Abstract
During the last ten years, the worldwide interest in using insects as food and feed has surged. Edible insects fall within the category of novel foods, i.e., the category of food not consumed in significant amounts in the European Union before 15 May 1997 (the date of entry into force of Regulation (EC) No. 258/1997, later repealed by Regulation (EU) No. 2283/2015). One of the most promising insect species to be raised for food is the house cricket (Acheta domesticus). In this study, the rearing of a stock of house crickets was studied over a period of four months. The microbiological quality of the farm was studied using swabs on the surface of the rearing boxes to analyze the trend over time of different populations of microorganisms (total aerobic mesophilic microbiota, Lactobacillus spp., enterococci, Staphylococcus spp., Enterobacteriaceae, total coliforms, Pseudomonas spp. and molds). The presence of four antimicrobial resistance genes (aph, blaZ, sul1, and tetM) was investigated by polymerase chain reaction. A production scheme was also developed in order to obtain a cricket-based flour, which was analyzed for its microbiological and chemical-centesimal profile. The results obtained in this study demonstrate that the contamination increases with time and that a proper management of the farming system for insects is of the utmost importance, as it is for conventional farm animals such as ungulates, poultry, and rabbits. The old-fashioned adage “all full, all empty” for the farming system summarizes the need for proper cleaning and disinfection of the structures at the end of each production cycle.
1. Introduction
In the face of the continual evolution of society, food habits and the variety of tastes commonly available are frequently replaced by innovative products or products from foreign cultures on the consumer’s table. Considering both the increase in population expected within 2050, reaching 9 billion people, and the parallel increase in the demand for proteins of animal origin, new protein sources have been suggested, including edible insects [1].
Edible insects fall within the category of novel foods, i.e., the category of food not consumed in significant amounts in the European Union before 15 May 1997 (the date of entry into force of Regulation (EC) No. 258/1997, later repealed by Regulation (EU) No. 2283/2015) [2]. A risk analysis is conducted for each novel food entering the European market and each new product or ingredient is subjected to the scientific opinion of EFSA (European Food Safety Authority) and requires formal authorization from the European Commission. These unconventional foods also have to guarantee they comply with the parameters of health and hygiene, safety, and quality, even in the absence of specific legislation.
The practice of consuming insects (or arthropods) is known as entomophagy, a term that first appeared in the English language in 1871. Although this is a little-known practice in western countries [3], it is familiar for over two billion people in the world. There is a list of more of 2000 edible species, which has been updated by Wageningen University and is continually on the increase [4]. Since 2012, the Food and Agriculture Organisation uses the term to focus attention on this topic, although the large variety of characteristics and species involved lead us to believe more specific terms will be coined in future. In some parts of the world, they are considered a natural source of sustenance and are naturally harvested in some places (e.g., Cambodia), whereas in others they constitute a delicacy (e.g., the chapulines—fried grasshoppers in Mexico) and some countries in Europe currently dispense them in the form of protein bars or in Michelin starred restaurants. The consumer is often unaware he is eating insects, as in the case of the colorant (E120) derived from Dactylopius coccus or cochineal.
So far, applications to EFSA for the following species have been submitted: Hermetia illucens (black soldier fly), Alphitobius diaperinus (lesser mealworm larvae), Acheta domesticus (house cricket), Gryllodes sigillatus (cricket), Locusta migratoria (locust), and Tenebrio molitor (yellow mealworm). Recently, Tenebrio molitor larvae have been considered by EFSA as fit for human consumption [5]. EFSA has also produced a scientific opinion on the risk profile related to the production and consumption of insects as food and feed [6]. The opinion, in the form of a risk profile, presents potential biological and chemical hazards as well as allergenicity and environmental hazards associated with farmed insects used as food and feed taking into account the entire chain, from farming to the final product.
From a nutritional point of view, insects represent an interesting source of nutrients such as vitamins, mineral salts, and proteins in particular [7,8]. Insects are also considered to have excellent food conversion rates (how much feed must be provided for each kg of food produced), higher relative growth, and lower greenhouse gas emissions when compared with pigs and cattle [9,10].
As a result, interest in the possibility of breeding insects for food has increased in recent years. The purpose of this study was to study a prototype of a cricket (Acheta domesticus) farm for food purposes, with particular attention to the microbiological characteristics of the rearing environment, the presence of genes encoding for antibiotic resistance and the characteristics of the final product (cricket flour). Considerations have been made regarding animal welfare, in particular, to avoid overpopulation and consequent cannibalism phenomena and to avoid suffering at the time of slaughter.
2. Materials and Methods
2.1. Crickets Rearing
The rearing of a stock of house crickets (Acheta domesticus) was studied over a period of four months. The rearing temperature was kept constant at around 26 °C in a conditioned chamber. Temperature and humidity during the entire period were recorded using a data-logger. The crickets (500 adults per box) were reared in plastic containers of 70 cm × 40 cm × 40 cm equipped with a special lid to prevent the escape of the insects. Considering a crawl space of 2800 cm2 in each box, we had 1 cricket per 5.6 cm2. This density of population was chosen to avoid overpopulation and consequent cannibalism phenomena that greatly increase when crowding exceeds 1 cricket per 2.5 cm2 [11]. Peat was used both as litter and for the ovipositional trays. The diet consisted of bran, vegetables, and fresh fruit (mainly apple). Water was provided via soaked sponges changed every other day. Eggs were collected once a week and the ovipositional trays (with 3–4 cm of peat) were kept at a temperature of 30 °C, and the humidity was adjusted between 70–75% twice a day using a water vaporizer. Eggs hatched in two weeks. Nymphs were reared apart for about two weeks (the amount of time needed to complete the first three molts and to reach a length of 1.1–1.3 cm). Then, they were moved to the boxes with the other subjects. The number of molts in the course of development varied from 6 to 12 (about 3-day intervals between each other).
2.2. Microbiological Analysis
Once a week, sterile swabs were used to collect samples from a total of nine rearing boxes on a surface of 100 cm2 each. Samples were transported to the laboratory in a refrigerated container. Tenfold dilutions were prepared in sterile tubes with 9 mL of Maximum Recovery Diluent (MRD, Oxoid, Basingstoke, Hampshire, UK). Dilutions were inoculated in triplicate on different culture media. The total aerobic and mesophilic microbiota was determined on Plate Count Agar (PCA; Oxoid) at 30 °C for 72 h; Lactobacillus spp. on Man, Rogosa and Sharpe Agar (MRS; Oxoid) pH 5.5, at 30 °C for 72 h under anaerobic conditions (Gas generating kit, Oxoid); enterococci on enterococcus agar (ENT; Oxoid), at 37 °C for 48 h; Staphylococcus spp. on Baird Parker agar (BP; Oxoid) containing Egg Yolk Tellurite (Oxoid) at 37 °C for 48 h; Enterobacteriaceae on violet red bile glucose agar (VRBG; Oxoid) at 37 °C for 24 h; total coliforms on violet red bile lactose agar (VRBL; Oxoid) at 37 °C for 24 h; Pseudomonas spp. on pseudomonas agar base (PS103; Oxoid) at 37 °C for 24 h; molds on Chloramphenicol Yeast Glucose Agar (CYG; HiMedia) at 25 °C for 72 h. The colonies were then counted on all the plates, using a colony count viewer (Petri light, PBI, Milan) and colony counter pen (Colony Count, PBI, Milan). All values were converted into logs and the arithmetic mean was calculated for each sampling. Samples were then divided into two groups: samples from day 0 to day 28 were grouped in the “first cycle” group, while samples from day 42 to day 63 were grouped in the “second cycle” group. This division was chosen because the period between day 28 and day 42 corresponds to the addition of the young crickets to the rearing boxes. Statistical analyses were performed with StatView 5.0.1 for Mac OS (SAS Institute, Inc., Cary, NC, USA). Unpaired comparison by unpaired t-test was performed to determine if the likelihood of observed differences between the two groups (bacterial counts for the first cycle, and bacterial counts for the second cycle) occurred by chance. The chances are reported as p values which are given in the box plots for each microbial group.
2.3. Antimicrobial Resistance Genes Research by PCR
Naturally dead crickets were taken from the nine different rearing boxes to check for the presence of antimicrobial resistance genes. Samples were frozen in liquid nitrogen and ground in order to obtain a homogeneous pulverized pool. The HipurA™ Insect DNA Purification Kit from the HiMedia company (Mumbai, India) was used to extract the DNA. The quantification of the extracted genetic material was performed using the NanoDrop™ Lite spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA) with 1 μL of sample. The DNA amplification was conducted on a volume of 25 μL using 12.5 μL of RED Taq (10 mM Tris HCl, pH 8.3, 50 mM KCl, 1.5 mMMg Cl2, 0.001% of gelatin, 0.2 m Meach of deoxyribonucleoside triphosphate), 0.5 μL (1 μM) of each primer, 5 μL of extracted DNA and 6.5 μL of H2O. The PCR reaction was carried out in a thermocycler Gene Amp, PCR System, 9700 Gold (Applied Biosystems, Foster City, CA, USA). The primers and the amplification conditions used are listed in Table 1. The amplifications were analyzed by an electrophoretic run on 1.5% agarose gel containing ethidium bromide (0.5 μg/mL); 10 μL of each PCR sample was loaded with 2 μL of 6× loading buffer (Fermentas-VWR-Italy) and 5 μL of marker PCR as reference DNA (Fermentas-VWR-Italy); the run was carried out at a voltage of 100 V for about 1 h in TBE 10× (Trizma base, boric acid, EDTA 0.5 MpH 8). At the end of the run, the bands were viewed with the UV transilluminator (Fotodine 3–3102 Celbio, Milan, Italy).

Table 1.
Primers and the amplification conditions used.
2.4. Flour Production
The following production scheme has been developed in order to obtain a flour-based on crickets: 24 h before the start of the production process, the food is removed from the rearing boxes in such a way as to facilitate insect purging. After this period, crickets were collected from the various boxes and transported to the laboratory. They were put into a bag that was seal closed and placed in the freezer at −20 °C for 24 h. Insects are ectothermic, which means that in cold temperatures, their metabolism slows down until death. The insects go into a cold-induced coma from which they do not recover, so there is no violent death or change in state [16]. The first step to be carried out in the laboratory is washing. The crickets undergo three washes in running water. Then, they are weighed and pasteurized in boiling water for about 5 min. This step is essential to decrease the microbial load present on the surface of the insects. Once the pasteurization time has elapsed, they are placed in a dryer (for the tests carried out, a dryer that worked at a temperature of 55 °C was used), distributed evenly over the entire surface of the plate, overnight. After the time necessary to obtain an adequate weight loss, all parts of the cricket were ground into flour using a mortar and pestle. The flour obtained was dark green/straw yellow in color, with a sweet smell, similar to hazelnut but slightly acrid, was packaged in conditions of absolute sterility with the aim of subsequently subjecting it to microbiological and chemical-centesimal analyses.
3. Results
The temperature and relative humidity of the rearing room during the entire period were recorded by the chamber data-logger and are reported in Figure 1. The mean temperature was 27.7 ± 2.4 °C and the mean relative humidity was 45 ± 8.5%.
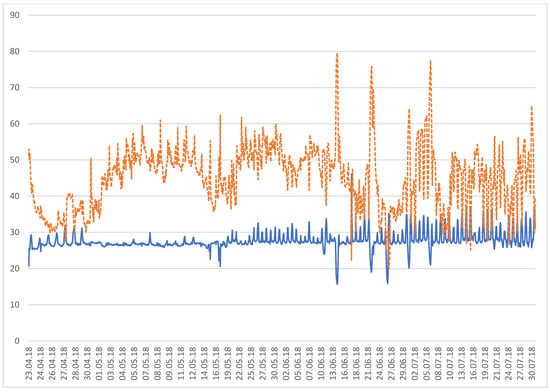
Figure 1.
Temperature (°C, continuous line) and relative humidity (%, dashed line) of the rearing room.
The trend over time of the means calculated for the different bacterial populations (expressed as log cfu/cm2) in the rearing boxes is shown in Table 2. The total aerobic mesophilic count was 4.44 ± 1.14 cfu/cm2 at t0 and reached 5.38 ± 0.28 cfu/cm2 at t63, with its maximum at t49 with a concentration of 5.61 ± 1.06 cfu/cm2. Pseudomonas spp. count was 3.63 ± 1.44 cfu/cm2 at t0 and reached 3.71 ± 0.89 cfu/cm2 at t63, with its maximum at t49 with a concentration of 4.92 ± 0.45 cfu/cm2. The Enterobacteriaceae count was 3.49 ± 0.64 cfu/cm2 at t0 and reached 4.68 ± 0.33 cfu/cm2 at t63, with its maximum at t49 with a concentration of 5.32 ± 0.55 cfu/cm2. The total coliforms count was 3.56 ± 0.66 cfu/cm2 at t0 and reached 4.38 ± 0.81 cfu/cm2 at t63, with its maximum at t49 with a concentration of 4.66 ± 0.51 cfu/cm2. The enterococci count was 4.28 ± 0.82 cfu/cm2 at t0 and reached 4.60 ± 0.68 cfu/cm2 at t56, with its maximum at t28 with a concentration of 5.49 ± 0.34 cfu/cm2. Lactobacillus spp. count was 4.70 ± 0.97 cfu/cm2 at t0 and reached 5.08 ± 0.53 cfu/cm2 at t63, with its maximum at t49 with a concentration of 5.86 ± 1.12 cfu/cm2. Staphylococcus spp. count was 3.74 ± 0.99 cfu/cm2 at t0 and reached 4.77 ± 0.46 cfu/cm2 at t63, which was also its maximum concentration. The mold count was 2.06 ± 1.24 cfu/cm2 at t0 and reached 3.14 ± 0.32 cfu/cm2 at t63, which was also its maximum concentration. p-values for total aerobic mesophilic microbiota, lactobacilli, Enterobacteriaceae, total coliforms, staphylococci, and enterococci showed that counts were significantly higher in the second cycle (Figure 2, Figure 3 and Figure 4).

Table 2.
The trend over time of the means calculated for the different bacterial populations (expressed as log cfu/cm2) on the surface of the rearing boxes.
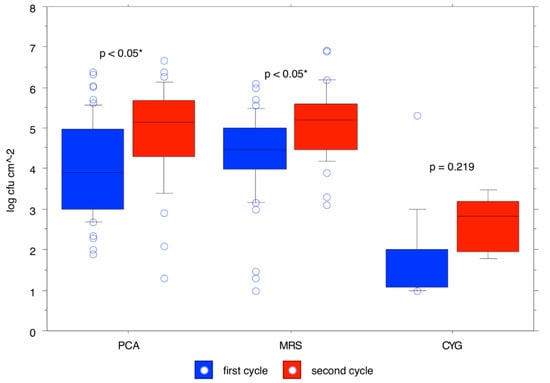
Figure 2.
The likelihood of observed differences between bacterial counts for the first cycle and bacterial counts for the second cycle for total aerobic mesophilic microbiota, Lactobacillus spp. and molds.
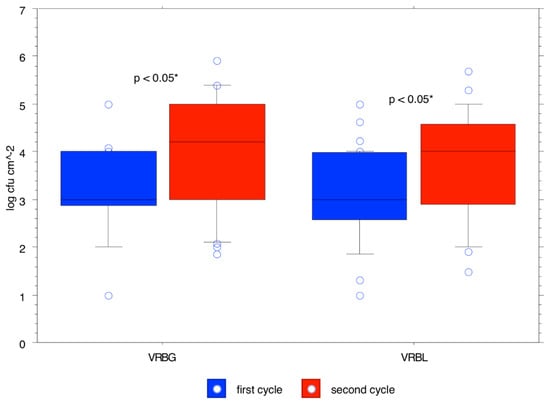
Figure 3.
Likelihood of observed differences between bacterial counts for the first cycle and bacterial counts for the second cycle for Enterobacteriaceae and total coliforms.
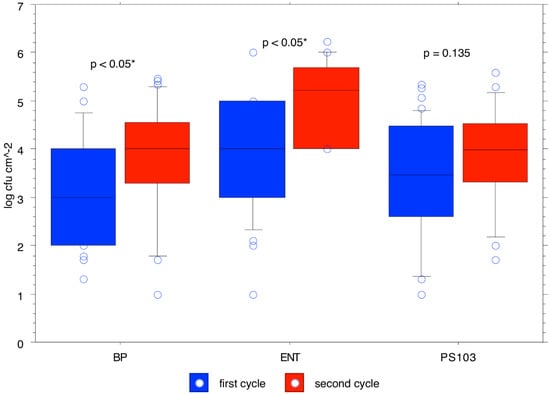
Figure 4.
Likelihood of observed differences between bacterial counts for the first cycle and bacterial counts for the second cycle for Staphylococcus spp., enterococci, and Pseudomonas spp.
The research by PCR of the genes that encode for antimicrobial resistance showed no positives for aph, blaZ, and sul1. Instead, four out of nine samples were positive for the presence of the tetM gene.
The chemical-centesimal analyses of the flour showed the following results: carbohydrates 9.5 g/100 g, ashes 3.1 g/100 g, dietary fiber <0.5 g/100 g, total fat 15 g/100 g, saturated fats 4.4 g/100 g, proteins 58.9 g/100 g, NaCl 0.61 g/100 g, humidity 13.5%, and energy value 408.6 kcal/100 g. The microbiological analysis of the flour did not highlight the presence of bacterial populations of interest within the detection limits of the methodology used.
4. Discussion
Currently, European legislation does not provide specific microbiological criteria for whole insects or insect products for human consumption. We decided to use the bacterial load of the surface as a process hygiene criterion and the bacterial load of the cricket flour as a food safety criterion. Some authors suggested using the total aerobic bacterial count provided by the European Commission Regulation (EC) No. 2073/2005 [17] for ground beef as a guideline for food safety and final product hygiene values [18]. The total aerobic bacterial counts for crickets reported in the literature vary in a range from 104 cfu/g to 108 cfu/g. This high variability reflects the differences in the protocol used for rearing and processing the insects before the transformation in the final product [19]. Considering that the whole animals are used to produce food, including their gut, a common practice also applied by the authors in the present work was to decrease the microbial loads by applying fasting 24–48 h before the kill step. If not applied, the microbial load for whole crickets reported in the literature is much higher (up to 1012 cfu/g) [20] if confronted with the limits provided by the law. In this study, we decided to focus on the microbial load of the surfaces of the rearing boxes. If compared with the limits provided by the EC Regulation 2073/2005 for the total aerobic count and Enterobacteriaceae count on the surface of the carcasses of different species, the results obtained in this study are interesting. The total aerobic count was lower than the upper limit provided (5.0 log cfu/cm2) in seven out of nine of the time analyzed. The level of contamination was higher for Enterobacteriaceae, which were compliant only in four out of nine cases. Results obtained during this study confirm the high microbial diversity in crickets, as reported by other authors [21,22]. The presence of fungal species (molds in particular) has been reported both by breeders in insect-farming facilities and in rearing experiments at the Swedish University of Agricultural Sciences (SLU) without involving any major mortality [23]. Other authors reported that yeast and mold counts for crickets were above the Good Manufacturing Practice (GMP) limits for raw meat [18,19]. The statistical analysis demonstrated that counts were significantly higher in the second cycle for total aerobic mesophilic microbiota, lactobacilli, Enterobacteriaceae, total coliforms, staphylococci, and enterococci if compared to the first cycle. This is due to the continued presence of the animals (of all ages and growth stages) in the boxes themselves. This suggests that an “all full, all empty” approach with disinfection of the rearing environment is advisable to avoid excessive and unwanted increases in the bacterial load.
Many insect species are known as vectors for bacteria that bring genes encoding for antimicrobial resistance [24,25]. Our results are consistent with a study by Milanovic et al. [26], which investigated the presence of antimicrobial resistance genes in edible insects by using both classic- and nested-polymerase chain reaction and reported the presence of tetracycline resistance genes in cricket samples.
Regarding the hygienic characteristics of the cricket flour produced as described above, at a legislative level, there are no specific items that provide precise parameters. If we compare the results obtained in this study with the parameters provided by the regulations for cereal and mixed flours, we can note that the values of Enterobacteriaceae (which must be less than 1000 cfu/g) and Salmonella (absent in 25 g) are widely respected. Likewise, cricket flour met the parameters set for powdered milk and powdered whey. The fact that the microbiological analyses carried out on cricket flour showed that the bacterial populations was reduced by pasteurization below the detection limit of the method used suggests that this product does not present any harm for food safety if correctly handled and stored.
5. Conclusions
During the last ten years, the worldwide interest in using insects as food and feed surged. One of the most promising insect species to be raised for food is the house cricket (Acheta domesticus). Our study is, to the best of our knowledge, the first organic approach for the definition of (i) environmental contamination level for farmed insects, (ii) insect load for animal welfare and prevention of cannibalism and contamination, and (iii) animal stunning for animal welfare consideration.
Our study, therefore, proved that the contamination increases with time and that proper management of the farming system for insects is of the utmost importance, as it is for conventional farm animals such as ungulates, poultry, and rabbits. The old-fashioned adage “all full, all empty” for the farming system summarizes the need for proper cleaning and disinfection of the structures at the end of each production cycle. Moreover, now that insects have been authorized for sale in many countries, it is mandatory to have available data and know-how for food safety of insects. In Europe, Tenebrio molitor larvae have been considered by EFSA as fit for human consumption [5].
In recent years there has been a plethora of papers describing microbiota, and microbial diversity of edible insects by metagenomic sequencing [27,28,29]. These works, while interesting from a zoological and entomologic point of view, are deceiving when used as tools for food safety. Insects can carry over the environmental contamination and act either as a reservoir or, very probably, as vectors for human pathogens. We do strongly believe that the correct approach for the definition of food safety standard of insects for human consumption is similar to any HACCP (Hazard Analysis and Critical Control Point), GMP (Good Manufacturing), and the more recent HARPC (Hazard Analysis and Risk-based Preventive Controls) introduced by the US FDA (Food and Drug Administration) with the FSMA (Food Safety Modernization Act).
Author Contributions
Conceptualization, B.T.C.-G. and L.G.; methodology, B.T.C.-G. and L.G.; formal analysis, B.T.C.-G., L.G., and S.B.; writing—original draft preparation, L.G., B.T.C.-G., and A.C.; writing—review and editing, L.G., B.T.C.-G., M.K., and S.E.-A.; supervision, C.M.S. and J.G.-D.; project administration, B.T.C.-G.; funding acquisition, B.T.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EFSA (European Food Safety Authority), grant number GP/EFSA/ENCO/2018/05_GA6.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations (FAO). Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013. [Google Scholar]
- Anonymous. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods; 2015. [Google Scholar]
- House, J. Consumer acceptance of insect-based foods in the Netherlands: Academic and commercial implications. Appetite 2016, 107, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Jongema, Y. List of Edible Insects of the World; Laboratory of Entomology, Wageningen University: Wageningen, The Netherlands,, 2017; Available online: https://www.wur.nl/upload_mm/8/a/6/0fdfc700–3929–4a74–8b69-f02fd35a1696_Worldwide%20list%20of%20edible%20insects%202017.pdf (accessed on 14 June 2021).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Allergens, F.; Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- Efsa Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- Wang, D.; Bai, Y.-Y.; Li, J.-H.; Zhang, C.-X. Nutritional value of the field cricket (gryllus testaceus walker). Insect Sci. 2004, 11, 275–283. [Google Scholar] [CrossRef]
- Raheem, D.; Raposo, A.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Carrascosa, C. Entomophagy: Nutritional, ecological, safety and legislation aspects. Food Res. Int. 2019, 126, 108672. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans—A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef]
- Patton, R.L. Growth and Development Parameters for Acheta domesticus1,2. Ann. Entomol. Soc. Am. 1978, 71, 40–42. [Google Scholar] [CrossRef]
- Kao, S.J.; You, I.; Clewell, D.B.; Donabedian, S.M.; Zervos, M.J.; Petrin, J.; Shaw, K.J.; Chow, J.W. Detection of the high-level aminoglycoside resistance gene aph(2′′)-Ib in Enterococcus faecium. Antimicrob. Agents Chemother. 2000, 44, 2876–2879. [Google Scholar] [CrossRef]
- Martineau, F.; Picard, F.J.; Grenier, L.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Multiplex PCR assays for the detection of clinically relevant antibiotic resistance genes in staphylococci isolated from patients infected after cardiac surgery. The ESPRIT Trial. J. Antimicrob. Chemother. 2000, 46, 527–534. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Crotti, S.; Costarelli, S.; Rondini, C.; Karama, M.; Bennett, P. Detection of tet(M) gene from raw milk by rapid DNA extraction followed by a two-step PCR with nested primers. J. Food Prot. 2004, 67, 2833–2838. [Google Scholar] [CrossRef]
- Kozak, G.K.; Boerlin, P.; Janecko, N.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009, 75, 559–566. [Google Scholar] [CrossRef]
- Gjerris, M.; Gamborg, C.; Röcklinsberg, H. Ethical aspects of insect production for food and feed. J. Insects Food Feed 2016, 2, 101–110. [Google Scholar] [CrossRef]
- Anonymous. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; 2005. [Google Scholar]
- Caparros Megido, R.; Desmedt, S.; Blecker, C.; Béra, F.; Haubruge, É.; Alabi, T.; Francis, F. Microbiological Load of Edible Insects Found in Belgium. Insects 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, e16082. [Google Scholar] [CrossRef] [PubMed]
- Cazemier, A.E.; Hackstein, J.H.P.; Op den Camp, H.J.M.; Rosenberg, J.; van der Drift, C. Bacteria in the Intestinal Tract of Different Species of Arthropods. Microb. Ecol. 1997, 33, 189–197. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Metagenetic analysis of the bacterial communities of edible insects from diverse production cycles at industrial rearing companies. Int. J. Food Microbiol. 2017, 261, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; Wynants, E.; Crauwels, S.; Verreth, C.; Viaene, N.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial Dynamics during Industrial Rearing, Processing, and Storage of Tropical House Crickets (Gryllodes sigillatus) for Human Consumption. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Boomsma, J.J.; Jensen, A.B.; Meyling, N.V.; Eilenberg, J. Evolutionary interaction networks of insect pathogenic fungi. Annu. Rev. Entomol. 2014, 59, 467–485. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Sechi, P.; Karama, M.; Ciavarella, R.; Pipistrelli, M.V.; Goretti, E.; Elia, A.C.; Gardi, T.; Pallottini, M.; Rossi, R.; et al. Cross-sectional study to identify risk factors associated with the occurrence of antimicrobial resistance genes in honey bees Apis mellifera) in Umbria, Central Italy. Environ. Sci. Pollut. Res. Int. 2020, 27, 9637–9645. [Google Scholar] [CrossRef] [PubMed]
- Mariano, V.; McCrindle, C.M.; Cenci-Goga, B.; Picard, J.A. Case-control study to determine whether river water can spread tetracycline resistance to unexposed impala (Aepyceros melampus) in Kruger National Park (South Africa). Appl. Environ. Microbiol. 2009, 75, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Milanović, V.; Osimani, A.; Pasquini, M.; Aquilanti, L.; Garofalo, C.; Taccari, M.; Cardinali, F.; Riolo, P.; Clementi, F. Getting insight into the prevalence of antibiotic resistance genes in specimens of marketed edible insects. Int. J. Food Microbiol. 2016, 227, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Clementi, F. The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol. 2017, 62, 15–22. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Garofalo, C.; Cardinali, F.; Roncolini, A.; Sabbatini, R.; De Filippis, F.; Ercolini, D.; Gabucci, C.; Petruzzelli, A.; et al. Revealing the microbiota of marketed edible insects through PCR-DGGE, metagenomic sequencing and real-time PCR. Int. J. Food Microbiol. 2018, 276, 54–62. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).