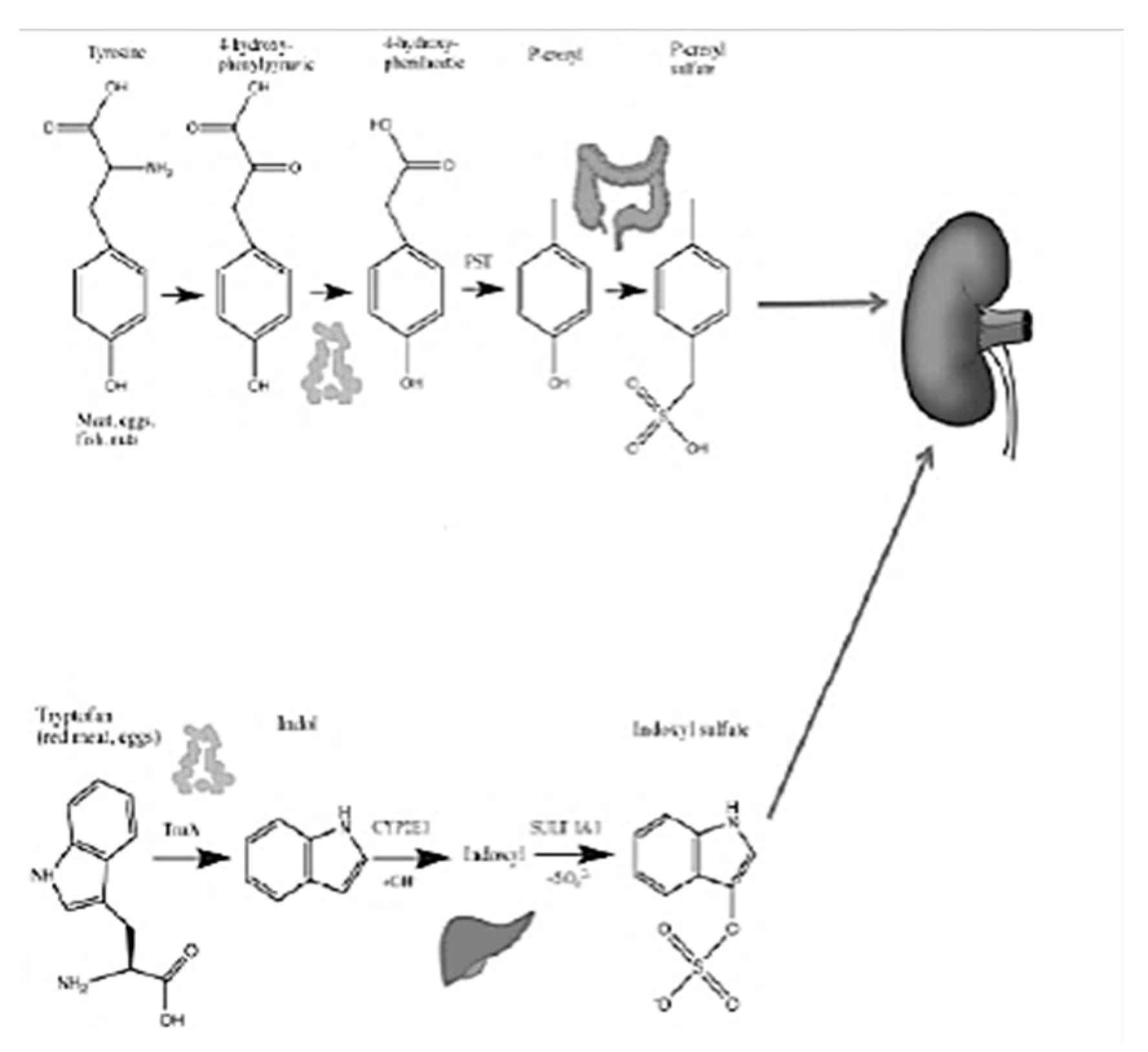
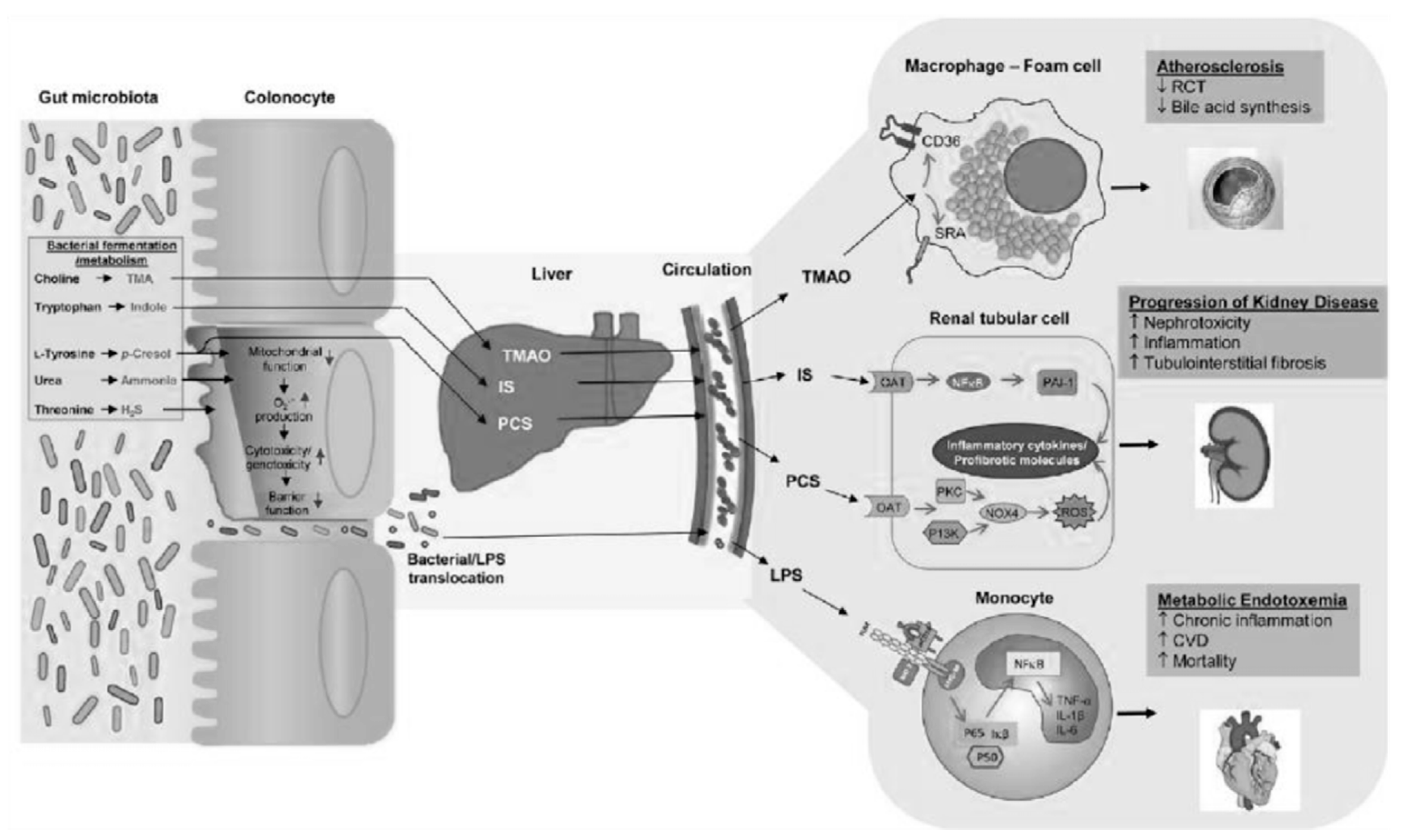
The relationship between microbiota and chronic kidney disease (CKD) has long been known [1]. With the progressive loss of its function, the kidney loses the ability to eliminate both substances from the human metabolism and those of its symbiote, the intestinal microbiota. Some of these substances are included in the category of uremic toxins: among those of intestinal derivation, the main and most studied are p-cresyl sulfate (PCS) and weaile sulphate (IS) (Figure 1).
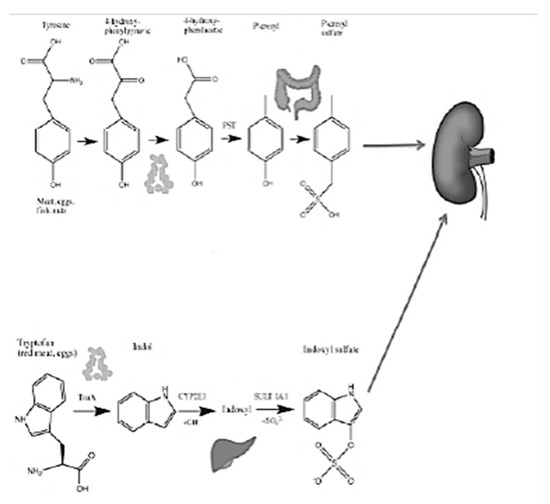
Figure 1.
PCS and IS (De Mauri, AOU della Carità, Novara, Italy).
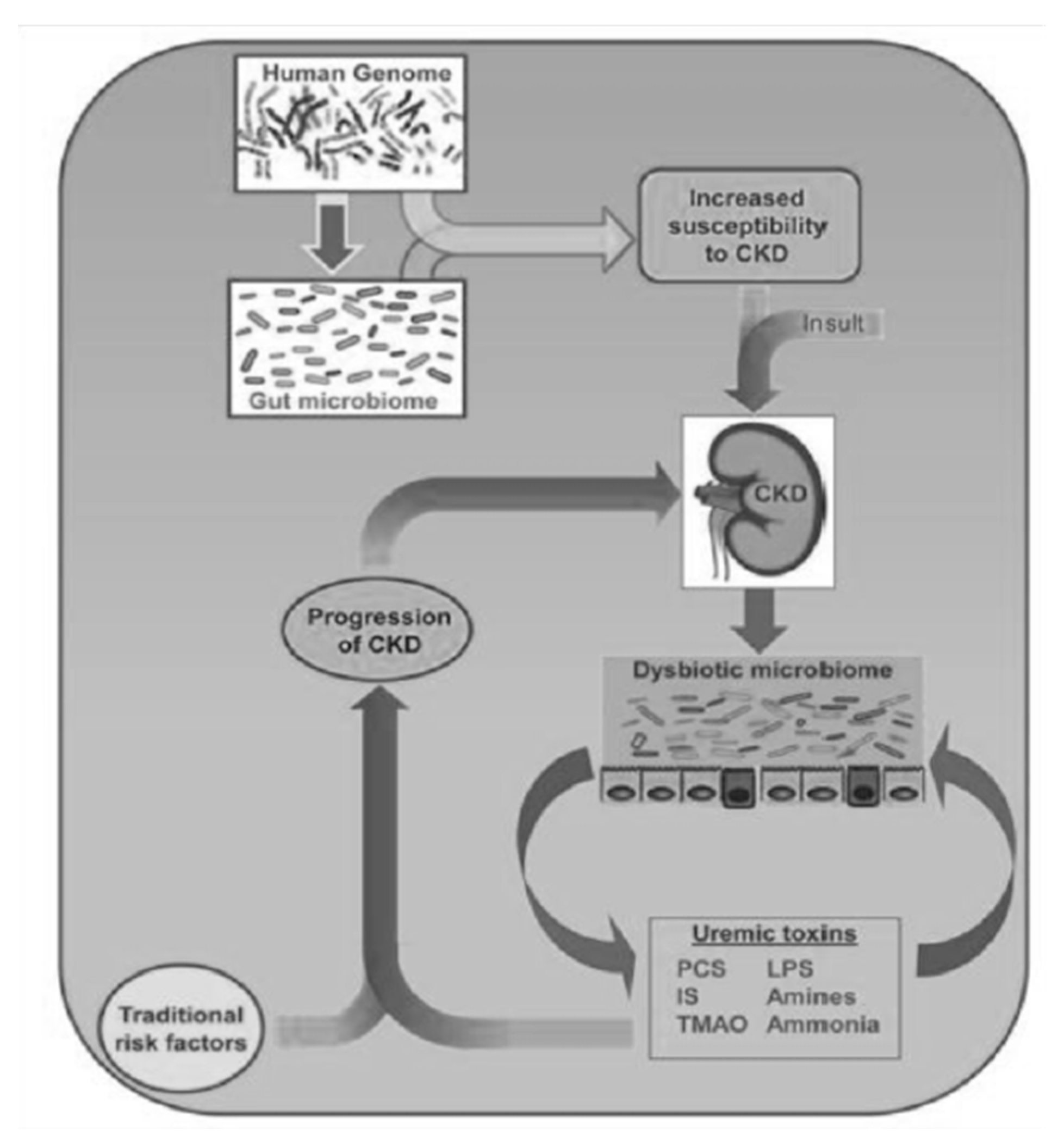
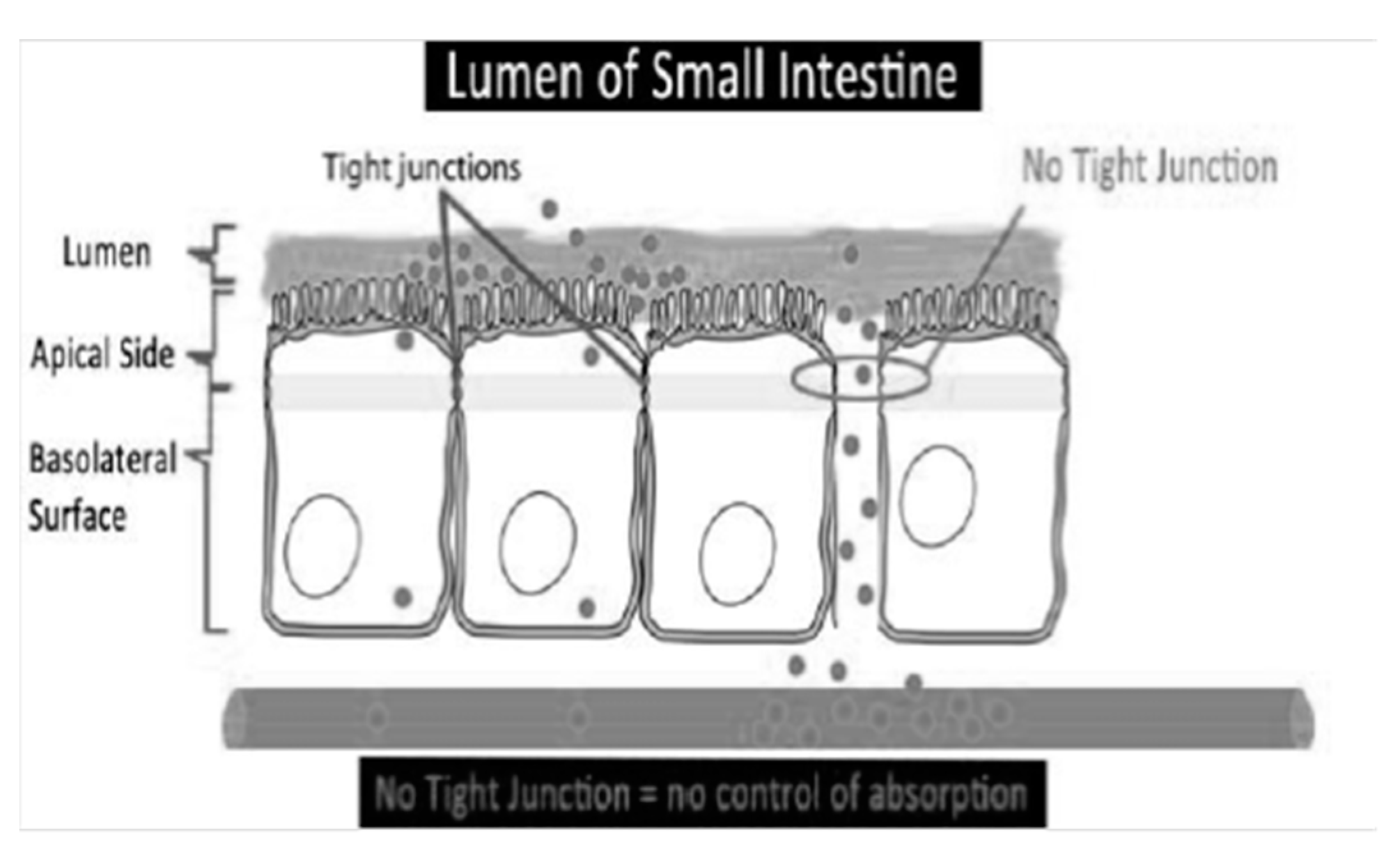
In addition to their toxicity in CKD, caused by their accumulation in the circulation, they have a strong greed for albumin, so they are not effectively eliminated using dialysis and contribute to the aggravation of uremic intoxication in dialysis patients. It is therefore clear that in nephrology, there is a great need not only to increase its dialysis clearance, but also to reduce its production. Here, the intestinal microbiota kicks in, the composition of which, in CKD patients, is completely different from that of healthy subjects [2]; this imbalance is called “dysbiosis” (Figure 2). The dysbiosis induced by uremia is attributable to a series of factors: with a decline in renal function, the colon assumes the role of an excretory organ, and the excretion of urea in the same organ changes the chemical microenvironment. The consequent increase in the pH of the colon exerts a selective pressure in favor of urease-positive species, which are responsible for the conversion of urea into ammonia. This causes the degradation of the protective mucus layer and an alteration of intestinal permeability due to the destruction of tight junctions (Figure 3).
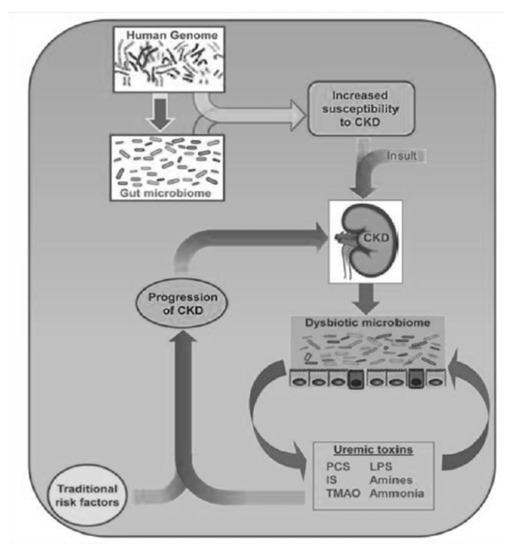
Figure 2.
Ramenzani et al.: Role of the gut microbiome in uremia: a potential therapeutic target—AJKD, 2016.
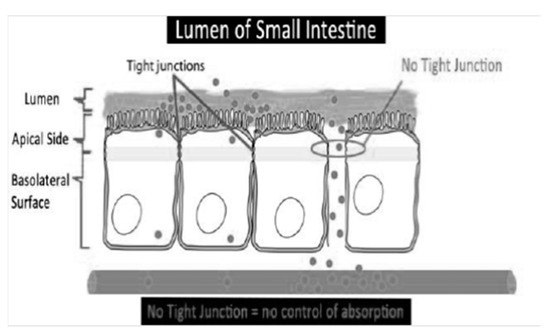
Figure 3.
Tight junctions (De Mauri, AOU della Carità, Novara, Italy).
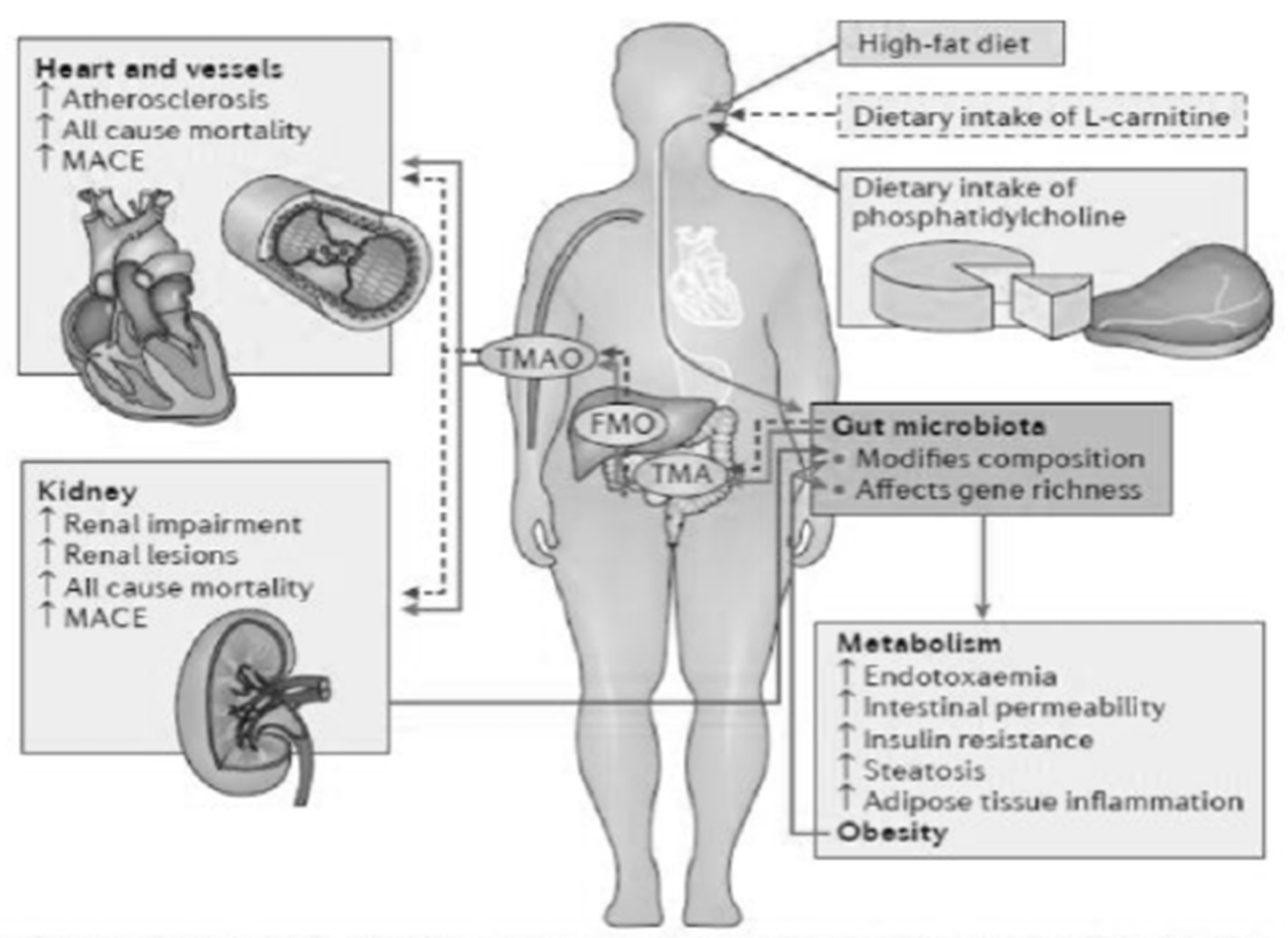
As a consequence of this, there is the passage of bacterial material through the mucosa and the activation of a local and systemic chronic inflammatory mechanism, due to the translocation of bacterial fragments in the circulation, as indicated by the blood presence of bacterial DNA of intestinal species, highlighted by some scientific studies [3,4]. Depending on the substrate that bacteria use to obtain energy, the microbiota can follow two main metabolic pathways, saccharolytic or proteolytic. The first pathway should prevail in a healthy intestine; however, in the event of food imbalances characterized by a lack of complex carbohydrates, the lack of substrate available for fermentation favors the imbalance towards the second pathway, in which bacteria use amino acids that do not have an anabolic function, but for energy purposes, with the consequent production of uremic toxins. Obviously, the balance between saccharolytic fermentation and proteolytic putrefaction should be in favor of the former, due to the different physiological effects of the metabolites downstream of the two pathways. In saccharolytic fermentation, the production of short chain fatty acids occurs, which, in addition to inhibiting the growth of pathobionts, are endowed with trophic action for the colon epithelium and local and systemic endocrine action (Figure 4). They are also characterized by anti-inflammatory activity, directly exercised both through signaling in some immune cells including neutrophils, and, thanks to induction (through epigenetic mechanisms), in the differentiation of T Reg lymphocytes with the activation of a tolerogenic phenotype, rather than proinflammatory. The anti-inflammatory action is also indirect, through the upregulation of tight junctions, a phenomenon that improves the functionality of the intestinal barrier with consequent systemic anti-inflammatory action. On the other hand, the products of protein putrefaction induce toxic effects: the most studied metabolites are PCS, IS and TMAO (trimethylamine N-oxide). The latter is a derivative of the catabolism of products essentially of animal origin, containing choline, phosphatidylcholine, carnitine and betaine.
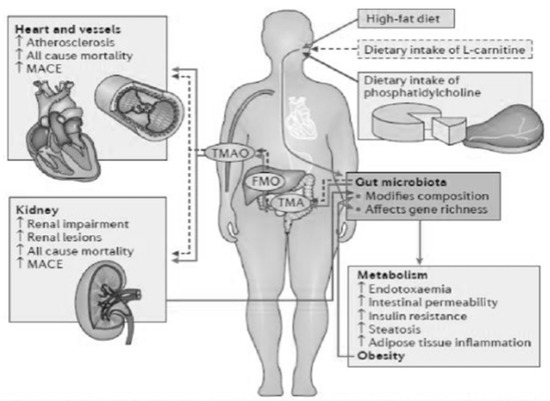
Figure 4.
Toward the comprehensive understanding of the gut ecosystem via metabolomics-based integrated omics approach, Wanping et al., Semin Immunopathol 2014.
PCS and IS which reach levels in CKD patients that are even a hundred times higher than those in healthy subjects, are derived from the degradation of aromatic amino acids, such as tryptophan, phenylalanine and tyrosine [5]. These substances are characterized by pro-inflammatory, pro-inflammatory and oxidative stress induction activity at the kidney level, but above all, at the cardiovascular level. To complicate matters, in CKD, due to the altered biochemical conditions of the colon already described, an expansion of the proteolytic bacterial populations and a reduction in the sucrarolytic protective populations occur, which favor a vicious circle of the increased production of intestinal uremic toxins [6]. Moreover, dysbiosis is worsened by the traditional nutritional management of nephropathic patients, especially those in more advanced stages, which involves a tight restriction of fiber intake, with further imbalance of the microbial metabolism in the proteolytic direction (Figure 1). The reduction in fiber intake in fact decreases the substrate for saccharolytic fermentation; fiber also favors intestinal transit, the slowdown of which constitutes a risk factor for the development of CKD, given that increased transit favors, among other things, the excretion of nitrogen compounds (Figure 5).
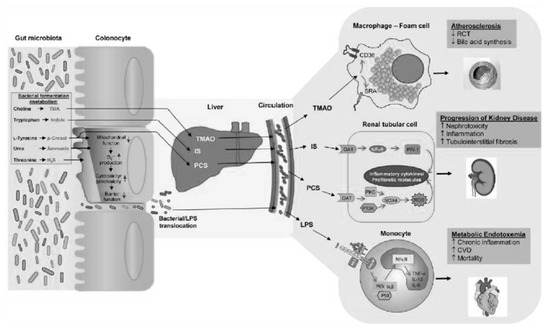
Figure 5.
Ramenzani et al.: Role of the gut microbiome in uremia: a potential therapeutic target-AJKD, 2016.
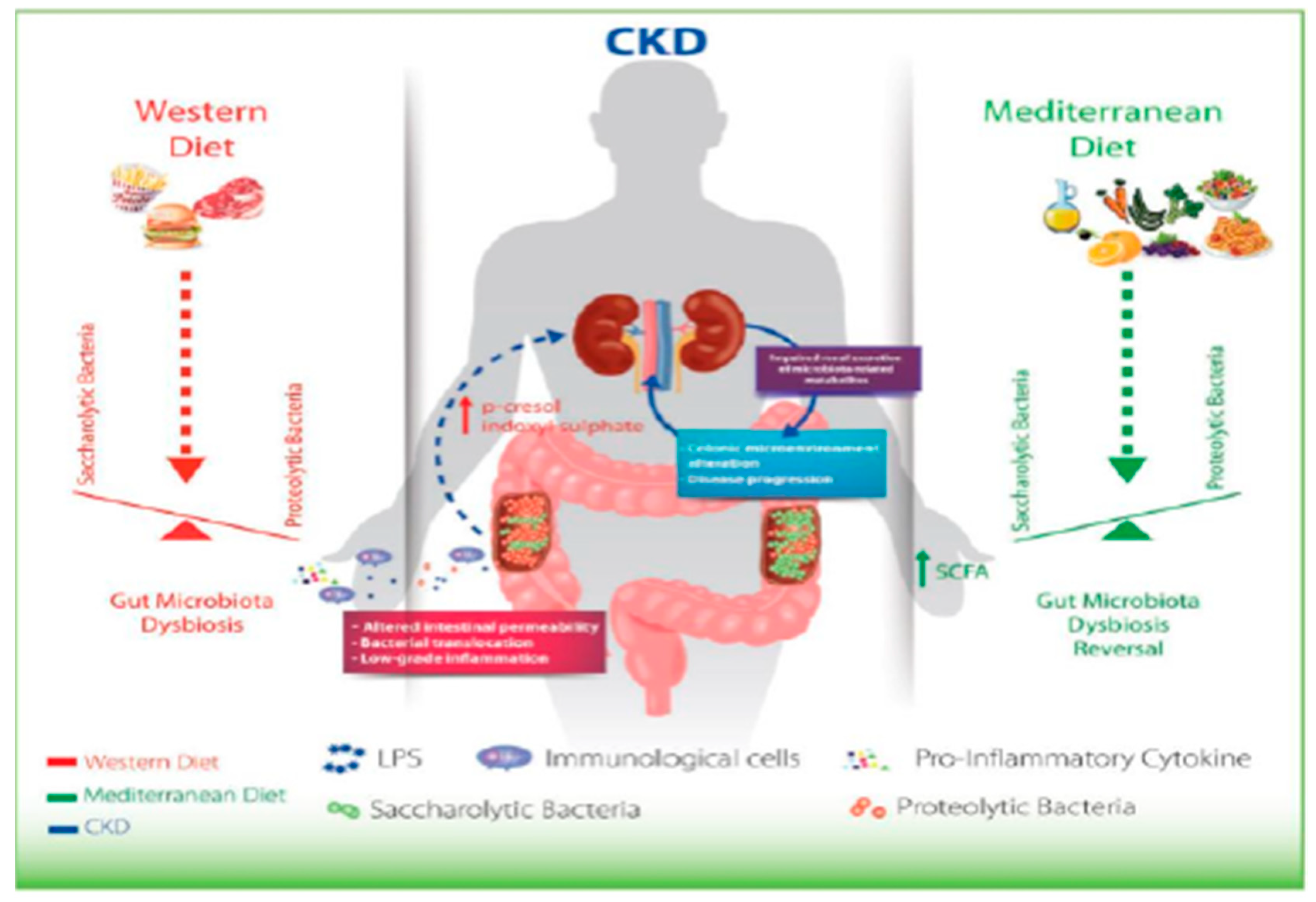
In CKD, the microbiota must be therefore considered a new, non-traditional risk factor, which can be changed due to, for example, to the inclusion of fiber in the diet, as in the case, for example, of the Mediterranean diet. (Figure 6).
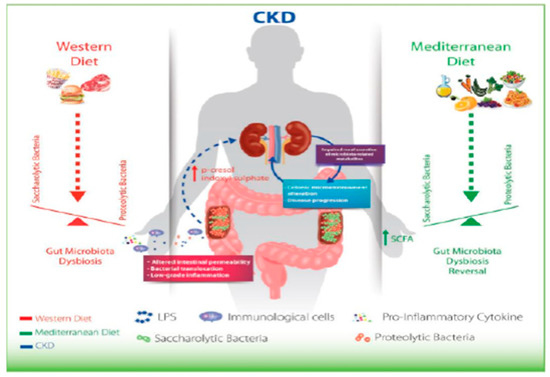
Figure 6.
Montemurro et al., KBPR 2014.
Lastly, although it is not traditionally considered to be optimal for nephropathic patients, a naturally prebiotic diet could be considered, which is capable of shifting the balance in a sucrolytic direction, and also contains nutraceutical compounds with antioxidant and anti-inflammatory properties [7]. In CKD, the ideal diet is represented by the restriction of proteins and salt and by a good intake of dietary fiber. A specialist dietician is responsible for customizing this diet based on the clinical and nutritional characteristics of each individual patient. In general, this type of dietetic–nutritional treatment has several potential benefits, confirmed by the scientific literature [8]: it contrasts intestinal dysbiosis, increases short chain fatty acids in the colon, reduces the intestinal permeability, has greater alkalizing power, decreases the production of uremic toxins, improves the intestinal transit, and has also beneficial effects in terms of reductions in creatininemia and azotemia.
There is a correlation between the levels of intestinal uremic toxins and the progression of CKD towards dialysis. The hope is that, with proper nutritional management, combined where necessary with symbiotic integration, the progression of the disease can be slowed down, with significant positive effects on the quality of life and life expectancy of nephropathic patients and effects on the reduction in health costs associated with dialysis treatment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cosola, C. Ruolo Del Microbiota Nelle Malattie Renali. In Microbioma Microbiota: Ricerca & Clinica; Supplemento al n1/2018; Pacini Editore S.r.l.: Pisa, Italy, 2018. [Google Scholar]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, H.J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Jiang, H.; Shi, K.; Ren, Y.; Zhang, P.; Cheng, S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton) 2012, 17, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Funk, B.A.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J. Am. Soc. Nephrol. 2014, 25, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the gut microbiome in uremia: A potential therapeutic target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montemurno, E.; Cosola, C.; Dalfino, G.; Daidone, G.; de angelis, M.; Gobbetti, M.; Gesualdo, L. A Mediterranean Diet, please! Kidney Blood Press Res. 2014, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Gesualdo, L.; Cosola, C.; Gallieni, M.; Egidi, M.F.; Fusaro, M. Non-traditional aspects of renal diets: Focus on fiber, alkali and vitamin K1 intake. Nutrients 2017, 9, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).