Clean to Prevent, Monitor to Protect: A Scoping Review on Strategies for Monitoring Cleaning in Hospitals to Prevent HAIs
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
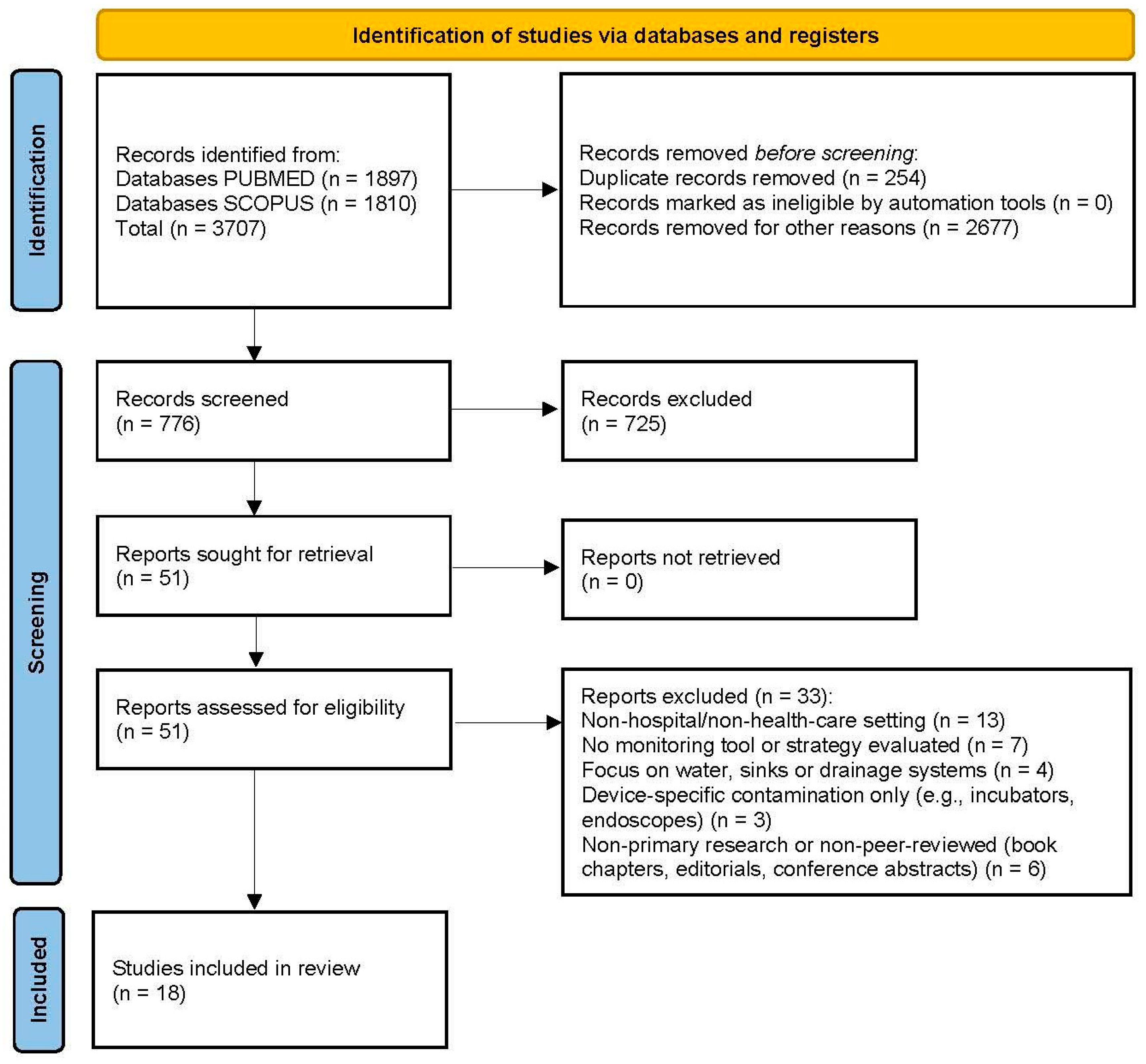
2.4. Selection Process
2.5. Data Extraction and Synthesis
3. Results
3.1. Monitoring Tools and Technologies
3.2. Targeted Pathogens in Environmental Contamination
3.3. Implementation Contexts for Cleaning and Monitoring Interventions
3.4. Reported Outcomes and Effectiveness of Monitoring and Cleaning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Control, European Centre for Disease Prevention and Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals. Available online: https://www.ecdc.europa.eu/en/publications-data/PPS-HAI-AMR-acute-care-europe-2022-2023 (accessed on 1 May 2025).
- Facciola, A.; Pellicano, G.F.; Visalli, G.; Paolucci, I.A.; Rullo, E.V.; Ceccarelli, M.; D’Aleo, F.; Di Pietro, A.; Squeri, R.; Nunnari, G.; et al. The Role of the Hospital Environment in the Healthcare-Associated Infections: A General Review of the Literature. Eur. Rev. Med. Pharmacol. Sci. 2019, 3, 1266–1278. [Google Scholar]
- Chawla, H.; Anand, P.; Garg, K.; Bhagat, N.; Varmani, S.G.; Bansal, T.; McBain, A.J.; Marwah, R.G. A Comprehensive Review of Microbial Contamination in the Indoor Environment: Sources, Sampling, Health Risks, and Mitigation Strategies. Front. Public. Health 2023, 11, 1285393. [Google Scholar] [CrossRef]
- Leistner, R.; Kohlmorgen, B.; Brodzinski, A.; Schwab, F.; Lemke, E.; Zakonsky, G.; Gastmeier, P. Environmental Cleaning to Prevent Hospital-Acquired Infections on Non-Intensive Care Units: A Pragmatic, Single-Centre, Cluster Randomized Controlled, Crossover Trial Comparing Soap-Based, Disinfection and Probiotic Cleaning. EClinicalMedicine 2023, 59, 101958. [Google Scholar] [CrossRef]
- Carling, P.C. Health Care Environmental Hygiene: New Insights and Centers for Disease Control and Prevention Guidance. Infect. Dis. Clin. N. Am. 2021, 3, 609–629. [Google Scholar] [CrossRef]
- Kubde, D.; Badge, A.K.; Ugemuge, S.; Shahu, S. Importance of Hospital Infection Control. Cureus 2023, 12, e50931. [Google Scholar] [CrossRef] [PubMed]
- Cobrado, L.; Silva-Dias, A.; Azevedo, M.M.; Rodrigues, A.G. High-Touch Surfaces: Microbial Neighbours at Hand. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 11, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Implementation Manual to Prevent and Control the Spread of Carbapenem-Resistant Organisms at the National and Health Care Facility Level: Interim Practical Manual Supporting Implementation of the Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Dancer, S.J. Controlling Hospital-Acquired Infection: Focus on the Role of the Environment and New Technologies for Decontamination. Clin. Microbiol. Rev. 2014, 4, 665–690. [Google Scholar] [CrossRef] [PubMed]
- Assadian, O.; Harbarth, S.; Vos, M.; Knobloch, J.K.; Asensio, A.; Widmer, A.F. Practical Recommendations for Routine Cleaning and Disinfection Procedures in Healthcare Institutions: A Narrative Review. J. Hosp. Infect. 2021, 113, 104–114. [Google Scholar] [CrossRef]
- Ferreira, A.M.; de Andrade, D.; Rigotti, M.A.; de Almeida, M.T.; Guerra, O.G.; Santos Junior, A.G.D. Assessment of Disinfection of Hospital Surfaces Using Different Monitoring Methods. Rev. Lat. Am. Enferm. 2015, 3, 466–474. [Google Scholar] [CrossRef]
- Han, J.H.; Sullivan, N.; Leas, B.F.; Pegues, D.A.; Kaczmarek, J.L.; Umscheid, C.A. Cleaning Hospital Room Surfaces to Prevent Health Care–Associated Infections. Ann. Intern. Med. 2015, 8, 598–607. [Google Scholar] [CrossRef]
- Browne, K.; Mitchell, B.G. Multimodal Environmental Cleaning Strategies to Prevent Healthcare-Associated Infections. Antimicrob. Resist. Infect. Control 2023, 1, 83. [Google Scholar] [CrossRef]
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhao, F.; Simoes, M. Current and Emergent Strategies for Disinfection of Hospital Environments. J. Antimicrob. Chemother. 2013, 12, 2718–2732. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Wilson, F.; Dancer, S.J.; McGregor, A. Methods to Evaluate Environmental Cleanliness in Healthcare Facilities. Healthc. Infect. 2013, 1, 23–30. [Google Scholar] [CrossRef]
- Carling, P.C.; Parry, M.F.; Bruno-Murtha, L.A.; Dick, B. Improving Environmental Hygiene in 27 Intensive Care Units to Decrease Multidrug-Resistant Bacterial Transmission. Crit. Care Med. 2010, 4, 1054–1059. [Google Scholar] [CrossRef]
- Napoli, C.; Tafuri, S.; Montenegro, L.; Cassano, M.; Notarnicola, A.; Lattarulo, S.; Montagna, M.T.; Moretti, B. Air Sampling Methods to Evaluate Microbial Contamination in Operating Theatres: Results of a Comparative Study in an Orthopaedics Department. J. Hosp. Infect. 2012, 2, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Birnbach, D.J.; Lubarsky, D.A.; Arheart, K.L.; Fajardo-Aquino, Y.; Rosalsky, M.; Cleary, T.; Depascale, D.; Coro, G.; Namias, N.; et al. Decreasing Operating Room Environmental Pathogen Contamination through Improved Cleaning Practice. Infect. Control Hosp. Epidemiol. 2012, 9, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Sui, W.; Wang, J.; Wang, H.; Wang, M.; Huang, Y.; Zhuo, J.; Lu, X. Comparing the Transmission Potential of Methicillin-Resistant Staphylococcus aureus and Multidrug-Resistant Acinetobacter Baumannii among Inpatients Using Target Environmental Monitoring. Am. J. Infect. Control 2013, 5, 411–415. [Google Scholar] [CrossRef]
- Snyder, G.M.; Holyoak, A.D.; Leary, K.E.; Sullivan, B.F.; Davis, R.B.; Wright, S.B. Effectiveness of Visual Inspection Compared with Non-Microbiologic Methods to Determine the Thoroughness of Post-Discharge Cleaning. Antimicrob. Resist. Infect. Control 2013, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Creamer, E.; Shore, A.C.; Deasy, E.C.; Galvin, S.; Dolan, A.; Walley, N.; McHugh, S.; Fitzgerald-Hughes, D.; Sullivan, D.J.; Cunney, R.; et al. Air and Surface Contamination Patterns of Meticillin-Resistant Staphylococcus aureus on Eight Acute Hospital Wards. J. Hosp. Infect. 2014, 3, 201–208. [Google Scholar] [CrossRef]
- Yuen, J.W.; Chung, T.W.; Loke, A.Y. Methicillin-Resistant Staphylococcus aureus (MRSA) Contamination in Bedside Surfaces of a Hospital Ward and the Potential Effectiveness of Enhanced Disinfection with an Antimicrobial Polymer Surfactant. Int. J. Environ. Res. Public Health 2015, 3, 3026–3041. [Google Scholar] [CrossRef]
- Sbarra, A.N.; Harris, A.D.; Johnson, J.K.; Madger, L.S.; O’Hara, L.M.; Jackson, S.S.; Thom, K.A. Guidance on Frequency and Location of Environmental Sampling for Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 2018, 3, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Doll, M.; Stevens, M.; Bearman, G. Environmental Cleaning and Disinfection of Patient Areas. Int. J. Infect. Dis. 2018, 67, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Sereia, A.F.R.; Christoff, A.P.; Cruz, G.N.F.; da Cunha, P.A.; da Cruz, G.C.K.; Tartari, D.C.; Zamparette, C.P.; Klein, T.C.R.; Masukawa, I.I.; Silva, C.I.; et al. Healthcare-Associated Infections-Related Bacteriome and Antimicrobial Resistance Profiling: Assessing Contamination Hotspots in a Developing Country Public Hospital. Front. Microbiol. 2021, 12, 711471. [Google Scholar] [CrossRef]
- Wei, L.; Wu, L.; Wen, H.; Feng, Y.; Zhu, S.; Liu, Y.; Tang, L.; Doughty, E.; van Schaik, W.; McNally, A.; et al. Spread of Carbapenem-Resistant Klebsiella pneumoniae in an Intensive Care Unit: A Whole-Genome Sequence-Based Prospective Observational Study. Microbiol. Spectr. 2021, 1, e0005821. [Google Scholar] [CrossRef]
- Monteiro, A.; Cardoso, J.; Guerra, N.; Ribeiro, E.; Viegas, C.; Cabo Verde, S.; Sousa-Uva, A. Exposure and Health Effects of Bacteria in Healthcare Units: An Overview. Appl. Sci. 2022, 4, 1958. [Google Scholar] [CrossRef]
- Ziegler, M.J.; Babcock, H.H.; Welbel, S.F.; Warren, D.K.; Trick, W.E.; Tolomeo, P.; Omorogbe, J.; Garcia, D.; Habrock-Bach, T.; Donceras, O.; et al. Stopping Hospital Infections with Environmental Services (SHINE): A Cluster-Randomized Trial of Intensive Monitoring Methods for Terminal Room Cleaning on Rates of Multidrug-Resistant Organisms in the Intensive Care Unit. Clin. Infect. Dis. 2022, 7, 1217–1223. [Google Scholar] [CrossRef]
- Keneh, N.K.; Kenmoe, S.; Bowo-Ngandji, A.; Akoachere, J.T.K.; Kamga, H.G.; Ndip, R.N.; Ebogo-Belobo, J.T.; Kengne-Ndé, C.; Mbaga, D.S.; Tendongfor, N.; et al. Methicillin-Resistant Staphylococcus aureus Carriage among Neonate Mothers, Healthcare Workers, and Environmental Samples in Neonatal Intensive Care Units: A Systematic Review. Biomed. Res. Int. 2024, 2024, 5675786. [Google Scholar] [CrossRef]
- Dhar, S.; Jinadatha, C.; Kilgore, P.E.; Henig, O.; Divine, G.W.; Todter, E.N.; Coppin, J.D.; Carter, M.J.; Chopra, T.; Egbert, S.; et al. Lowering the Acquisition of Multidrug-Resistant Organisms (MDROS) with Pulsed-Xenon (LAMP) Study: A Cluster-Randomized, Controlled, Double-Blinded, Interventional Crossover Trial. Clin. Infect. Dis. 2024, 4, 1024–1030. [Google Scholar] [CrossRef]
- Hamed, N.M.H.; Deif, O.A.; El-Zoka, A.H.; Abdel-Atty, M.M.; Hussein, M.F. The Impact of Enhanced Cleaning on Bacterial Contamination of the Hospital Environmental Surfaces: A Clinical Trial in Critical Care Unit in an Egyptian Hospital. Antimicrob. Resist. Infect. Control 2024, 1, 138. [Google Scholar] [CrossRef]
- de Bastiani, D.C.; Silva, C.V.; Christoff, A.P.; Cruz, G.N.F.; Tavares, L.D.; de Araújo, L.S.R.; Tomazini, B.M.; Arns, B.; Piastrelli, F.T.; Cavalcanti, A.B.; et al. 16s rRNA Amplicon Sequencing and Antimicrobial Resistance Profile of Intensive Care Units Environment in 41 Brazilian Hospitals. Front. Public Health 2024, 12, 1378413. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, S.; Accorgi, D.; D’Ancona, F. Tools and Strategies for Monitoring Hospital Environmental Hygiene Services. J. Hosp. Infect. 2025, 159, 52–61. [Google Scholar] [CrossRef]
- Safarabadi, M.; Motallebirad, T.; Azadi, D.; Jadidi, A. Healthcare-Associated Infections in Iranian Pediatric and Adult Intensive Care Units: A Comprehensive Review of Risk Factors, Etiology, Molecular Epidemiology, Antimicrobial Sensitivity, and Prevention Strategies During the COVID-19 Pandemic. J. Intensive Care Med. 2025, 8, 839–848. [Google Scholar] [CrossRef]
- Suleyman, G.; Alangaden, G.; Bardossy, A.C. The Role of Environmental Contamination in the Transmission of Nosocomial Pathogens and Healthcare-Associated Infections. Curr. Infect. Dis. Rep. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, P.M.S.; Duarte, M.L.; Imoto, A.M.; Atallah, Á.N.; Franco, E.S.B.; Peccin, M.S.; Taminato, M. Environmental Cleaning to Prevent COVID-19 Infection. A Rapid Systematic Review. Sao Paulo Med. J. 2020, 6, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Nante, N.; Ceriale, E.; Messina, G.; Lenzi, D.; Manzi, P. Effectiveness of Atp Bioluminescence to Assess Hospital Cleaning: A Review. J. Prev. Med. Hyg. 2017, 2, E177–E183. [Google Scholar]
- Fattorini, M.; Ceriale, E.; Nante, N.; Lenzi, D.; Manzi, P.; Basagni, C.; Messina, G. Use of a Fluorescent Marker for Assessing Hospital Bathroom Cleanliness. Am. J. Infect. Control 2016, 9, 1066–1068. [Google Scholar] [CrossRef]
- Parry, M.F.; Sestovic, M.; Renz, C.; Pangan, A.; Grant, B.; Shah, A.K. Environmental Cleaning and Disinfection: Sustaining Changed Practice and Improving Quality in the Community Hospital. Antimicrob. Steward. Healthc. Epidemiol. 2022, 1, e113. [Google Scholar] [CrossRef]
- Rock, C.; Xie, A.; Andonian, J.; Hsu, Y.J.; Osei, P.; Keller, S.C.; Gurses, A.P.; Trexler, P.; Maragakis, L.L.; Cosgrove, S.E. Evaluation of Environmental Cleaning of Patient Rooms: Impact of Different Fluorescent Gel Markers. Infect. Control Hosp. Epidemiol. 2019, 1, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Havill, N.L.; Dumigan, D.G.; Golebiewski, M.; Balogun, O.; Rizvani, R. Monitoring the Effectiveness of Hospital Cleaning Practices by Use of an Adenosine Triphosphate Bioluminescence Assay. Infect. Control Hosp. Epidemiol. 2009, 7, 678–684. [Google Scholar] [CrossRef]
- Burnham, J.P.; Shives, E.R.; Warren, D.K.; Han, J.H.; Babcock, H.M. Assessment of Percent Positive Agreement between Fluorescent Marker and Atpase for Environmental Cleaning Monitoring During Sequential Application in an Intensive Care Unit. Am. J. Infect. Control 2020, 4, 454–455. [Google Scholar] [CrossRef]
- Montgomery, A.; Hammer, K.; Guerrero, D.; Lo, T.S. Evaluation of Adenosine Triphosphate Bioluminescence-Based System in Rooms Occupied by Patients with Methicillin Resistant Staphylococcus aureus. Infect. Med. 2022, 3, 217–220. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L.; Havill, H.L.; Mangione, E.; Dumigan, D.G.; Moore, B.A. Comparison of Fluorescent Marker Systems with 2 Quantitative Methods of Assessing Terminal Cleaning Practices. Infect. Control Hosp. Epidemiol. 2011, 12, 1187–1193. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium Difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 7, e1–e48. [Google Scholar] [CrossRef]
- Sehulster, L.; Chinn, R.Y. Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations of Cdc and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm. Rep. 2003, 10, 1–42. [Google Scholar]
- Organisation for Economic Co-operation and Development. Stemming the Superbug Tide; Studies, OECD Health Policy; Organisation for Economic Co-operation and Development: Paris, France, 2018. [Google Scholar]
- Lucy, H. Embracing a One Health Framework to Fight Antimicrobial Resistance; OECD Health Policy Studies; Organisation for Economic Co-operation and Development: Paris, France, 2023. [Google Scholar]
- Flouchi, R.; Elmniai, A.; El Far, M.; Touzani, I.; El Hachlafi, N.; Fikri-Benbrahim, K. Microbiological Monitoring of the Environment Using the Association Rules Approach and Disinfection Procedure Evaluation in a Hospital Center in Morocco. J. Environ. Public Health 2021, 2021, 7682042. [Google Scholar] [CrossRef]
- Ahmad, S.; Lohiya, S.; Taksande, A.; Meshram, R.J.; Varma, A.; Vagha, K. A Comprehensive Review of Innovative Paradigms in Microbial Detection and Antimicrobial Resistance: Beyond Traditional Cultural Methods. Cureus 2024, 6, e61476. [Google Scholar] [CrossRef]
- Amodeo, D.; Manzi, P.; De Palma, I.; Puccio, A.; Nante, N.; Barcaccia, M.; Marini, D.; Pietrella, D. Efficacy of Violet-Blue (405 Nm) LED Lamps for Disinfection of High-Environmental-Contact Surfaces in Healthcare Facilities: Leading to the Inactivation of Microorganisms and Reduction of MRSA Contamination. Pathogens 2023, 12, 1338. [Google Scholar] [CrossRef]
- Boyce, J.M. Hand and Environmental Hygiene: Respective Roles for MRSA, Multi-Resistant Gram Negatives, Clostridioides difficile, and Candida spp. Antimicrob. Resist. Infect. Control 2024, 1, 110. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, S.; Salamon, I.; Girolamini, L.; Pascale, M.R.; Marino, F.; Derelitto, C.; Caligaris, L.; Paghera, S.; Ferracin, M.; Cristino, S. Surfaces Environmental Monitoring of Sars-Cov-2: Loop Mediated Isothermal Amplification (LAMP) and Droplet Digital PCR (Ddpcr) in Comparison with Standard Reverse-Transcription Quantitative Polymerase Chain Reaction (Rt-QPCR) Techniques. PLoS ONE 2025, 2, e0317228. [Google Scholar] [CrossRef]
- Orach, C.G. Health Equity: Challenges in Low Income Countries. Afr. Health Sci. 2009, 2 (Suppl. S2), S49–S51. [Google Scholar]
- Hung, I.C.; Chang, H.Y.; Cheng, A.; Chen, M.W.; Chen, A.C.; Ting, L.; Lai, Y.H.; Wang, J.T.; Chen, Y.C.; Sheng, W.H. Implementation of Human Factors Engineering Approach to Improve Environmental Cleaning and Disinfection in a Medical Center. Antimicrob. Resist. Infect. Control 2020, 1, 17. [Google Scholar] [CrossRef]
- Williams, A.; Aguilar, M.R.; Arachchillage, K.G.G.P.; Chandra, S.; Rangan, S.; Gupta, S.G.; Vivancos, J.M.A. Biosensors for Public Health and Environmental Monitoring: The Case for Sustainable Biosensing. ACS Sustain. Chem. Eng. 2024, 28, 10296–10312. [Google Scholar] [CrossRef]
- McElvania, E.; Mindel, S.; Lemstra, J.; Brands, K.; Patel, P.; Good, C.E.; Morel, D.; Orny, C.; Volle, J.M.; Desjardins, M.; et al. Automated Detection of Methicillin-Resistant Staphylococcus aureus with the MRSA Chrom Imaging Application on Bd KIESTRA Total Lab Automation System. J. Clin. Microbiol. 2024, 5, e0144523. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Tran, N.K.S.; Nguyen, H.A.; Chon, N.M.; Trinh, K.T.L.; Lee, N.Y. Recent Advances in Portable Devices for Environmental Monitoring Applications. Biomicrofluidics 2024, 5, 051501. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Cohen, C.C.; Løyland, B.; Larson, E.L. Transmission of Health Care-Associated Infections from Roommates and Prior Room Occupants: A Systematic Review. Clin. Epidemiol. 2017, 9, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Wohrley, J.D.; Bartlett, A.H. The Role of the Environment and Colonization in Healthcare-Associated Infections. In Healthcare-Associated Infections in Children: A Guide to Prevention and Management; Springer: Cham, Switzerland, 2018; pp. 17–36. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Simmons, S.; Dale, C., Jr.; Ghantoji, S.S.; Rodriguez, M.; Gubb, J.; Stachowiak, J.; Stibich, M. The Role of the Healthcare Environment in the Spread of Multidrug-Resistant Organisms: Update on Current Best Practices for Containment. Ther. Adv. Infect. Dis. 2014, 2, 79–90. [Google Scholar] [CrossRef] [PubMed]
| Selected References | Setting and Study Design | Monitoring Tools | Pathogens Target | Main Results |
|---|---|---|---|---|
| Carling et al. (2010) [16] | 27 ICUs (USA), multicenter interventional study | Cleaning audit, feedback, infection monitoring | MRSA * VRE MDRO | Enhanced cleaning significantly reduces MDRO transmission |
| Napoli et al. (2012) [17] | Orthopedics Operating Room (Italy), comparative study | Air sampling: active vs. passive methods | MRSA | Identify differences between air sampling methods; MRSA detected in the air |
| Munoz-Price et al. (2012) [18] | 2 Operating Rooms (USA), pre-post intervention study | Surface microbiological counts, environmental swabs | Bacterial pathogens | Enhanced operation significantly reduces environmental microbial load |
| Sui et al. (2013) [19] | Inpatient wards (China), prospective observational study | Targeted surface monitoring, environmental swabs | MRSA A. baumannii | A. baumannii persists longer than MRSA in the environment, with differential risk of transmission |
| Snyder et al. (2013) [20] | Tertiary care hospital (USA), prospective study | ATP vs. FM vs. Cultures vs. visual inspection | Bacterial pathogens | Visual inspection is not inferior to ATP/FM in predicting cleanliness (sensitivity ~60–70%) |
| Creamer et al. (2014) [21] | 8 acute care wards (Ireland), observational study | Surface swabs, active/passive air plates | MRSA | MRSA is frequently found near patients and in the air; pattern of spread identified |
| Yuen et al. (2015) [22] | Inpatient ward (Hong Kong), crossover trial | Surface swabs pre/post advanced disinfection | MRSA | Antimicrobial polymer and cleaning routine reduced MRSA compared to standard cleaning (hypochlorite) |
| Sbarra et al. (2018) [23] | Guidelines (USA), case review | Recommendations for surface sampling | A. baumannii | Optimal areas and frequencies for A. baumannii sampling suggested |
| Doll et al. (2018) [24] | Narrative review (USA) | ATP, FM, UV literature summary | C. difficile Bacterial pathogens | Multimodal approach recommended; Audit and feedback improve hygiene |
| Sereia et al. (2021) [25] | University Hospital (Brazil), metagenomics | 16S sequencing and surface shotgun metagenomics | Acinetobacter Enterobacteriaceae MDRO | Hotspots contaminated by opportunistic pathogens linked to local antibiotic use identified |
| Wei et al. (2021) [26] | ICUs (China, UK), prospective WGS study | Surface swabs + WGS | KPC | The hospital environment (ICU) contributes to the spread and environmental cloning of KPC strains |
| Monteiro et al. (2022) [27] | Literature review (Portugal) | Summary of environmental bacterial exposure | MRSA P. aeruginosa C. difficile | Need for continuous environmental monitoring to reduce microbiological risks highlighted |
| Ziegler et al. (2022) [28] | 6 ICUs (USA), cluster-RCT | ATP vs. UV markers for terminal room cleaning | MRSA VRE C. difficile MDRO | ATP feedback effective in reducing MDRO infections/colonizations; slightly increased room cleaning time |
| Keneh et al. (2024) [29] | Systematic review, NICU (Cameroon) | Summary of environmental MRSA contamination | MRSA | In the neonatal intensive care unit, 16.6% of environmental samples tested positive for MRSA; targeted hygiene measures recommended |
| Dhar et al. (2024) [30] | 9 hospitals (USA), cluster-RCT crossover (LAMP study) | Pulse-xenon UV after terminal cleaning | MRSA VRE MDRO | Pulse UV disinfection does not significantly reduce MDRO safety compared to standard cleaning |
| Hamed et al. (2024) [31] | Neurosurgery ICU (Egypt), quasi-experimental study | Checklist + enhanced cleaning training; surface swabs | Gram-negative MDRO | Enhanced treatment significantly reduces surface Gram-negative bacteria and decreases the incidence of nosocomial infections |
| de Bastiani et al. (2024) [32] | 41 ICUs (Brazil), multicenter molecular study | 16S rRNA sequencing, qPCR for surface resistance genes | Bacterial pathogens resistant | Significant bacterial diversity and the presence of widespread resistance genes identified |
| Gastaldi et al. (2025) [33] | International scoping review | ATP, FM, microbiological cultures, direct observation, new digital technologies | MRSA VRE C. difficile Gram-negative MDRO | Multimodal integration of traditional and innovative techniques for hospital environmental hygiene monitoring recommended |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santella, B.; Donato, A.; Fortino, L.; Satriani, V.; Ferrara, R.F.; Santoro, E.; Longanella, W.; Franci, G.; Capunzo, M.; Boccia, G. Clean to Prevent, Monitor to Protect: A Scoping Review on Strategies for Monitoring Cleaning in Hospitals to Prevent HAIs. Infect. Dis. Rep. 2025, 17, 120. https://doi.org/10.3390/idr17050120
Santella B, Donato A, Fortino L, Satriani V, Ferrara RF, Santoro E, Longanella W, Franci G, Capunzo M, Boccia G. Clean to Prevent, Monitor to Protect: A Scoping Review on Strategies for Monitoring Cleaning in Hospitals to Prevent HAIs. Infectious Disease Reports. 2025; 17(5):120. https://doi.org/10.3390/idr17050120
Chicago/Turabian StyleSantella, Biagio, Antonio Donato, Luigi Fortino, Vittoria Satriani, Rosaria Flora Ferrara, Emanuela Santoro, Walter Longanella, Gianluigi Franci, Mario Capunzo, and Giovanni Boccia. 2025. "Clean to Prevent, Monitor to Protect: A Scoping Review on Strategies for Monitoring Cleaning in Hospitals to Prevent HAIs" Infectious Disease Reports 17, no. 5: 120. https://doi.org/10.3390/idr17050120
APA StyleSantella, B., Donato, A., Fortino, L., Satriani, V., Ferrara, R. F., Santoro, E., Longanella, W., Franci, G., Capunzo, M., & Boccia, G. (2025). Clean to Prevent, Monitor to Protect: A Scoping Review on Strategies for Monitoring Cleaning in Hospitals to Prevent HAIs. Infectious Disease Reports, 17(5), 120. https://doi.org/10.3390/idr17050120










