Glucosylceramides from Cladosporium and Their Roles in Fungi–Plant Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
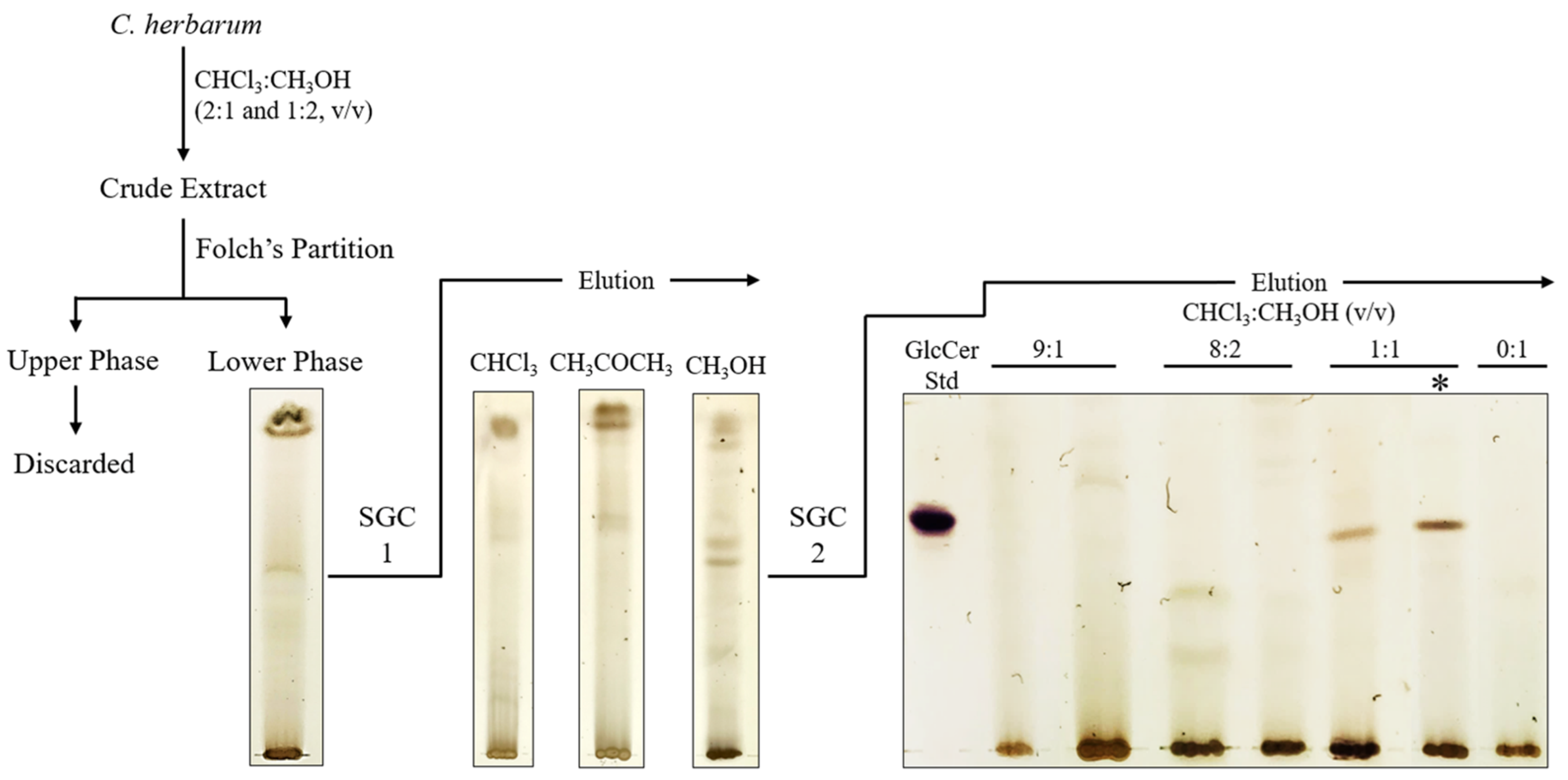
2.2. Extraction and Purification of Glycosphingolipids
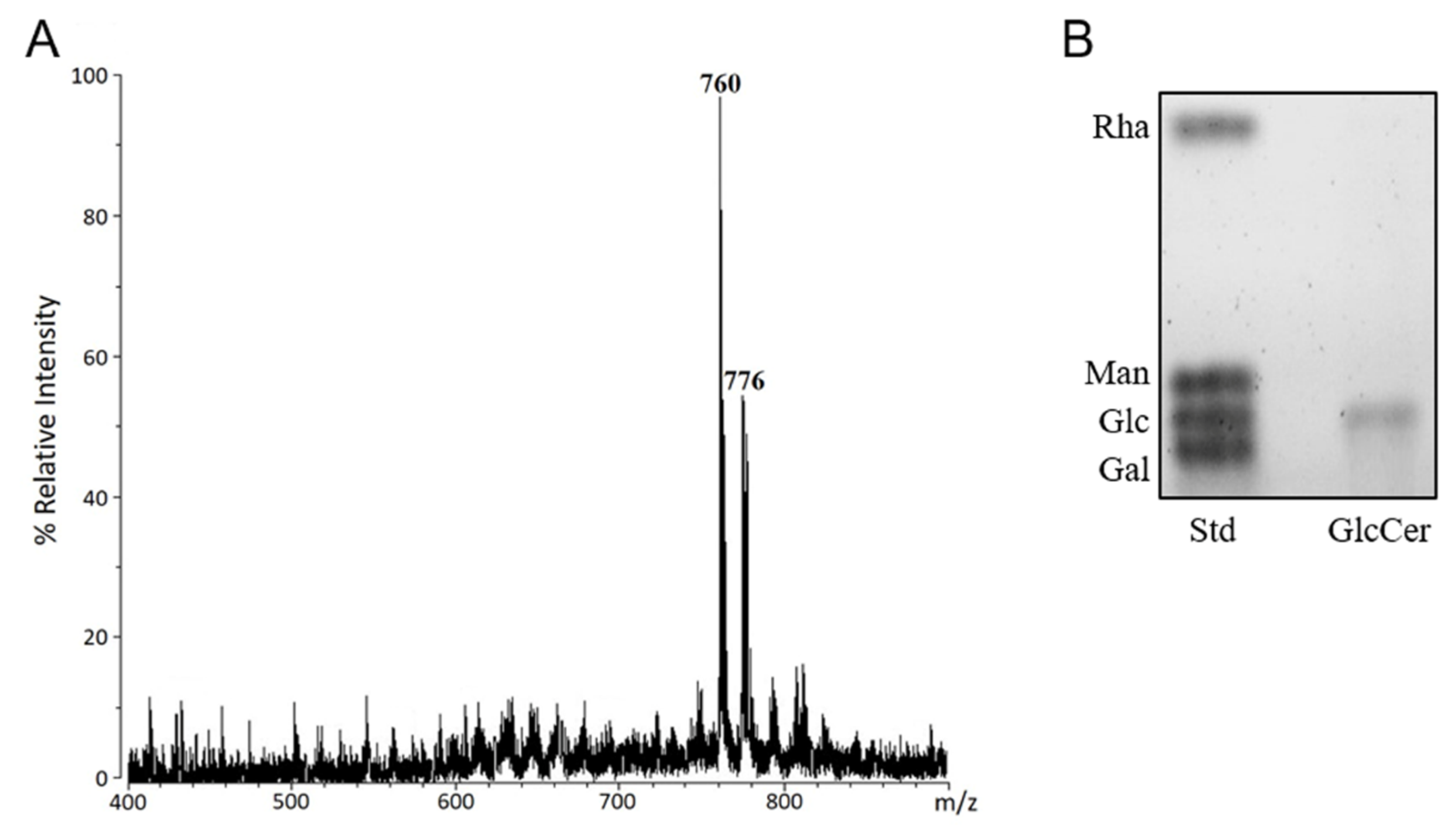
2.3. Sugar Analysis
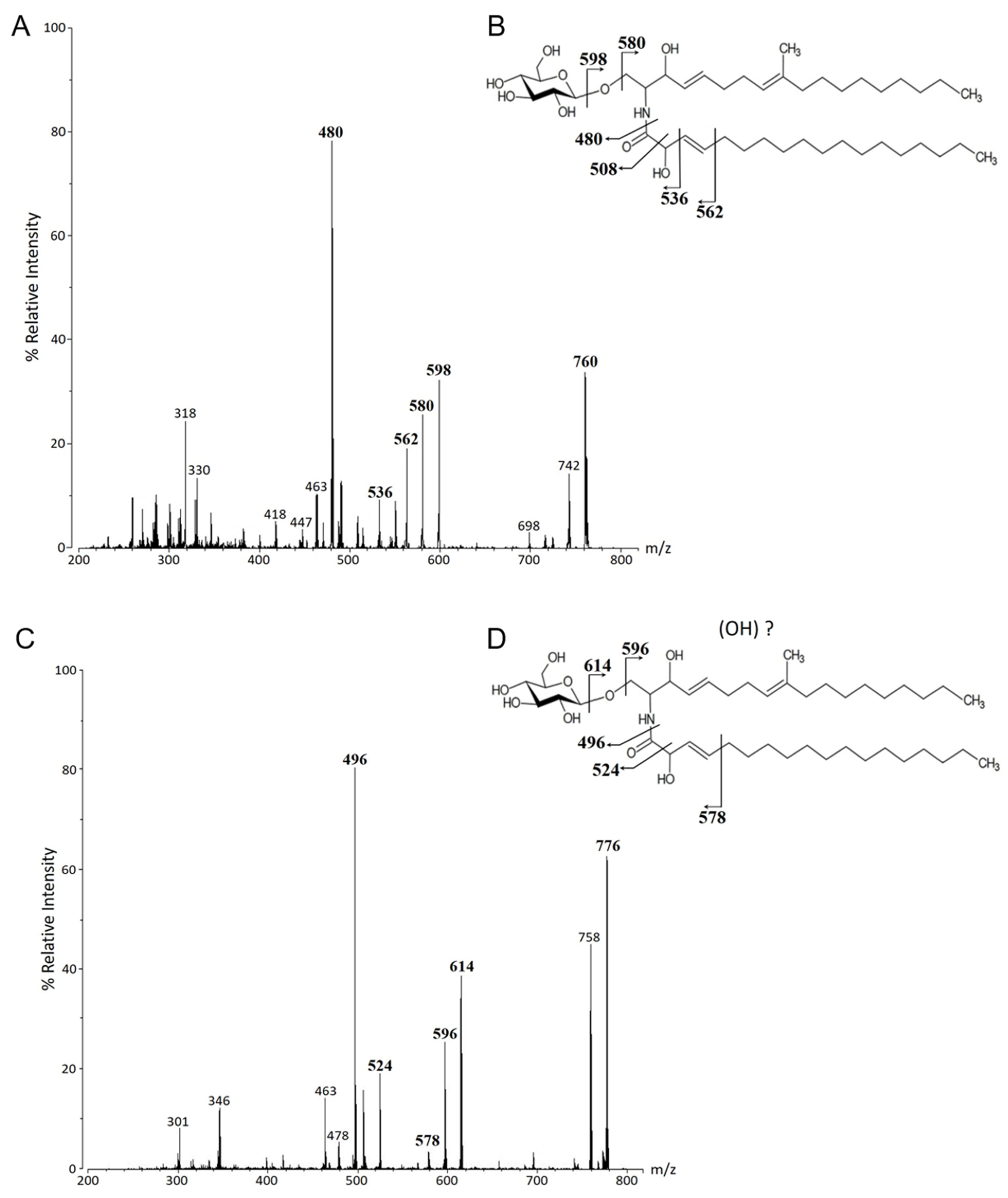
2.4. ESI-MS Analysis
2.5. Reactivity of GlcCer with Monoclonal Anti-Glccer Antibodies (Mabs)
2.6. Immunolocalization of GlcCer on the Surface of C. herbarum and C. resinae
2.7. Plant Culture
2.8. GlcCer Pulverization on P. edulis
2.9. Histochemical Detection of Reactive Species Oxygen (ROS)
2.10. Evaluation of Gene Expression by Real-Time RT-qPCR
2.11. Plant Developmental Parameter Evaluation
2.12. Statistical Analysis
3. Results
3.1. Structural Characterization of Glycosphingolipids from C. herbarum
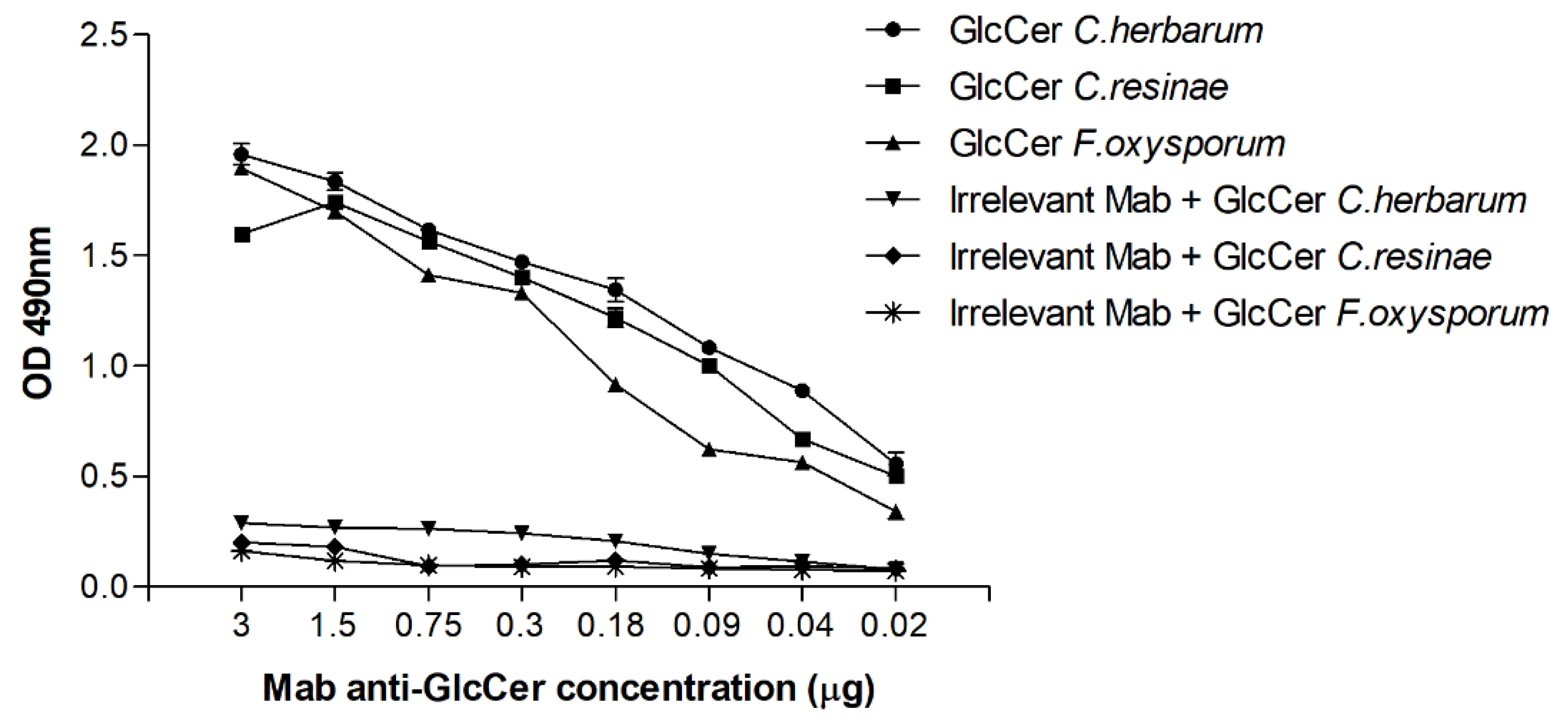
3.2. Reactivity of GlcCer with Monoclonal Antibodies (Mabs) Anti-GlcCer
3.3. Immunolocalization of GlcCer on the Surface of C. herbarum
3.4. Histochemical Detection of Reactive Oxygen Species (ROS) in P. edulis after GlcCer Treatment
3.5. Evaluation of Gene Expression

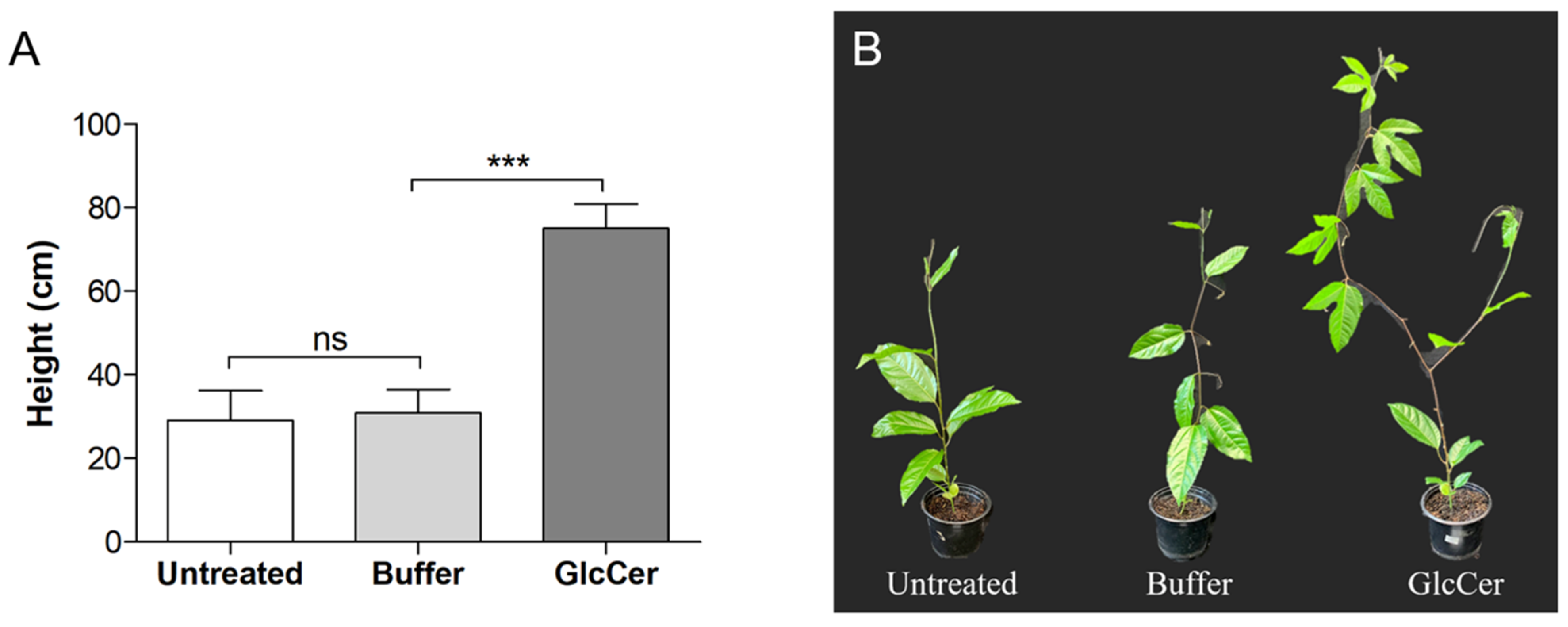
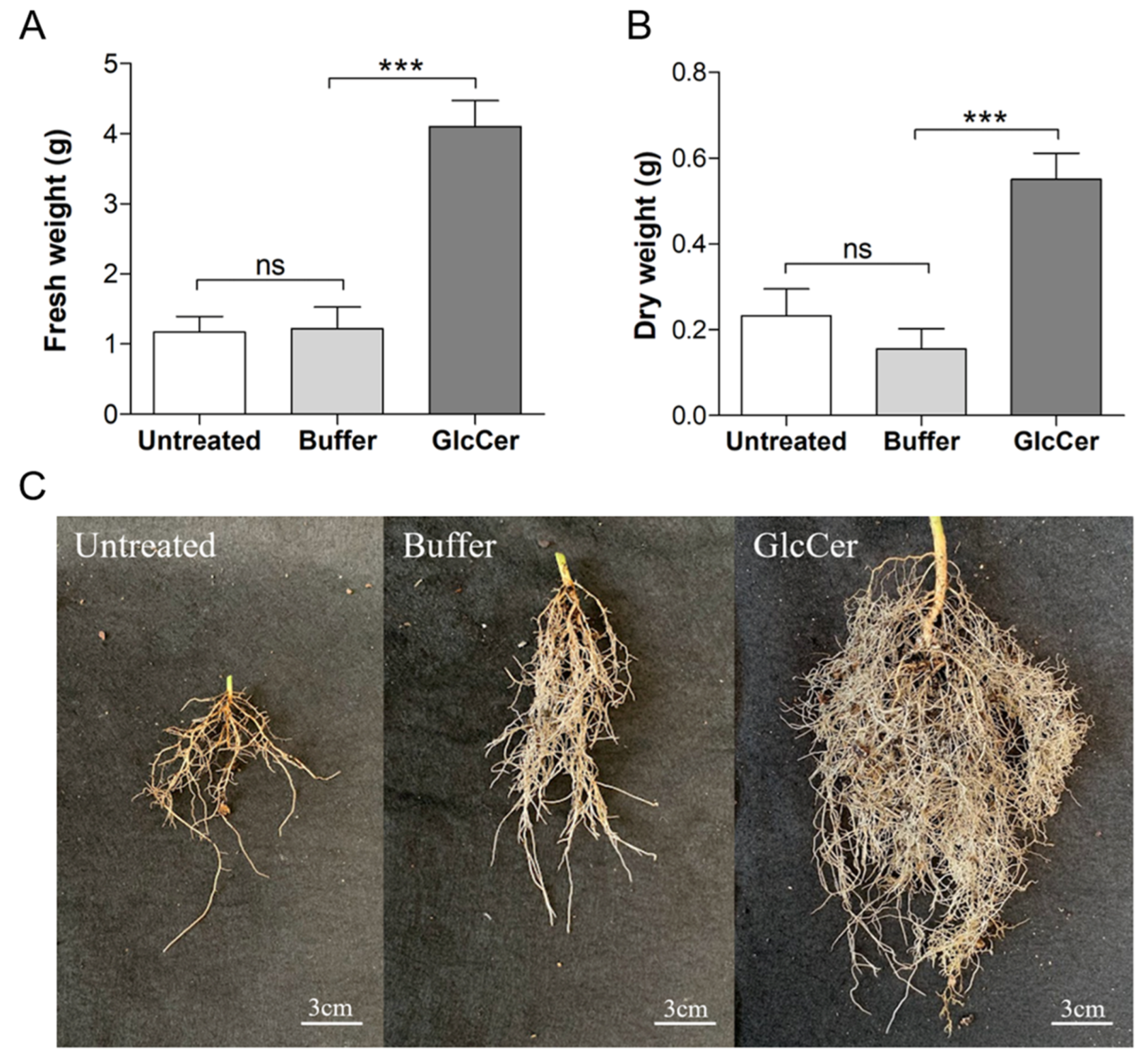
3.6. Evaluation of Plant Developmental Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bensch, K.; Braun, U. Taxonomic revision of the genus Cladosporium s. lat. 6. New species, reallocations to and synonyms of Cercospora, Fusicladium, Passalora, Septonema and Stenella. Nova Hedwig. 2007, 84, 189–208. [Google Scholar] [CrossRef]
- Seifert, K.A.; Hughes, S.J.; Boulay, H.; Louis-Seize, G. Taxonomy, nomenclature and phylogeny of three cladosporium-like hyphomycetes, Sorocybe resinae, Seifertia azaleae and the Hormoconis anamorph of Amorphotheca resinae. Stud. Mycol. 2007, 58, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Rancé, I.; Fournier, J.; Esquerré-Tugayé, M.T. The incompatible interaction between Phytophthora parasitica var. nicotianae race 0 and tobacco is suppressed in transgenic plants expressing antisense lipoxygenase sequences. Proc. Natl. Acad. Sci. USA 1998, 95, 6554–6559. [Google Scholar] [CrossRef]
- Latgé, J.P. Tasting the fungal cell wall. Cell Microbiol. 2010, 12, 863–872. [Google Scholar] [CrossRef]
- Barreto-Bergter, E.; Pinto, M.R.; Rodrigues, M.L. Structure and biological functions of fungal cerebrosides. An. Acad. Bras. Cienc. 2004, 76, 67–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barreto-Bergter, E.; Sassaki, G.L.; de Souza, L.M. Structural analysis of fungal cerebrosides. Front. Microbiol. 2011, 2, 239. [Google Scholar] [CrossRef]
- Naveen, J.; Hariprasad, P.; Nayaka, S.C.; Niranjana, S.R. Cerebroside mediated elicitation of defense response in chilli (Capsicum annuum L.) against Colletotrichum capsici infection. J. Plant Interact. 2013, 8, 65–73. [Google Scholar] [CrossRef]
- Umemura, K.; Ogawa, N.; Yamauchi, T.; Iwata, M.; Shimura, M.; Koga, J. Cerebroside elicitors found in diverse phytopathogens activate defense responses in rice plants. Plant Cell Physiol. 2000, 41, 676–683. [Google Scholar] [CrossRef]
- Bernardino, M.C.; Couto, M.; Vaslin, M.F.S.; Barreto-Bergter, E. Antiviral activity of glucosylceramides isolated from Fusarium oxysporum against Tobacco mosaic virus infection. PLoS ONE 2020, 15, e0242887. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Rollin-Pinheiro, R.; Liporagi-Lopes, L.C.; de Meirelles, J.V.; Souza, L.M.; Barreto-Bergter, E. Characterization of Scedosporium apiospermum glucosylceramides and their involvement in fungal development and macrophage functions. PLoS ONE 2014, 9, e98149. [Google Scholar] [CrossRef] [PubMed]
- Boas, M.H.; da Silva, M.C.; de Oliveira, T.G.; Travassos, L.R.; Bergter, E.B. Reactivity of chagasic sera with crude and highly purified glycosphingolipid fractions from Trypanosoma cruzi epimastigotes. J. Clin. Lab. Anal. 1994, 8, 260–266. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.F.; Rodrigues, M.L.; Farias, S.E.; Almeida, I.C.; Pinto, M.R.; Barreto-Bergter, E. Glucosylceramides in Colletotrichum gloeosporioides are involved in the differentiation of conidia into mycelial cells. FEBS Lett. 2004, 561, 137–143. [Google Scholar] [CrossRef]
- Calixto, R.O.; Rollin-Pinheiro, R.; da Silva, M.I.; Liporagi-Lopes, L.C.; Vieira, J.M.; Sassaki, G.L.; Barreto-Bergter, E. Structural analysis of glucosylceramides (GlcCer) from species of the Pseudallescheria/Scedosporium complex. Fungal Biol. 2016, 120, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Nimrichter, L.; Cerqueira, M.D.; Leitão, E.A.; Miranda, K.; Nakayasu, E.S.; Almeida, S.R.; Almeida, I.C.; Alviano, C.S.; Barreto-Bergter, E.; Rodrigues, M.L. Structure, cellular distribution, antigenicity, and biological functions of Fonsecaea pedrosoi ceramide monohexosides. Infect. Immun. 2005, 73, 7860–7868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xisto, M.; Henao, J.E.M.; Dias, L.D.S.; Santos, G.M.P.; Calixto, R.O.R.; Bernardino, M.C.; Taborda, C.P.; Barreto-Bergter, E. Glucosylceramides From Lomentospora prolificans Induce a Differential Production of Cytokines and Increases the Microbicidal Activity of Macrophages. Front. Microbiol. 2019, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Santos-Jiménez, J.L.; de Barros Montebianco, C.; Vidal, A.H.; Ribeiro, G.S.; Barreto-Bergter, E.; Vaslin, M.F.S. A fungal glycoprotein mitigates passion fruit woodiness disease caused by Cowpea aphid-borne mosaic virus (CABMV) in Passiflora edulis. Front. Microbiol. 2022, 67, 75–87. [Google Scholar] [CrossRef]
- Wang, N.; Liu, M.; Guo, L.; Yang, X.; Qiu, D. A Novel Protein Elicitor (PeBA1) from Bacillus amyloliquefaciens NC6 Induces Systemic Resistance in Tobacco. Int. J. Biol. Sci. 2016, 12, 757–767. [Google Scholar] [CrossRef]
- Kim, D.S.; Ryu, J.W.; Son, M.Y.; Oh, J.H.; Chung, K.S.; Lee, S.; Lee, J.J.; Ahn, J.H.; Min, J.S.; Ahn, J.; et al. A liver-specific gene expression panel predicts the differentiation status of in vitro hepatocyte models. Hepatology 2017, 66, 1662–1674. [Google Scholar] [CrossRef]
- Son, M.Y.; Jung, C.R.; Kim, D.S.; Cho, H.S. Comparative in silico profiling of epigenetic modifiers in human tissues. Mol. Biol. Rep. 2018, 45, 309–314. [Google Scholar] [CrossRef]
- Souto, A.; Cordeiro, M.; Rosado, L.; Santos, C.E.; Bruckner, C.H. Non-destructive estimation of leaf area in passion fruit (Passiflora edulis L.). Aust. J. Crop Sci. 2017, 11, 1534–1538. [Google Scholar] [CrossRef]
- Duarte, R.S.; Polycarpo, C.R.; Wait, R.; Hartmann, R.; Bergter, E.B. Structural characterization of neutral glycosphingolipids from Fusarium species. Biochim. Biophys. Acta 1998, 1390, 186–196. [Google Scholar] [CrossRef]
- Villas Boas, M.H.S.; Egge, H.; Pohlentz, G.; Hartmann, R.; Barreto Bergter, E. Structural determination of N-2′-hydroxyoctadecenoyl-1-O-β-d-glucopyranosyl-9-methyl-4,8-sphingadienine from species of Aspergillus. Chem. Phys. Lipids 1994, 70, 11–19. [Google Scholar] [CrossRef]
- Haq, I.U.; Calixto, R.O.d.R.; Yang, P.; dos Santos, G.M.P.; Barreto-Bergter, E.; van Elsas, J.D. Chemotaxis and adherence to fungal surfaces are key components of the behavioral response of Burkholderia terrae BS001 to two selected soil fungi. FEMS Microbiol. Ecol. 2016, 92, fiw164. [Google Scholar] [CrossRef] [PubMed]
- Rollin-Pinheiro, R.; Singh, A.; Barreto-Bergter, E.; Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 2016, 8, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Cahoon, E.B.; Thokala, M.; Kaur, J.; Li, J.; Shah, D.M. Sphingolipid C-9 methyltransferases are important for growth and virulence but not for sensitivity to antifungal plant defensins in Fusarium graminearum. Eukaryot Cell 2009, 8, 217–229. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, M.; Wang, W.; Ruan, R.; Ma, H.; Mao, C.; Li, H. Glucosylceramides are required for mycelial growth and full virulence in Penicillium digitatum. Biochem. Biophys. Res. Commun. 2014, 455, 165–171. [Google Scholar] [CrossRef]
- Caneppa, A.; de Meirelles, J.V.; Rollin-Pinheiro, R.; Dutra Xisto, M.; Liporagi-Lopes, L.C.; Souza, L.; Villela Romanos, M.T.; Barreto-Bergter, E. Structural Differences Influence Biological Properties of Glucosylceramides from Clinical and Environmental Isolates of Scedosporium aurantiacum and Pseudallescheria minutispora. J. Fungi 2019, 5, 62. [Google Scholar] [CrossRef]
- Pinto, M.R.; Rodrigues, M.L.; Travassos, L.R.; Haido, R.M.; Wait, R.; Barreto-Bergter, E. Characterization of glucosylceramides in Pseudallescheria boydii and their involvement in fungal differentiation. Glycobiology 2002, 12, 251–260. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Travassos, L.R.; Miranda, K.R.; Franzen, A.J.; Rozental, S.; de Souza, W.; Alviano, C.S.; Barreto-Bergter, E. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 2000, 68, 7049–7060. [Google Scholar] [CrossRef]
- Rochetti, V.P.; Rollin-Pinheiro, R.; de Oliveira, E.B.; Xisto, M.; Barreto-Bergter, E. Glucosylceramide Plays a Role in Fungal Germination, Lipid Raft Organization and Biofilm Adhesion of the Pathogenic Fungus Scedosporium aurantiacum. J. Fungi 2020, 6, 345. [Google Scholar] [CrossRef]
- Koga, J.; Yamauchi, T.; Shimura, M.; Ogawa, N.; Oshima, K.; Umemura, K.; Kikuchi, M.; Ogasawara, N. Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. J. Biol. Chem. 1998, 273, 31985–31991. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Ciani, S.; Schachtman, D.P. A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol. Plant 2010, 3, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Kim, Y.J.; Lee, S.C.; Hong, J.K.; Hwang, B.K. Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiol. 2007, 145, 890–904. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lazar, T.; Taiz, L.; Zeiger, E. Plant physiology. 3rd edn. Ann. Bot. 2003, 91, 750–751. [Google Scholar] [CrossRef]
- Boava, L.P.; Cristofani-Yaly, M.; Stuart, R.M.; Machado, M.A. Expression of defense-related genes in response to mechanical wounding and Phytophthora parasitica infection in Poncirus trifoliata and Citrus sunki. Physiol. Mol. Plant Pathol. 2011, 76, 119–125. [Google Scholar] [CrossRef]
- Orozco-Cárdenas, M.L.; Narváez-Vásquez, J.; Ryan, C.A. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 2001, 13, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Zeier, J. Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 2013, 4, 30. [Google Scholar] [CrossRef]
- Nimrichter, L.; Rodrigues, M.L. Fungal glucosylceramides: From structural components to biologically active targets of new antimicrobials. Front. Microbiol. 2011, 2, 212. [Google Scholar] [CrossRef]
- Sonnino, S.; Mauri, L.; Chigorno, V.; Prinetti, A. Gangliosides as components of lipid membrane domains. Glycobiology 2007, 17, 1r–13r. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xisto, M.I.D.d.S.; Bernardino, M.C.; Rollin-Pinheiro, R.; Montebianco, C.B.; da Silva, A.P.; Calixto, R.O.R.; Mattos, B.B.; Vaslin, M.F.S.; Barreto-Bergter, E. Glucosylceramides from Cladosporium and Their Roles in Fungi–Plant Interaction. Microbiol. Res. 2022, 13, 350-365. https://doi.org/10.3390/microbiolres13030028
Xisto MIDdS, Bernardino MC, Rollin-Pinheiro R, Montebianco CB, da Silva AP, Calixto ROR, Mattos BB, Vaslin MFS, Barreto-Bergter E. Glucosylceramides from Cladosporium and Their Roles in Fungi–Plant Interaction. Microbiology Research. 2022; 13(3):350-365. https://doi.org/10.3390/microbiolres13030028
Chicago/Turabian StyleXisto, Mariana Ingrid Dutra da Silva, Mariana Collodetti Bernardino, Rodrigo Rollin-Pinheiro, Caroline Barros Montebianco, Andrêina Paula da Silva, Renata Oliveira Rocha Calixto, Bianca Braz Mattos, Maite Freitas Silva Vaslin, and Eliana Barreto-Bergter. 2022. "Glucosylceramides from Cladosporium and Their Roles in Fungi–Plant Interaction" Microbiology Research 13, no. 3: 350-365. https://doi.org/10.3390/microbiolres13030028
APA StyleXisto, M. I. D. d. S., Bernardino, M. C., Rollin-Pinheiro, R., Montebianco, C. B., da Silva, A. P., Calixto, R. O. R., Mattos, B. B., Vaslin, M. F. S., & Barreto-Bergter, E. (2022). Glucosylceramides from Cladosporium and Their Roles in Fungi–Plant Interaction. Microbiology Research, 13(3), 350-365. https://doi.org/10.3390/microbiolres13030028






