The Underestimated Threat—Mycobacterium Genavense Infection: A Case Report
Abstract
1. Introduction
2. Materials and Methods
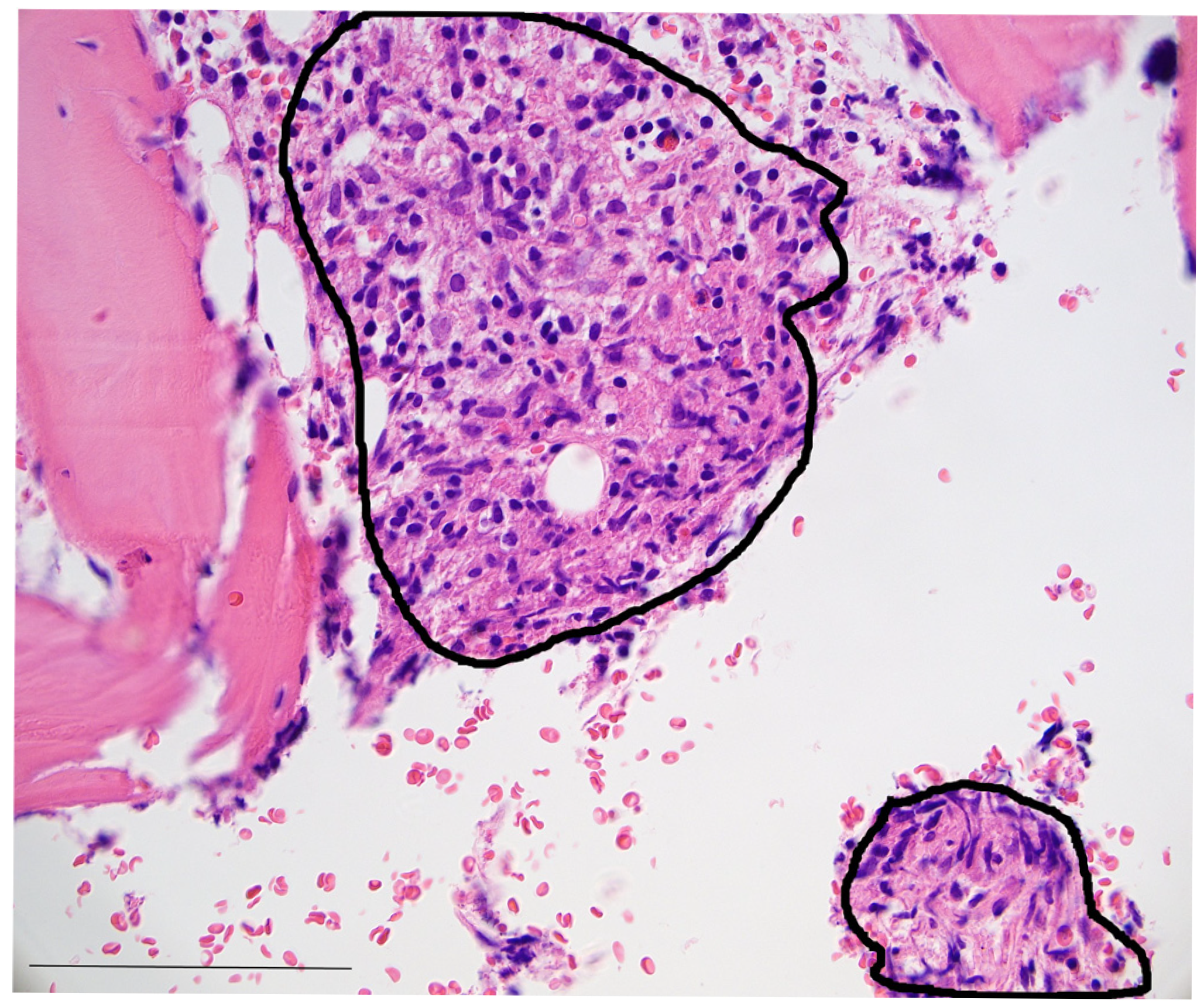
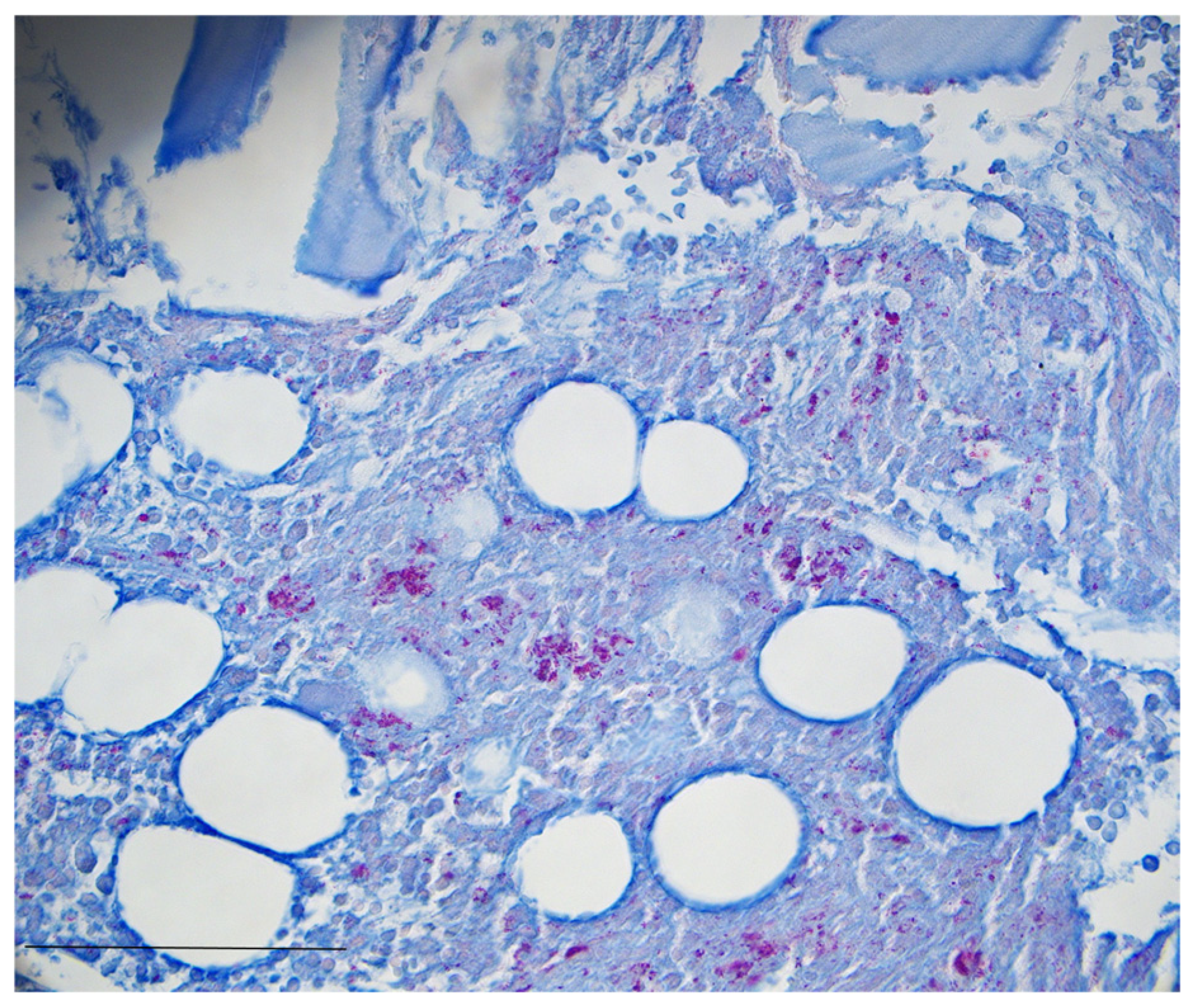
3. Case Presentation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, S.-H.; Lai, C.-C.; Huang, S.-H.; Hung, C.-C.; Hsueh, P.-R. Mycobacterial bone marrow infections at a medical centre in Taiwan, 2001–2009. Epidemiol. Infect. 2013, 142, 1524–1532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, S.C.; Kang, M.J.; Han, C.H.; Lee, S.M.; Kim, C.J.; Lee, J.M.; Kang, Y.A. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: A nationwide population-based study. BMC Pulm. Med. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.M.; Queensland Mycobacterial Reference Laboratory. Queensland Mycobacterial Reference, Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg. Infect. Dis. 2010, 16, 1576–1583. [Google Scholar] [CrossRef]
- Ricotta, E.E.; Adjemian, J.; Blakney, R.A.; Lai, Y.L.; Kadri, S.S.; Prevots, D.R. Extrapulmonary Nontuberculous Mycobacteria Infections in Hospitalized Patients, United States, 2009–2014. Emerg. Infect. Dis. 2021, 27, 845–852. [Google Scholar] [CrossRef]
- Corbett, C.; Finger, P.; Heiß-Neumann, M.; Bohnert, J.; Eder, I.B.; Eisele, M.; Friesen, I.; Kaasch, A.J.; Kehrmann, J.; Lang, R.; et al. Development of prevalence and incidence of non-tuberculous mycobacteria in German laboratories from 2016 to 2020. Emerg. Microbes Infect. 2023, 12, 2276342. [Google Scholar] [CrossRef]
- Blanc, P.; Dutronc, H.; Peuchant, O.; Dauchy, F.-A.; Cazanave, C.; Neau, D.; Wirth, G.; Pellegrin, J.-L.; Morlat, P.; Mercié, P.; et al. Nontuberculous Mycobacterial Infections in a French Hospital: A 12-Year Retrospective Study. PLoS ONE 2016, 11, e0168290. [Google Scholar] [CrossRef]
- Omori, K.; Kitagawa, H.; Yamaguchi, K.; Sakamoto, S.; Horimasu, Y.; Masuda, T.; Miyamoto, S.; Nakashima, T.; Iwamoto, H.; Fujitaka, K.; et al. Clinical characteristics of extrapulmonary nontuberculous mycobacteria infections in comparison with pulmonary infections: A single-center, retrospective study in Japan. J. Infect. Chemother. 2023, 29, 875–881. [Google Scholar] [CrossRef]
- Zweijpfenning, S.M.H.; Hoefsloot, W.; Ingen, J. Geographic Distribution of Nontuberculous Mycobacteria Isolated from Clinical Specimens: A Systematic Review. Semin. Respir. Crit. Care Med. 2018, 39, 336–342. [Google Scholar] [CrossRef]
- Chen, H.-H.; Lin, C.-H.; Chao, W.-C. Mortality association of nontuberculous mycobacterial infection requiring treatment in Taiwan: A population-based study. Ther. Adv. Respir. Dis. 2022, 16, 17534666221103213. [Google Scholar] [CrossRef]
- Loebinger, M.R.; Quint, J.K.; van der Laan, R.; Obradovic, M.; Chawla, R.; Kishore, A.; van Ingen, J. Risk Factors for Nontuberculous Mycobacterial Pulmonary Disease: A Systematic Literature Review and Meta-Analysis. Chest 2023, 164, 1115–1124. [Google Scholar] [CrossRef]
- Chai, J.; Han, X.; Mei, Q.; Liu, T.; Walline, J.H.; Xu, J.; Liu, Y.; Zhu, H. Clinical Characteristics and Mortality of Non-tuberculous Mycobacterial Infection in Immunocompromised vs. Immunocompetent Hosts. Front. Med. 2022, 9, 884446. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.M.; Vu, A.; Challener, D.; Rivera, C.G.; Shweta, F.; Zeuli, J.D.; Temesgen, Z. Approach to the diagnosis and treatment of non-tuberculous mycobacterial disease. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 24, 100244. [Google Scholar] [CrossRef] [PubMed]
- Wetzstein, N.; Kessel, J.; Bingold, T.M.; Carney, J.; Graf, C.; Koch, B.F.; Meier, F.; Baumgarten, J.; Küpper-Tetzel, C.P.; Khodamoradi, Y.; et al. High overall mortality of Mycobacterium genavense infections and impact of antimycobacterial therapy: Systematic review and individual patient data meta-analysis. J. Infect. 2022, 84, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ratnatunga, C.N.; Lutzky, V.P.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Field, M.; Bell, S.C.; Thomson, R.M.; Miles, J.J. The Rise of Non-Tuberculosis Mycobacterial Lung Disease. Front. Immunol. 2020, 11, 303. [Google Scholar] [CrossRef]
- Chindam, A.; Vengaldas, S.; Srigiri, V.R.; Syed, U.; Kilaru, H.; Chenimilla, N.P.; Kilaru, S.C.; Patil, E. Challenges of diagnosing and treating non-tuberculous mycobacterial pulmonary disease [NTM-PD]: A case series. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 25, 100271. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Lange, C.; Böttger, E.C.; Cambau, E.; E Griffith, D.; Guglielmetti, L.; van Ingen, J.; Knight, S.L.; Santin, M.; E Stout, J.; Tortoli, E.; et al. Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases. Lancet Infect. Dis. 2022, 22, e178–e190. [Google Scholar] [CrossRef]
- Wright, W.F.; Auwaerter, P.G. Fever and Fever of Unknown Origin: Review, Recent Advances, and Lingering Dogma. Open Forum Infect. Dis. 2020, 7, ofaa132. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kinjo, T.; Motooka, D.; Nabeya, D.; Jung, N.; Uechi, K.; Horii, T.; Iida, T.; Fujita, J.; Nakamura, S. Comprehensive subspecies identification of 175 nontuberculous mycobacteria species based on 7547 genomic profiles. Emerg. Microbes Infect. 2019, 8, 1043–1053. [Google Scholar] [CrossRef]
- Piersimoni, C.; Scarparo, C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg. Infect. Dis. 2009, 15, 1351. [Google Scholar] [CrossRef]
- Bottger, E.; Teske, A.; Kirschner, P.; Bost, S.; Hirschel, B.; Chang, H.; Beer, V. Disseminated “Mycobacterium genavense” infection in patients with AIDS. Lancet 1992, 340, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Gil-Brusola, A.; Escandell, A.; Blanes, M.; Gobernado, M. Mycobacterium genavense Infections in a Tertiary Hospital and Reviewed Cases in Non-HIV Patients. Pathol. Res. Int. 2014, 2014, 371370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hassanzadeh, A.; Hasannezhad, M.; Abbasian, L.; Ghaderkhani, S.; Ameli, F.; Allahdadi, M. Disseminated mycobacterium genavense infection with central nervous system involvement in an HIV patient: A case report and literature review. BMC Infect. Dis. 2024, 24, 437. [Google Scholar] [CrossRef]
- Singh, M.; Heincelman, M. Disseminated Nontuberculous Mycobacterium Presenting as Chronic Diarrhea and Wasting. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221101860. [Google Scholar] [CrossRef]
- Schmidt, V.; Köhler, H.; Heenemann, K.; Möbius, P. Mycobacteriosis in Various Pet and Wild Birds from Germany: Pathological Findings, Coinfections, and Characterization of Causative Mycobacteria. Microbiol. Spectr. 2022, 10, e0045222. [Google Scholar] [CrossRef]
- Trauth, J.; Discher, T.; Fritzenwanker, M.; Imirzalioglu, C.; Arnold, T.; Steiner, D.; Richter, E.; Crisponi, L.; Grimbacher, B.; Herold, S. Hodgkin Lymphoma after Disseminated Mycobacterium genavense Infection, Germany. Emerg. Infect. Dis. 2022, 28, 1506–1509. [Google Scholar] [CrossRef]
- Thomsen, V.Ø.; Dragsted, U.B.; Bauer, J.; Fuursted, K.; Lundgren, J. Disseminated Infection with Mycobacterium genavense: A Challenge to Physicians and Mycobacteriologists. J. Clin. Microbiol. 1999, 37, 3901–3905. [Google Scholar] [CrossRef]
- Charles, P.; Lortholary, O.; Dechartres, A.; Doustdar, F.; Viard, J.P.; Lecuit, M.; Gutierrez, M.C.; French Mycobacterium genavense Study Group. Mycobacterium genavense infections: A retrospective multicenter study in France, 1996–2007. Medicine 2011, 90, 223–230. [Google Scholar] [CrossRef]
- Ogata, R.; Kido, T.; Takeda, K.; Nemoto, K.; Heima, R.; Takao, M.; Miyashita, R.; Ozasa, M.; Tokito, T.; Okuno, D.; et al. Disseminated Mycobacterium genavense Infection Mimicking Sarcoidosis: A Case Report and Review of Literature on Japanese Patients. Microorganisms 2023, 11, 2145. [Google Scholar] [CrossRef]
- Denicolò, S.; Laydevant, S.; Fink, J.; Geiger, C.; Pizzini, A.; Sarcletti, M.; Zschocke, J.; Bellmann-Weiler, R.; Weiss, G.; Tancevski, I. Sarcoid-like lesions obfuscating the diagnosis of disseminated Mycobacterium genavense infection in a patient with IL-12Rβ1-associated immunodeficiency. BMC Infect. Dis. 2022, 22, 770. [Google Scholar] [CrossRef]
- Ulrichs, T.; Lefmann, M.; Reich, M.; Morawietz, L.; Roth, A.; Brinkmann, V.; A Kosmiadi, G.; Seiler, P.; Aichele, P.; Hahn, H.; et al. Modified immunohistological staining allows detection of Ziehl–Neelsen-negative Mycobacterium tuberculosis organisms and their precise localization in human tissue. J. Pathol. 2005, 205, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Pfyffer, G.E.; Wittwer, F. Incubation time of mycobacterial cultures: How long is long enough to issue a final negative report to the clinician? J. Clin. Microbiol. 2012, 50, 4188–4189. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Sim, B.R.; Park, Y.; Yong, S.H.; Shin, S.J.; Kang, Y.A. Identification of nontuberculous mycobacteria isolated from household showerheads of patients with nontuberculous mycobacteria. Sci. Rep. 2022, 12, 8648. [Google Scholar] [CrossRef]
- Telenti, A.; Marchesi, F.; Balz, M.; Bally, F.; Böttger, E.C.; Bodmer, T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 1993, 31, 175–178. [Google Scholar] [CrossRef]
- Kirschner, P.; Springer, B.; Vogel, U.; Meier, A.; Wrede, A.; Kiekenbeck, M.; Bange, F.C.; Böttger, E.C. Genotypic identification of mycobacteria by nucleic acid sequence determination: Report of a 2-year experience in a clinical laboratory. J. Clin. Microbiol. 1993, 31, 2882–2889. [Google Scholar] [CrossRef]
- Nick, J.A.; Malcolm, K.C.; Hisert, K.B.; Wheeler, E.A.; Rysavy, N.M.; Poch, K.; Caceres, S.; Lovell, V.K.; Armantrout, E.; Saavedra, M.T.; et al. Culture independent markers of nontuberculous mycobacterial (NTM) lung infection and disease in the cystic fibrosis airway. Tuberculosis 2022, 138, 102276. [Google Scholar] [CrossRef]
- Böttger, E.C. Mycobacterium genavense: An emerging pathogen. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef]
- Malhotra, A.M.; Arias, M.; Backx, M.; Gadsby, J.; Goodman, A.; Gourlay, Y.; Milburn, H.; Moncayo-Nieto, O.L.; Shimmin, D.; Dedicoat, M.; et al. Extrapulmonary nontuberculous mycobacterial infections: A guide for the general physician. Clin. Med. 2024, 24, 100016. [Google Scholar] [CrossRef]
- Dumic, I.; Lutwick, L. Successful treatment of rapid growing mycobacterial infections with source control alone: Case series. IDCases 2021, 26, e01332. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tsubata, Y.; Shiratsuki, Y.; Hotta, T.; Hamaguchi, M.; Isobe, T. Nontuberculous Mycobacterium-associated immune reconstitution inflammatory syndrome in a non-HIV immunosuppressed patient. Respirol. Case Rep. 2022, 10, e0918. [Google Scholar] [CrossRef] [PubMed]
- Andréjak, C.; Thomsen, V.Ø.; Johansen, I.S.; Riis, A.; Benfield, T.L.; Duhaut, P.; Sørensen, H.T.; Lescure, F.X.; Thomsen, R.W. Nontuberculous Pulmonary Mycobacteriosis in Denmark Incidence and Prognostic Factors. Am. J. Respir. Crit. Care Med. 2010, 181, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Takayanagi, N.; Kanauchi, T.; Miyahara, Y.; Yanagisawa, T.; Sugita, Y. Prognostic Factors of 634 HIV-Negative Patients with Mycobacterium avium Complex Lung Disease. Am. J. Respir. Crit. Care Med. 2012, 185, 575–583. [Google Scholar] [CrossRef]
- Kotilainen, H.; Valtonen, V.; Tukiainen, P.; Poussa, T.; Eskola, J.; Järvinen, A. Clinical symptoms and survival in non-smoking and smoking HIV-negative patients with non-tuberculous mycobacterial isolation. Scand. J. Infect. Dis. 2010, 43, 188–196. [Google Scholar] [CrossRef]
| Baseline Characteristics | Reference Values | ||
|---|---|---|---|
| Age | 73 years | ||
| Gender | Male | ||
| Occupation | Pensioner, pigeon fancier | ||
| Symptoms | Reoccurring fever, pronounced weakness, pancytopenia, reduced vigilance | ||
| Medical history | BMI 31.2 kg/m², rheumatoid arthritis, myocardial infarction, aortic aneurysm, cholecystolithiasis, diverticulosis, splenomegaly, spondylodesis | ||
| Family history | None | ||
| Medication | Acetylsalicylic acid Prasugrel Bisoprolol Doxycycline Folic acid Methotrexate Leflunomide Prednisolone Oxycodone Pantoprazole Rosuvastatin Spironolactone Tamsulosin | 100 mg/day 10 mg/day 2.5 mg/day 200 mg/day 5 mg/day Paused, 20 mg/week 20 mg/day 20 mg/day 40 mg/day 40 mg/day 20 mg/day 25 mg/day 0.4 mg/day | |
| Initial laboratory results | |||
| Leucocytes | 2.05 × 103/µL | [3.91–10.9 × 103/µL] | |
| Neutrophils | 81.8% | 41–70.7% | |
| Lymphocytes | 11.1% | 19.1–47.9% | |
| Monocytes | 5.2% | 5.2–15.2% | |
| Eosinophils | 1.2% | 0.6–7.6% | |
| Basophils | 0.6% | 0.1–1.2% | |
| Hemoglobin | 8.1 g/dL | [13.5–16.9 g/dL] | |
| Thrombocytes | 101 × 103/µL | [166–308 × 103/µL | |
| Bilirubin | 1.6 mg/dL | [<1.2 mg/dL] | |
| Serum creatinine | 1.22 mg/dL | [<0.9 mg/dL] | |
| C-reactive protein | 3.2 mg/dL | [<0.5 mg/dL] | |
| Initial blood pressure | 95/66 mmHg | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonnenberg, J.; Gabriels, G.; Olaru, I.D.; Mühl, S.; Fischer, J.; Pavenstädt, H.; Trebicka, J.; Peiffer, K.-H.; Tepasse, P.-R. The Underestimated Threat—Mycobacterium Genavense Infection: A Case Report. Infect. Dis. Rep. 2025, 17, 60. https://doi.org/10.3390/idr17030060
Sonnenberg J, Gabriels G, Olaru ID, Mühl S, Fischer J, Pavenstädt H, Trebicka J, Peiffer K-H, Tepasse P-R. The Underestimated Threat—Mycobacterium Genavense Infection: A Case Report. Infectious Disease Reports. 2025; 17(3):60. https://doi.org/10.3390/idr17030060
Chicago/Turabian StyleSonnenberg, Jannik, Gert Gabriels, Ioana Diana Olaru, Sebastian Mühl, Julia Fischer, Hermann Pavenstädt, Jonel Trebicka, Kai-Henrik Peiffer, and Phil-Robin Tepasse. 2025. "The Underestimated Threat—Mycobacterium Genavense Infection: A Case Report" Infectious Disease Reports 17, no. 3: 60. https://doi.org/10.3390/idr17030060
APA StyleSonnenberg, J., Gabriels, G., Olaru, I. D., Mühl, S., Fischer, J., Pavenstädt, H., Trebicka, J., Peiffer, K.-H., & Tepasse, P.-R. (2025). The Underestimated Threat—Mycobacterium Genavense Infection: A Case Report. Infectious Disease Reports, 17(3), 60. https://doi.org/10.3390/idr17030060






