, H.; Spiteller, P.; Spitelle, M. Melanocrocin, a polyene pigment from Melanogaster broomeianus (Basidiomycetes). Zeitsch. Naturforsch. 2001, 56C, 495–498. [Google Scholar] [CrossRef]
- Zhan, Z.J.; Yue, J.M. New glycosphingolipids from the fungus Catathelasma ventricosa. J. Nat. Prod. 2003, 66, 1013–1016. [Google Scholar] [CrossRef]
- Manam, R.R.; Teisan, S.; White, D.J.; Nicholson, B.; Grodberg, J. Lajollamycin, a nitro-tetraene spiro-beta-lactone-gamma-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J. Nat. Prod. 2005, 68, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Coughlin, J.M.; Ju, J.; Zhu, D.; Wendt-Pienkowski, E.; Zhou, X.; Wang, Z.; Shen, B.; Deng, Z. Oxazolomycin biosynthesis in Streptomyces albus JA3453 featuring an "acyltransferase-less" type I polyketide synthase that incorporates two distinct extender units. J. Biol. Chem. 2010, 285, 20097–20108. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.G.; Trippier, P.C.; Yaqoob, M.; Wang, Z. The oxazolomycins: A structurally novel class of bioactive compounds. Curr. Drug Discov. Technol. 2004, 1, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Tonew, E.; Tonew, M.; Gräfe, U.; Zöpel, P. On the antiviral activity of diffusomycin (oxazolomycin). Acta Virol. 1992, 36, 166–172. [Google Scholar]
- Kanzaki, H.; Wada, K.; Nitoda, T.; Kawazu, K. Novel bioactive oxazolomycin isomers produced by Streptomyces albus JA3453. Biosci. Biotechnol. Biochem. 1998, 62, 438–442. [Google Scholar] [CrossRef][Green Version]
- Ryu, G.; Hwang, S.; Kim, S.K. 16-Methyloxazolomycin, a new antimicrobial and cytotoxic substance produced by a Streptomyces sp. J. Antibiot. 1997, 50, 1064–1066. [Google Scholar] [CrossRef]
- Ogura, M.; Nakayama, H.; Furihata, K.; Shimazu, A.; Seto, H.; Otake, N. Structure of a new antibiotic curromycin A produced by a genetically modified strain of Streptomyces hygroscopicus, a polyether antibiotic producing organism. J. Antibiot. 1985, 38, 669–673. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Akimoto, M.; Ishikawa, A.; Izawa, M.; Shin-Ya, K. Curromycin A as a GRP78 downregulator and a new cyclic dipeptide from Streptomyces sp. J. Antibiot. 2016, 69, 187–188. [Google Scholar] [CrossRef]
- Nakamura, M.; Honma, H.; Kamada, M.; Ohno, T.; Kunimoto, S.; Ikeda, Y.; Kondo, S.; Takeuchi, T. Inhibitory effect of curromycin A and B on human immunodeficiency virus replication. J. Antibiot. 1994, 47, 616–618. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kondo, S.; Naganawa, H.; Hattori, S.; Hamada, M.; Takeuchi, T. New triene-beta-lactone antibiotics, triedimycins A and B. J. Antibiot. 1991, 44, 453–455. [Google Scholar] [CrossRef]
- Guo, J.-P.; Zhu, C.-Y.; Zhang, C.-P.; Chu, Y.-S.; Wang, Y.-L.; Zhang, J.-X.; Wu, D.-K.; Zhang, K.-Q.; Niu, X.-M. Thermolides, potent nematocidal pks-nrps hybrid metabolites from thermophilic fungus Talaromyces thermophilus. J. Am. Chem. Soc. 2012, 134, 20306–20309. [Google Scholar] [CrossRef] [PubMed]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as alternatives for biological control. Part II: Metabolites from nematophagous basidiomycetes and non-nematophagous fungi. Appl. Microbiol. Biotechnol. 2016, 100, 3813–3824. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.; Martin, M.T.; Servy, C.; Cortial, S.; Lopes, P. Isolation and characterization of α,β-unsaturated γ-lactono-hydrazides from Streptomyces sp. J. Nat. Prod. 2012, 75, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.; Martin, M.T.; Iorga, B.I.; Adelin, E.; Servy, C.; Cortial, S.; Ouazzani, J. Isolation and characterization of unusual hydrazides from Streptomyces sp. impact of the cultivation support and extraction procedure. J. Nat. Prod. 2013, 76, 142–149. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological and predicted activities of natural azo compounds. Nat. Prod. Bioprospect. 2017, 7, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.M.; Maurya, R.; Vijayakumar, E.K.; Chatterjee, S.; Blumbach, J.; Ganguli, B.N. Alisamycin, a new antibiotic of the manumycin group. I. Taxonomy, production, isolation and biological activity. J. Antibiot. 1991, 44, 1289–1293. [Google Scholar] [CrossRef]
- Silva, L.R.; da Silva-Júnior, E.F. Inhibiting the “Undruggable” RAS/farnesyltransferase (FTase) cancer target by manumycin-related natural products. Current Med. Chem. 2022, 29, 189–211. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakagawa, M.; Fujita, T.; Tanimori, S.; Nakayama, M. Nisamycin, a new manumycin group antibiotic from Streptomyces sp. K106. II. Structure determination and structure-activity relationships. J. Antibiot. 1994, 47, 1110–1115. [Google Scholar] [CrossRef]
- Omura, S.; Kitao, C.; Tanaka, H.; Oiwa, R.; Takahashi, Y. A new antibiotic, asukamycin, produced by Streptomyces. J. Antibiot. 1976, 29, 876–881. [Google Scholar] [CrossRef]
- Caglioti, L.; Misiti, D.; Mondelli, R.; Selva, A.; Arcamone, F.; Cassinelli, G. The structure of neoantimycin. Tetrahedron 1969, 25, 2193–2221. [Google Scholar] [CrossRef]
- Caglioti, L.; Ciranni, G.; Misiti, D.; Arcamone, F.; Minghetti, A. Biosynthesis of the 3,4-dihydroxy-2,2-dimethyl-5-phenylvaleric acid residue of neoantimycin. J. Chem. Soc. Perkin 1 1972, 9, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zvanych, R.; Vanner, S.A.; Wang, W.; Magarvey, N.A. Chemical variation from the neoantimycin depsipeptide assembly line. Bioorg. Med. Chem. Lett. 2013, 23, 5123–5127. [Google Scholar] [CrossRef] [PubMed]
- Umeda, Y.; Chijiwa, S.; Furihata, K.; Furihata, K.; Sakuda, S.; Nagasawa, H. Prunustatin A, a novel GRP78 molecular chaperone down-regulator isolated from Streptomyces violaceoniger. J. Antibiot. 2005, 58, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L.; Nogawa, T.; Okano, A.; Futamura, Y.; Kawatani, M. Unantimycin A, a new neoantimycin analog isolated from a microbial metabolite fraction library. J. Antibiot. 2016, 69, 456–458. [Google Scholar] [CrossRef]
- Hosoya, T.; Hirokawa, T.; Takagi, M.; Shin-ya, K. Trichostatin analogues JBIR-109, JBIR-110, and JBIR-111 from the marine sponge-derived Streptomyces sp. RM72. J. Nat. Prod. 2012, 75, 285–289. [Google Scholar] [CrossRef]
- Dewey, R.S.; Arison, B.H.; Hannah, J.; Shih, D.H.; Albers-Schönberg, G. The structure of efrotomycin. J. Antibiot. 1985, 38, 1691–1698. [Google Scholar] [CrossRef]
- Wax, R.; Maises, W.; Weston, R.; Birnbaum, J. Efrotomycin, a new antibiotic from Streptomyces lactamdurans. J. Antibiot. 1976, 29, 670–673. [Google Scholar] [CrossRef]
- Berger, J.; Lehr, H.H.; Teitel, S.; Maehr, H.; Grunberg, E. A new antibiotic X-5108 of Streptomyces origin. I. Production, isolation and properties. J. Antibiot. 1973, 26, 15–22. [Google Scholar] [CrossRef]
- Liu, C.; Hermann, T.; Miller, P.A. Feedback inhibition of the synthesis of an antibiotic: Aurodox (X-5108). J. Antibiot. 1977, 30, 244–251. [Google Scholar] [CrossRef]
- Yu, C.M.; Curtis, J.M.; Walter, J.A.; Wright, J.L.; Ayer, S.W.; Kaleta, J.; Querengesser, L.; Fathi-Afshar, Z.R. Potent inhibitors of cysteine proteases from the marine fungus Microascus longirostris. J. Antibiot. 1996, 49, 395–397. [Google Scholar] [CrossRef]
- Hoberg, K.A.; Cihlar, R.L.; Calderone, R.A. Characterization of cerulenin-resistant mutants of Candida albicans. Infect Immun. 1986, 51, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.Y.; Lee, J.S.; Yoo, K.S.; Oh, S.; Choe, E.; Lee, H.J. Fatty acid synthase inhibitor cerulenin inhibits topoisomerase I catalytic activity and augments SN-38-induced apoptosis. Apoptosis 2013, 18, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Thirkettle, J. SB-253514 and analogues; novel inhibitors of lipoprotein associated phospholipase A2 produced by Pseudomonas fluorescens DSM 11579. III. Biotransformation using naringinase. J. Antibiot. 2000, 53, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Thirkettle, J.; Alvarez, E.; Boyd, H.; Brown, M.; Diez, E.; Hueso, J.; Elson, S.; Fulston, M. SB-253514 and analogues; novel inhibitors of lipoprotein-associated phospholipase A2 produced by Pseudomonas fluorescens DSM 11579. I. Fermentation of producing strain, isolation and biological activity. J. Antibiot. 2000, 53, 664–669. [Google Scholar] [CrossRef]
- Sayed, A.M.; Abdel-Wahab, N.M.; Hassan, H.M.; Abdelmohsen, U.R. Saccharopolyspora: An underexplored source for bioactive natural products. J. Appl. Microbiol. 2019, 128, 314–329. [Google Scholar] [CrossRef]
- Masunaka, A.; Ohtani, K.; Peever, T.L.; Timmer, L.W.; Tsuge, T. An isolate of Alternaria alternata that is pathogenic to both tangerines and rough lemon and produces two host-selective toxins, ACT- and ACR-toxins. Phytopathology 2005, 95, 241–247. [Google Scholar] [CrossRef]
- Takaoka, S.; Kurata, M.; Harimoto, Y.; Hatta, R.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. Complex regulation of secondary metabolism controlling pathogenicity in the phytopathogenic fungus Alternaria alternata. New Phytol. 2014, 202, 1297–1309. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Borris, R.P.; Tran, T.T.; Jee, J.M.; Seow, H.F.; Cheah, H.Y.; Hoc, C.C.; Chang, L.C. Three new amides from Streptomyces sp. H7372. J. Braz. Chem. Soc. 2011, 22, 223–229. [Google Scholar] [CrossRef]
- Kunze, B.; Jansen, R.; Höfle, G.; Reichenbach, H. Crocacin, a new electron transport inhibitor from Chondromyces crocatus (myxobacteria). Production, isolation, physico-chemical and biological properties. J. Antibiot. 1994, 47, 881–886. [Google Scholar] [CrossRef]
- Capon, R.J.; Skene, C.; Lacey, E.; Gill, J.H.; Wicker, J.; Heiland, K.; Friedel, T. Lorneamides A and B: two new aromatic amides from a Southern Australian marine Actinomycete. J. Nat. Prod. 2000, 63, 1682–1683. [Google Scholar] [CrossRef]
- El-Naggar, M.Y.; El-Assar, S.A.; Abdul-Gawad, S.M. Solid-state fermentation for the production of meroparamycin by Streptomyces sp. strain MAR01. J. Microbiol. Biotechnol. 2009, 19, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Sawa, R.; Naganawa, H.; Muraoka, Y.; Aoyagi, T.; Takeuchi, T. Epostatin, new inhibitor of dipeptidyl peptidase II, produced by Streptomyces sp. MJ995-OF5 II. Structure elucidation. J. Antibiot. 1998, 51, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Lu, C.H.; Li, Y.Y.; Li, S.R.; Shen, Y.M. Cuevaenes C-E: Three new triene carboxylic derivatives from Streptomyces sp. LZ35ΔgdmAI. Beilstein J. Org. Chem. 2014, 10, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, N.; Naganawa, H.; Inuma, H.; Hamada, M.; Takeuchi, T.; Kanbe, T.; Hori, M. Thiazinotrienomycins, new ansamycin group antibiotics. J. Antibiot. 1995, 48, 471–478. [Google Scholar] [CrossRef]
- Evano, G.; Schaus, J.V.; Panek, J.S. A convergent synthesis of the macrocyclic core of cytotrienins: Application of RCM for macrocyclization. Org. Lett. 2004, 6, 525–528. [Google Scholar] [CrossRef]
- Huang, Y.F.; Li, L.H.; Tian, L.; Qiao, L.; Hua, H.M.; Pei, Y.H. Sg17-1-4, a novel isocoumarin from a marine fungus Alternaria tenuis Sg17-1. J. Antibiot. 2006, 59, 355–357. [Google Scholar] [CrossRef]
- Sasaki, T.; Igarashi, Y.; Saito, N.; Furumai, T. TPU-0031-A and B, new antibiotics of the novobiocin group produced by Streptomyces sp. TP-A0556. J. Antibiot. 2001, 54, 441–447. [Google Scholar] [CrossRef]
- Bu, Y.Y.; Yamazaki, H.; Ukai, K.; Namikoshi, M. Anti-mycobacterial nucleoside antibiotics from a marine-derived Streptomyces sp. TPU1236A. Mar. Drugs 2014, 12, 6102–6112. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, L.; Li, W.; Yang, Y.; Xu, L.; Ding, Z. Anti-mycobacterium tuberculosis active metabolites from an endophytic Streptomyces sp. YIM65484. Rec. Nat. Prod. 2015, 9, 196–200. [Google Scholar]
- Matsuda, S.; Adachi, K.; Matsuo, Y.; Nukina, M. Salinisporamycin, a novel metabolite from Salinispora arenicora. J. Antibiot. 2009, 62, 519–526. [Google Scholar] [CrossRef]
- Liu, J.; de Brabander, J.K. A concise total synthesis of saliniketal B. J. Am. Chem. Soc. 2009, 131, 12562–12563. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Murai, M.; Abe, M.; Ichimaru, N.; Haradа, T. Crucial structural factors and mode of action of polyene amides as inhibitors for mitochondrial NADH-Ubiquinone oxidoreductase (Complex I). Biochemistry 2007, 46, 10365–10372. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, E.; Jansen, R.; Kunze, B. Three new potent HIV-1 inhibitors from Myxobacteria. Antiviral Chem. Chemother. 1992, 2, 189–193. [Google Scholar] [CrossRef]
- Trowitzsch-Kienast, W.; Forche, E.; Wray, V. Antibiotika aus Gleitenden Bakterien, 45. Phenalamide, neue HIV-1-Inhibitoren aus Myxococcus stipitatus Mx s40. Liebigs Ann. Chem. 1992, 7, 659–664. [Google Scholar] [CrossRef]
- Raju, R.; Piggott, A.M.; Conte, M.; Tnimov, Z. Nocardiopsins: New FKBP12-binding macrolide polyketides from an Australian marine-derived actinomycete, Nocardiopsis sp. Chemistry-A European J. 2010, 16, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Piggott, A.M.; Quezada, M.; Capon, R.J. Nocardiopsins C and D and nocardiopyrone A: New polyketides from an Australian marine-derived Nocardiopsis sp. Tetrahedron 2013, 69, 692–698. [Google Scholar] [CrossRef]
- Wu, Y.; Seyedsayamdost, M.R. The polyene natural product thailandamide A inhibits fatty acid biosynthesis in Gram-positive and Gram-Negative bacteria. Biochemistry 2018, 57, 4247–4251. [Google Scholar] [CrossRef]
- Wozniak, C.E.; Lin, Z.; Schmidt, E.W. Thailandamide, a fatty acid synthesis antibiotic that is coexpressed with a resistant target gene. Antimicro. Agents Chemother. 2018, 62, e00463-18. [Google Scholar] [CrossRef]
- Kim, J.W.; Shin-ya, K.; Furihata, K.; Hayakawa, Y.; Seto, H. Oximidines I and II: novel antitumor macrolides from Pseudomonas sp. J. Org. Chem. 1999, 64, 153–155. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Tomikawa, T.; Shin-Ya, K.; Arao, N. Oximidine III, a new antitumor antibiotic against transformed cells from Pseudomonas sp. II. Structure elucidation. J. Antibiot. 2003, 56, 905–908. [Google Scholar] [CrossRef][Green Version]
- Kino, T.; Hatanaka, H.; Hashimoto, M.; Nishiyama, M.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; Imanaka, H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. 1987, 40, 1249–1255. [Google Scholar] [CrossRef]
- Lechner, A.; Wilson, M.C.; Ban, Y.H.; Hwang, J.Y.; Yoon, Y.J.; Moore, B.S. Designed biosynthesis of 36-methyl-FK506 by polyketide precursor pathway engineering. ACS Synth. Biol. 2013, 2, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Mcalpine, J.B.; Sørensen, D.; Ibrahim, A.; Aouidate, M. Genomic analyses lead to novel secondary metabolites. Part 3. ECO-0501, a novel antibacterial of a new class. J. Antibiot. 2006, 59, 533–542. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.B.; Bachmann, B.O.; Piraee, M.; Tremblay, S.; Alarco, A.M.; Zazopoulos, E.; Farnet, C.M. Microbial genomics as a guide to drug discovery and structural elucidation: ECO-02301, a novel antifungal agent, as an example. J. Nat. Prod. 2005, 68, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, S.C.; Danziger, L.H.; Rodvold, K.A. Clarithromycin and azithromycin: New macrolide antibiotics. Clin. Pharm. 1992, 11, 137–152. [Google Scholar]
- Fraschini, F.; Scaglione, F.; Demartini, G. Clarithromycin clinical pharmacokinetics. Clin-Pharmacokinet. 1993, 25, 189–204. [Google Scholar] [CrossRef]
- Alvarez-Elcoro, S.; Enzler, M.J. The macrolides: Erythromycin, clarithromycin, and azithromycin. Mayo Clin. Proceed. 1999, 74, 613–634. [Google Scholar] [CrossRef]
- Furumai, T.; Yamakawa, T.; Yoshida, R.; Igarashi, Y. Clethramycin, a new inhibitor of pollen tube growth with antifungal activity from Streptomyces hygroscopicus TP-A0623. I. Screening, taxonomy, fermentation, isolation and biological properties. J. Antibiot. 2003, 56, 700–704. [Google Scholar] [CrossRef][Green Version]
- Hong, H.; Fill, T.; Leadlay, P.F. A common origin for guanidinobutanoate starter units in antifungal natural products. Angew. Chem. Int. Ed. Engl. 2013, 52, 13096–13099. [Google Scholar] [CrossRef]
- Sun, F.; Xu, S.; Jiang, F. Genomic-driven discovery of an amidinohydrolase involved in the biosynthesis of mediomycin A. Appl. Microbiol. Biotechnol. 2018, 102, 2225–2234. [Google Scholar] [CrossRef]
- Friedrich, R.M.; GK Friestad. Inspirations from tetrafibricin and related polyketides: New methods and strategies for 1, 5-polyol synthesis. Nat. Prod. Rep. 2020, 37, 1229–1261. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Fraser, T.R. The connection of chemical constitution and physiological action. Trans. R. Soc. Edinb. 1868, 25, 224–242. [Google Scholar]
- Cros, A.F.A. Action de l’Alcohol Amylique Sur l’Organisme. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 1863. [Google Scholar]
- Richet, M.C. Note sur le rapport entre la toxicité et les propriétes physiques des corps. Compt. Rend. Soc. Biol. 1893, 45, 775–776. [Google Scholar]
- Meyer, H. Zur Theorie der AIkoholnarkose. Arch. Exp. Path. Pharm. 1899, 42, 109–118. [Google Scholar] [CrossRef]
- Overton, C.E. Studien Über Die Narkose; Fischer: Jena, Germany, 1901. [Google Scholar]
- Hammett, L.P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 1935, 17, 125–136. [Google Scholar] [CrossRef]
- Hammett, L.P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]
- Taft, R.W. Separation of polar, steric and resonance effects in reactivity. In Steric Effects in Organic Chemistry; Newman, M.S., Ed.; Wiley: Hoboken, NJ, USA, 1956; pp. 556–675. [Google Scholar]
- Hansch, C.; Fujita, T. p-σ-π Analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Exploring QSAR; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W., Jr. Computational methods in drug discovery. Pharm. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef]
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef]
- Kokh, D.B.; Amaral, M.; Bomke, J.; Grädler, U.; Musil, D. Estimation of drug-target residence times by τ-random acceleration molecular dynamics simulations. J. Chem. Theor. Comput. 2018, 14, 3859–3869. [Google Scholar] [CrossRef]
- Cherkasov, A.M.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I.; Cronin, M.; Dearden, J. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef] [PubMed]
- Poroikov, V.V. Computer-aided drug design: From discovery of novel pharmaceutical agents to systems pharmacology. Biochemistry 2020, 14, 216–227. [Google Scholar]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.V. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Antitumor profile of carbon-bridged steroids (CBS) and triterpenoids. Mar. Drugs 2021, 19, 324. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Antitumor and hepatoprotective activity of natural and synthetic neo steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’yakonov, D. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 524, 772–783. [Google Scholar] [CrossRef]
- Dembitsky, V.M. In silico prediction of steroids and triterpenoids as potential regulators of lipid metabolism. Mar. Drugs 2021, 19, 650. [Google Scholar] [CrossRef]
- Pounina, T.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Sulfated and sulfur-containing steroids and their pharmacological profile. Mar. Drugs 2021, 19, 240. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Hydrobiological aspects of saturated, methyl-branched, and cyclic fatty acids derived from aquatic ecosystems: Origin, distribution, and biological activity. Hydrobiology 2022, 1, 7. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Natural polyether ionophores and their pharmacological profile. Mar. Drugs 2022, 20, 292. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).