More than Mucositis: Pediatric RIME Following Co-Infection with SARS-CoV-2 and Mycoplasma pneumoniae—A Case Report and Mini-Review
Abstract
1. Introduction
2. Case Presentation
3. Discussion
3.1. RIME: Beyond Mycoplasma Pneumoniae
3.2. COVID-19 and the Evolving Immunopathogenesis of RIME
3.3. Distinguishing RIME from Other Mucocutaneous Syndromes
3.4. Clinical Management of RIME—Challenges and Considerations
- ✓
- RIME should be included in the differential diagnosis of mucositis, particularly following respiratory infections. Broad pathogen testing is essential when evaluating unexplained mucocutaneous eruptions.
- ✓
- Co-infections, especially involving viral pathogens, can exacerbate immune-mediated mucocutaneous syndromes and should be actively evaluated. Clinicians should remain vigilant for viral reactivations, such as varicella-zoster virus, in the setting of systemic illness.
- ✓
- Systemic corticosteroids may offer clinical benefit in severe cases, provided that appropriate antimicrobial coverage is ensured. However, their concomitant use with antimicrobial agents warrants careful monitoring, as immunosuppression may predispose to secondary infections or mask evolving complications. For this reason, the decision to initiate corticosteroid therapy should ideally involve multidisciplinary evaluation, and in some settings, additional authorization or oversight is required.
- ✓
- Empirical escalation to carbapenems, including meropenem, should be reserved for selected severe pediatric cases and guided by infectious disease consultation. Timely diagnostic reassessment is critical, as delays can impact antimicrobial decision-making. Strict adherence to antimicrobial stewardship principles is essential to minimize unnecessary broad-spectrum use.
- ✓
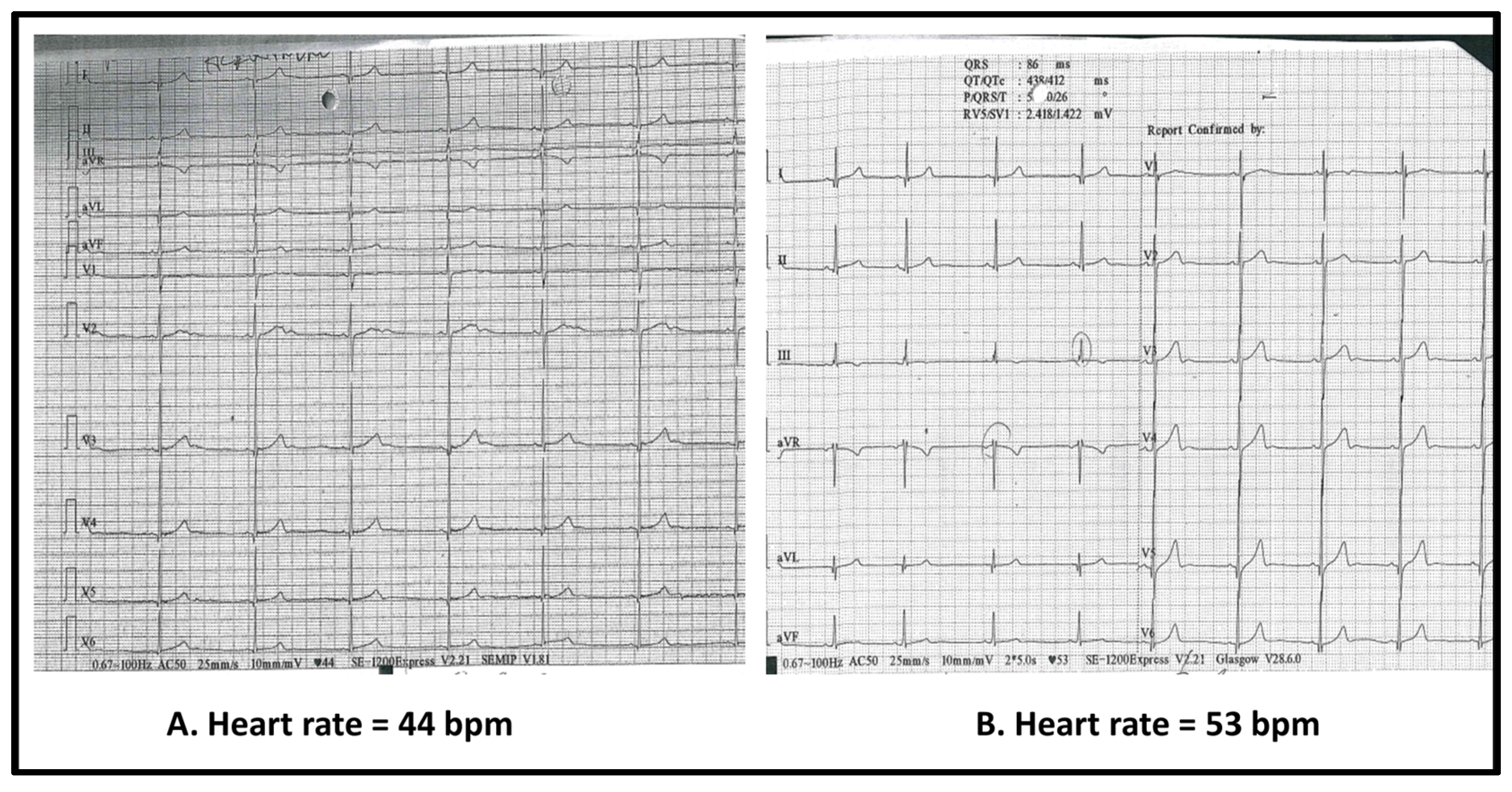
- Cardiac involvement, including bradycardia, should be monitored in the context of systemic illness. Unexplained bradycardia during systemic illness may be inflammatory in origin and, in the absence of structural heart disease, may not require intervention beyond monitoring.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASLO | Anti-streptolysin O |
| BSA | Body Surface Area |
| COVID-19 | Coronavirus Disease 2019 |
| DEN | Drug-induced Epidermal Necrolysis |
| ECG | Electrocardiogram |
| EF | Ejection Fraction |
| EM | Erythema Multiforme |
| ESR | Erythrocyte Sedimentation Rate |
| FiO2 | Fraction of Inspired Oxygen |
| GAS | Group A Streptococcus |
| GI | Gastrointestinal |
| HSV | Herpes Simplex Virus |
| ICU | Intensive Care Unit |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IV | Intravenous |
| IVIG | Intravenous Immunoglobulin |
| MIS-C | Multisystem Inflammatory Syndrome in Children |
| MIRM | Mycoplasma pneumoniae-Induced Rash and Mucositis |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| PCR | Polymerase Chain Reaction |
| RIME | Reactive Infectious Mucocutaneous Eruption |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SJS | Stevens–Johnson Syndrome |
| TEN | Toxic Epidermal Necrolysis |
| TORCH | Toxoplasmosis, Other agents, Rubella, Cytomegalovirus, Herpes simplex |
References
- Citti, C.; Blanchard, A. Mycoplasmas and their host: Emerging and re-emerging minimal pathogens. Trends Microbiol. 2013, 21, 196–203. [Google Scholar] [CrossRef]
- Parrott, G.L.; Kinjo, T.; Fujita, J. A compendium for Mycoplasma pneumoniae. Front. Microbiol. 2016, 7, 513. [Google Scholar] [CrossRef] [PubMed]
- Dalpke, A.; Zimmermann, S.; Schnitzler, P. Underdiagnosing of Mycoplasma pneumoniae infections as revealed by use of a respiratory multiplex PCR panel. Diagn. Microbiol. Infect. Dis. 2016, 86, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Narita, M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front. Microbiol. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Xiao, L.; Liu, Y.; Balish, M.F.; Atkinson, T.P. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin. Microbiol. Rev. 2017, 30, 747–809. [Google Scholar] [CrossRef]
- Schalock, P.C.; Dinulos, J.G. Mycoplasma pneumoniae-induced cutaneous disease. Int. J. Dermatol. 2009, 48, 673–681. [Google Scholar] [CrossRef]
- Canavan, T.N.; Mathes, E.F.; Frieden, I.; Shinkai, K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: A systematic review. J. Am. Acad. Dermatol. 2015, 72, 239–245. [Google Scholar] [CrossRef]
- Mayor-Ibarguren, A.; Feito-Rodriguez, M.; González-Ramos, J.; del Rosal-Rabes, T.; González-Sainz, F.J.; Sánchez-Orta, A.; de Lucas-Laguna, R. Mucositis secondary to Chlamydia pneumoniae infection: Expanding the Mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr. Dermatol. 2017, 34, 465–472. [Google Scholar] [CrossRef]
- Ramien, M.L. Reactive infectious mucocutaneous eruption: Mycoplasma pneumoniae-induced rash and mucositis and other parainfectious eruptions. Clin. Exp. Dermatol. 2021, 46, 420–429. [Google Scholar] [CrossRef]
- Goyal, A.; Hook, K. Two pediatric cases of influenza B–induced rash and mucositis: Stevens–Johnson syndrome or expansion of the M. pneumoniae-induced rash with mucositis spectrum? Pediatr. Dermatol. 2019, 36, 929–931. [Google Scholar] [CrossRef]
- Gámez-González, L.B.; Peña-Varela, C.; Ramírez-López, J.M.; Yamazaki-Nakashimada, M.A. Adenoviral-induced rash and mucositis: Expanding the spectrum of reactive infectious mucocutaneous eruption. Pediatr. Dermatol. 2021, 38, 306–308. [Google Scholar] [CrossRef]
- Mazori, D.R.; Nagarajan, S.; Glick, S.A. Recurrent reactive infectious mucocutaneous eruption (RIME): Insights from a child with three episodes. Pediatr. Dermatol. 2020, 37, 545–547. [Google Scholar] [CrossRef]
- Rodriguez, I.; Kwong, A.T.; Luu, M.; Worswick, S.D. A severe case of reactive infectious mucocutaneous eruption associated with two possible triggers: Coronavirus and group A streptococcus. Pediatr. Dermatol. 2025, 42, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Faludi, N.; Bíró, J. Target-like lesions with mucositis in a 13 year old adolescent due to co-infection with Mycoplasma pneumoniae and Varicella Zoster Virus: A case report and literature review. EMJ Rev. 2025, 10, 93–100. [Google Scholar] [CrossRef]
- Song, A.; Nicholson, C.; Maguiness, S. Recurrent reactive infectious mucocutaneous eruption (RIME) in two adolescents triggered by several distinct pathogens including SARS-CoV-2 and influenza A. Pediatr. Dermatol. 2021, 38, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Ryder, C.Y.; Pedersen, E.A.; Mancuso, J.B. Reactive infectious mucocutaneous eruption secondary to SARS-CoV-2. JAAD Case Rep. 2021, 18, 103–105. [Google Scholar] [CrossRef]
- ESGMAC MAPS Study Group. Global spatiotemporal dynamics of Mycoplasma pneumoniae re-emergence after COVID-19 pandemic restrictions: An epidemiological and transmission modelling study. Lancet Microbe. 2025, 6, 101019. [Google Scholar] [CrossRef]
- Ulmeanu, A.I.; Ciuparu, G.-E.; Matran, E.R. Characteristics of Mycoplasma pneumoniae Pneumonia in Romanian Children. Microorganisms 2025, 13, 883. [Google Scholar] [CrossRef]
- Toader, M.P.; Branisteanu, D.C.; Glod, M.; Esanu, I.M.; Branisteanu, C.I.; Capsa, M.-S.; Dimitriu, A.; Nicolescu, A.C.; Pinzariu, A.C.; Branisteanu, D.E. Mucocutaneous lesions associated with SARS-CoV-2 infection (Review). Exp. Ther. Med. 2022, 23, 258. [Google Scholar] [CrossRef]
- Martora, F.; Villani, A.; Fabbrocini, G.; Battista, T. COVID-19 and cutaneous manifestations: A review of the published literature. J. Cosmet. Dermatol. 2023, 22, 4–10. [Google Scholar] [CrossRef]
- Silva, K.R.P.D.; Giaccio, R.S.C.; de Aquino, B.M.; Van Moorsel, M.A.; Pires, F.M.; Hélène, C.M.; Hélène, S.M.F. Mucocutaneous disease: A child with extrapulmonary manifestation of mycoplasma infection. Einstein 2025, 23, eRC1138. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.; Gresham, L.; Spurr, A.; Gavigan, G. Reactive infectious mucocutaneous eruption following COVID-19 infection in vaccinated patients. JAAD Case Rep. 2023, 31, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ramien, M.L.; Mansour, D.; Shear, N.H. Management of drug-induced epidermal necrolysis (DEN) in pediatric patients: Moving from drug-induced Stevens-Johnson syndrome, overlap and toxic epidermal necrolysis to a single unifying diagnosis of DEN. Paediatr. Drugs. 2022, 24, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Ramien, M.; Bahubeshi, A.; Lara-Corrales, I.; Pope, E.; Levy, M.; Nopper, A.; Shear, N.; Eichenfield, L. Blistering severe cutaneous adverse reactions in children: Proposal for paediatric-focused clinical criteria. Br. J. Dermatol. 2021, 185, 447–449. [Google Scholar] [CrossRef]
- Alerhand, S.; Cassella, C.; Koyfman, A. Stevens-Johnson syndrome and toxic epidermal necrolysis in the pediatric population: A review. Pediatr. Emerg. Care. 2016, 32, 472–476. [Google Scholar] [CrossRef]
- Goldman, R.D. Erythema multiforme in children. Can. Fam. Physician 2022, 68, 507–508. [Google Scholar] [CrossRef]
- Rekhtman, S.; Tannenbaum, R.; Strunk, A.; Birabaharan, M.; Wright, S.; Garg, A. Mucocutaneous disease and related clinical characteristics in hospitalized children and adolescents with COVID-19 and multisystem inflammatory syndrome in children. J. Am. Acad. Dermatol. 2021, 84, 408–414. [Google Scholar] [CrossRef]
- Young, T.K.; Shaw, K.S.; Shah, J.K.; Noor, A.; Alperin, R.A.; Ratner, A.J.; Orlow, S.J.; Betensky, R.A.; Shust, G.F.; Kahn, P.J.; et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol. 2021, 157, 207–212. [Google Scholar] [CrossRef]
- Alawad, S.; Alsaeed, N.; Burnette, B.; Colantonio, M.; Kasson, L. Reactive infectious mucocutaneous eruption (RIME) in an adult male with Mycoplasma pneumoniae: A case report. Cureus 2025, 17, e78301. [Google Scholar] [CrossRef]
- Maredia, H.; Eseonu, A.; Grossberg, A.L.; Cohen, B.A. Recurrent Mycoplasma pneumoniae-associated reactive infectious mucocutaneous eruption responsive to systemic steroids: A case series. JAAD Case Rep. 2021, 11, 139–143. [Google Scholar] [CrossRef]
- Bowe, S.; O’Connor, C.; Gleeson, C.; Murphy, M. Reactive infectious mucocutaneous eruption in children diagnosed with COVID-19. Pediatr. Dermatol. 2021, 38, 1385–1386. [Google Scholar] [CrossRef]
- Cao, L.; Ji, Z.; Zhang, P.; Wang, J. Epidemiology and mortality predictors for severe childhood community-acquired pneumonia in ICUs: A retrospective observational study. Front. Pediatr. 2023, 11, 1031423. [Google Scholar] [CrossRef]
- Bradley, J.S.; Byington, C.L.; Shah, S.S.; Alverson, B.; Carter, E.R.; Harrison, C.; Kaplan, S.L.; Mace, S.E.; McCracken, G.H.; Moore, M.R.; et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 53, e25–e76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Hu, B.; Li, X.; Han, W.; Liang, Y.; Ma, X. A case report of Mycoplasma pneumoniae-induced fulminant myocarditis in a 15-year-old male leading to cardiogenic shock and electrical storm. Front. Cardiovasc. Med. 2024, 11, 1347885. [Google Scholar] [CrossRef]
- Formosa, G.M.; Bailey, M.; Barbara, C.; Muscat, C.; Grech, V. Mycoplasma pneumonia—An unusual cause of acute myocarditis in childhood. Images Paediatr. Cardiol. 2006, 8, 7–10. [Google Scholar]
- Ciccarelli, G.P.; Bruzzese, E.; Asile, G.; Vassallo, E.; Pierri, L.; De Lucia, V.; Guarino, A.; Vecchio, A.L.; Shemesh, E.; Raza, S.; et al. Bradycardia associated with multisystem inflammatory syndrome in children with COVID-19: A case series. Eur. Heart J. Case Rep. 2021, 5, ytab405. [Google Scholar] [CrossRef]
- Hallberg, T.C.; Bjorklund, A.R.; Slusher, T.M.; Rodgers, N. Sinus bradycardia in a toddler with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19. BMJ Case Rep. 2021, 14, e242058. [Google Scholar] [CrossRef]

| Parameter | Reference Range | Baseline (Regional Hospital) | Re-Evaluation (Regional Hospital) | Admission (Day 1, Our Center) | Follow-Up (Day 3) |
|---|---|---|---|---|---|
| Leukocytes (×103/µL) | 3.70–9.50 | 7.47 | 10.36 | 10.9 | 14.17 |
| Neutrophils (×103/µL) | 1.80–8.00 | 5.09 | 6.41 | 7.9 | 11.87 |
| Lymphocytes (×103/µL) | 2.00–6.50 | 1.63 | 2.62 | 1.87 | 1.52 |
| Monocytes (×103/µL) | 0.20–1.00 | 0.72 | 1.14 | 1.0 | 0.75 |
| Erythrocytes (×106/µL) | 4.30–6.00 | 4.71 | 4.97 | 4.5 | 3.68 |
| Platelets (×103/µL) | 150–450 | 212 | 273 | 359 | 410 |
| Hemoglobin (g/dL) | 13.0–18.0 | 13.8 | 14.5 | 13.4 | 12.7 |
| Alanine aminotransferase (ALT) (U/L) | 0–55 | 14 | 17 | 16 | 20.6 |
| Aspartate aminotransferase (AST) (U/L) | 5–34 | 39 | 35 | 25 | 20.6 |
| Urea (mg/dL) | 15–36 | 22 | 27 | 29.96 | 30.9 |
| Creatinine (mg/dL) | 0.72–1.25 | 0.7 | 0.63 | 0.47 | 0.46 |
| C-reactive protein (CRP) (mg/L) | 0–5 | 23.83 | 12.26 | 5.5 | 1.52 |
| D-dimers (mg/L FEU) | 0–0.5 | — | 0.98 | 0.82 | — |
| Authors (Year) | Patient Profile | Pathogens | Mucosal Involvement | Skin Findings | Treatment Approach | Outcome |
|---|---|---|---|---|---|---|
| Mayor Ibarguren et al. (2017) [8] | 6-year-old male | Chlamydia pneumoniae | Oral and genital erosions | No rash | Antibiotics + systemic corticosteroids | Full recovery |
| Goyal & Hook (2019) [10] | 16-year-old male | M. pneumoniae + Influenza B | Oral, genital, ocular mucositis | Discrete erythematous papules on the extremities | Antibiotics + steroid eye drops | Full recovery |
| 16-year-old female | M. pneumoniae + Influenza B | Oral, genital, ocular mucositis | No rash | Antibiotics + topical and systemic corticosteroids + vaginal antifungals | Full recovery | |
| Gámez González et al. (2021) [11] | 7- and 14-year-old males | Adenovirus | Severe oral and ocular mucositis | Targetoid lesions; <10% BSA | IVIG + systemic corticosteroids + Ganciclovir | Full recovery |
| Mazori et al. (2020) [12] | 8-year-old female | GAS + M. pneumoniae (1st episode) | Severe oral, ocular, and vulvar mucositis | Mild cutaneous involvement | Antibiotics + IVIG + systemic corticosteroids | Recurrent RIME (3 episodes) Full resolution after each episode |
| M. pneumoniae (2nd episode) | Oral mucositis | No rash | Antibiotics + supportive care | |||
| M. pneumoniae + Influenza B (3rd episode) | Oral mucositis | No rash | Antibiotics + supportive care | |||
| Rodriguez et al. (2025) [13] | 5-year-old female | Coronavirus NL63 + GAS | Oral, conjunctival, nasal mucositis | Severe mucocutaneous involvement | Antibiotics + Acyclovir + IVIG + etanercept | Full recovery; resolution by day 10 |
| Falludi et al. (2025) [14] | 13-year-old male | M. pneumoniae + Varicella-Zoster Virus | Ocular, oral, and urogenital mucositis | Vesicular and targetoid lesions (limbs, trunk, earlobe); sparing scalp/palms/soles | Antibiotics + Acyclovir + IVIG | Full recovery, but resolution after several weeks |
| Song et al. (2025) [15] | 13-year-old male | M. pneumoniae (1st episode) | Oral mucositis | Mild rash | Antibiotics | Recurrent RIME (4 episodes) Full resolution after each episode. |
| GAS (2nd episode) | Oral mucositis | Mild rash | Antibiotics + topical corticosteroids | |||
| Influenza A (3rd episode) | Oral mucositis | Mild rash | Antibiotics + antiviral (Oseltamivir) + topical and systemic corticosteroids | |||
| SARS-CoV-2 (4th episode) | Oral mucositis | Mild rash | Topical and systemic corticosteroids | |||
| 18-year-old female | M. pneumoniae | Ocular, oral, and genital ulcerations | Vesicular lesions, maculopapular rash (face, trunk, arm) | IVIG + antibiotics + topical and systemic corticosteroids | Recurrent RIME (3 episodes) Full recovery; re-epithelialization over 3 months; required ocular amniotic membrane grafts | |
| M. pneumoniae + Influenza A | Oral and genital mucositis | Erythematous eroded papules (buccal mucosa, lips, clitoral hood) | IVIG + antibiotics + topical and systemic corticosteroids | Full recovery; resolution after 1 week | ||
| Unknown infectious agent | Oral mucositis | No rash | IVIG + antibiotics + systemic corticosteroids | Full recovery; resolution after 2 weeks |
| Feature | RIME | SJS/TEN | Erythema Multiforme (EM) | MIS-C |
|---|---|---|---|---|
| Etiology | Infectious triggers, primarily Mycoplasma pneumoniae; also, other respiratory pathogens (e.g., Chlamydia pneumoniae, influenza, adenovirus, SARS-CoV-2) | Drug-induced hypersensitivity reactions, commonly from sulfonamides, anticonvulsants, NSAIDs | Mostly HSV infection-associated; occasionally other infections | Post-infectious inflammatory syndrome after SARS-CoV-2 infection |
| Onset | Subacute; follows respiratory illness by days to weeks | Acute; usually 1–3 weeks after drug initiation | Acute; days after HSV infection | Delayed, 2–6 weeks after COVID-19 infection |
| Mucosal Involvement | Prominent and often severe; usually affects ≥2 mucosal sites (oral, ocular, genital) | Severe mucositis involving multiple sites (oral, ocular, genital), often painful | Mild to moderate mucositis, predominantly oral and sometimes ocular | Variable mucosal involvement; oral mucositis is common |
| Skin Findings | Minimal to mild rash; may include sparse targetoid or papular lesions | Dusky macules, confluent erythema, blistering, epidermal detachment | Typical “target” lesions, mostly acral (hands/feet), classic iris-shaped | Polymorphic rash: maculopapular, urticarial, or purpuric lesions |
| Systemic Involvement | Occasionally present | Common systemic symptoms: fever, malaise, risk of sepsis, multi-organ dysfunction | Usually mild or absent systemic symptoms | Prominent systemic inflammation with cardiac dysfunction, shock, GI symptoms |
| Epidermal Necrosis | Absent | Present | Absent | Absent |
| Course | Self-limited but may recur with new infections; favorable prognosis | Potentially life-threatening; requires hospitalization and supportive care | Self-limited, resolving in weeks; rare recurrence | Requires hospitalization, intensive care often needed; generally good outcomes with treatment |
| Prognosis | Excellent with supportive care; recurrence possible | Variable; SJS mortality ~10%, TEN up to 30% | Excellent; self-resolving in most cases | Generally good with timely treatment; potential for severe complications |
| Diagnostic Clues | Preceding respiratory infection; mucositis predominates; mild or absent skin rash | Recent drug exposure; epidermal detachment; severe mucositis with painful erosions | History of HSV; characteristic target lesions; mild mucositis | Recent SARS-CoV-2 infection; systemic inflammation; multiorgan involvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grama, A.C.; Grama, O.; Mănescu, M.; Chinceșan, M. More than Mucositis: Pediatric RIME Following Co-Infection with SARS-CoV-2 and Mycoplasma pneumoniae—A Case Report and Mini-Review. Infect. Dis. Rep. 2025, 17, 121. https://doi.org/10.3390/idr17050121
Grama AC, Grama O, Mănescu M, Chinceșan M. More than Mucositis: Pediatric RIME Following Co-Infection with SARS-CoV-2 and Mycoplasma pneumoniae—A Case Report and Mini-Review. Infectious Disease Reports. 2025; 17(5):121. https://doi.org/10.3390/idr17050121
Chicago/Turabian StyleGrama, Alina Corina, Ovidiu Grama, Măriuca Mănescu, and Mihaela Chinceșan. 2025. "More than Mucositis: Pediatric RIME Following Co-Infection with SARS-CoV-2 and Mycoplasma pneumoniae—A Case Report and Mini-Review" Infectious Disease Reports 17, no. 5: 121. https://doi.org/10.3390/idr17050121
APA StyleGrama, A. C., Grama, O., Mănescu, M., & Chinceșan, M. (2025). More than Mucositis: Pediatric RIME Following Co-Infection with SARS-CoV-2 and Mycoplasma pneumoniae—A Case Report and Mini-Review. Infectious Disease Reports, 17(5), 121. https://doi.org/10.3390/idr17050121






