Stage-Specific Immune Responses to AgB T-Peptides in Patients with Cystic Echinococcosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Enrollment
2.3. AgB Peptides
2.4. Whole Blood Assay
2.5. Cytokine Analysis
2.6. Statistical Analysis
3. Results
3.1. Study Population
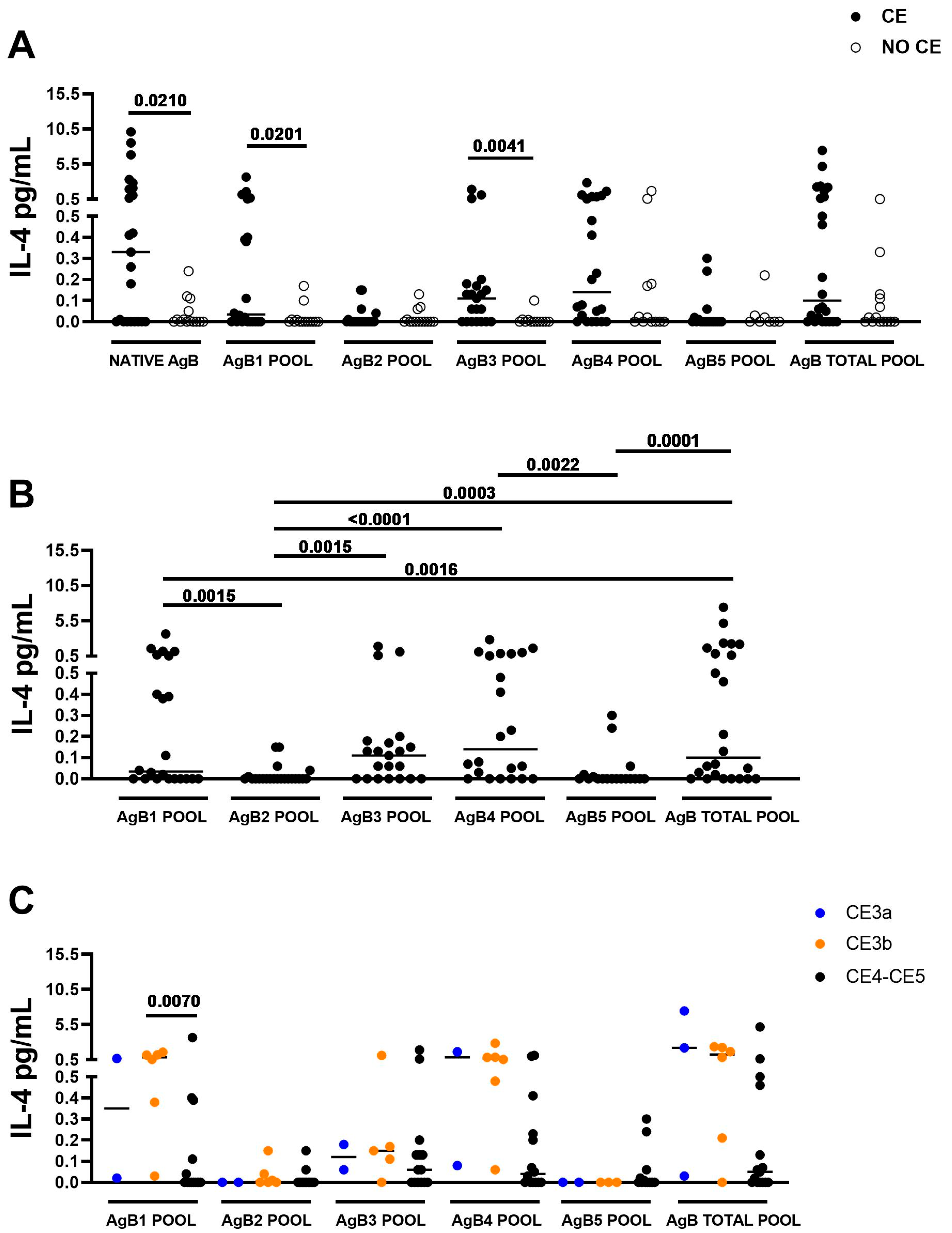
3.2. The IL-4 Response to AgB1 and to AgB3 Pools Is Associated with CE
3.3. The IL-4 Levels in Response to AgB1 Pool Are Significantly Higher in Presence of CE3b Cyst
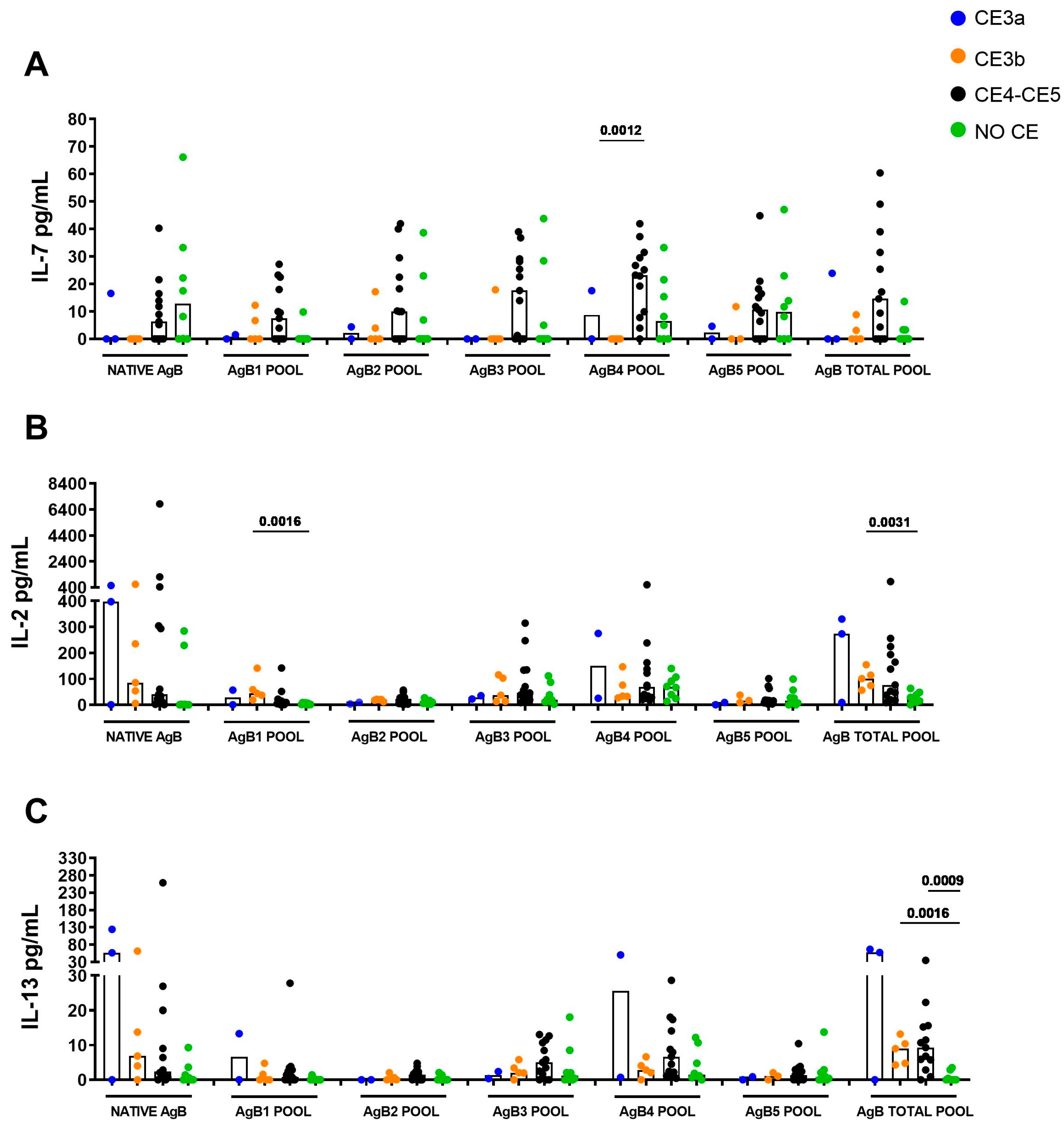
3.4. IL-7 Response Is Associated with CE4/CE5 Cysts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuitton, D.A.; McManus, D.P.; Rogan, M.T.; Romig, T.; Gottstein, B.; Naidich, A.; Tuxun, T.; Wen, H.; Menezes da Silva, A.; World Association of Echinococcosis. International Consensus on Terminology to Be Used in the Field of Echinococcoses. Parasite 2020, 27, 41. [Google Scholar] [CrossRef] [PubMed]
- Cucher, M.A.; Macchiaroli, N.; Baldi, G.; Camicia, F.; Prada, L.; Maldonado, L.; Avila, H.G.; Fox, A.; Gutiérrez, A.; Negro, P.; et al. Cystic Echinococcosis in South America: Systematic Review of Species and Genotypes of Echinococcus granulosus sensu lato in Humans and Natural Domestic Hosts. Trop. Med. Int. Health 2016, 21, 166–175. [Google Scholar] [CrossRef]
- Alvarez Rojas, C.A.; Romig, T.; Lightowlers, M.W. Echinococcus granulosus sensu lato Genotypes Infecting Humans—Review of Current Knowledge. Int. J. Parasitol. 2014, 44, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Deplazes, P. Biological, Epidemiological, and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.; Menezes da Silva, A.; Akhan, O.; Müllhaupt, B.; Vizcaychipi, K.A.; Budke, C.; Vuitton, D.A. The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv. Parasitol. 2017, 96, 259–369. [Google Scholar] [CrossRef]
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; de la Rue, M. Ecology and Life Cycle Patterns of Echinococcus Species. Adv. Parasitol. 2017, 95, 213–314. [Google Scholar] [CrossRef]
- Gottstein, B.; Soboslay, P.; Ortona, E.; Wang, J.; Siracusano, A.; Vuitton, D.A. Immunology of Alveolar and Cystic Echinococcosis (AE and CE). Adv. Parasitol. 2017, 96, 1–54. [Google Scholar] [CrossRef]
- Kern, P. Clinical Features and Treatment of Alveolar Echinococcosis. Curr. Opin. Infect. Dis. 2010, 23, 505–512. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Jenkins, D.J. Echinococcus as a Model System: Biology and Epidemiology. Int. J. Parasitol. 2014, 44, 865–877. [Google Scholar] [CrossRef]
- Stojkovic, M.; Gottstein, B.; Junghanss, T. 56—Echinococcosis. In Manson’s Tropical Infectious Diseases (Twenty-Third Edition); Farrar, J., Hotez, P.J., Junghanss, T., Kang, G., Lalloo, D., White, N.J., Eds.; W.B. Saunders: London, UK, 2014; pp. 795–819.e3. ISBN 978-0-7020-5101-2. [Google Scholar]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Writing Panel for the WHO-IWGE Expert Consensus for the Diagnosis and Treatment of Cystic and Alveolar Echinococcosis in Humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Stojkovic, M.; Zwahlen, M.; Teggi, A.; Vutova, K.; Cretu, C.M.; Virdone, R.; Nicolaidou, P.; Cobanoglu, N.; Junghanss, T. Treatment Response of Cystic Echinococcosis to Benzimidazoles: A Systematic Review. PLoS Negl. Trop. Dis. 2009, 3, e524. [Google Scholar] [CrossRef] [PubMed]
- Hosch, W.; Junghanss, T.; Stojkovic, M.; Brunetti, E.; Heye, T.; Kauffmann, G.W.; Hull, W.E. Metabolic Viability Assessment of Cystic Echinococcosis Using High-Field 1H MRS of Cyst Contents. NMR Biomed. 2008, 21, 734–754. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; De Silvestri, A.; Tamarozzi, F.; Cattaneo, F.; Lissandrin, R.; Brunetti, E. Medical Treatment versus “Watch and Wait” in the Clinical Management of CE3b Echinococcal Cysts of the Liver. BMC Infect. Dis. 2014, 14, 492. [Google Scholar] [CrossRef] [PubMed]
- Siles-Lucas, M.; Casulli, A.; Conraths, F.J.; Müller, N. Laboratory Diagnosis of Echinococcus Spp. in Human Patients and Infected Animals. Adv. Parasitol. 2017, 96, 159–257. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Mariconti, M.; Neumayr, A.; Brunetti, E. The Intermediate Host Immune Response in Cystic Echinococcosis. Parasite Immunol. 2016, 38, 170–181. [Google Scholar] [CrossRef]
- Carmena, D.; Benito, A.; Eraso, E. Antigens for the Immunodiagnosis of Echinococcus granulosus Infection: An Update. Acta Trop. 2006, 98, 74–86. [Google Scholar] [CrossRef]
- Monteiro, K.M.; Zaha, A.; Ferreira, H.B. Recombinant Subunits as Tools for the Structural and Functional Characterization of Echinococcus granulosus Antigen B. Exp. Parasitol. 2008, 119, 490–498. [Google Scholar] [CrossRef]
- Muzulin, P.M.; Kamenetzky, L.; Gutierrez, A.M.; Guarnera, E.A.; Rosenzvit, M.C. Echinococcus granulosus Antigen B Gene Family: Further Studies of Strain Polymorphism at the Genomic and Transcriptional Levels. Exp. Parasitol. 2008, 118, 156–164. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Liu, M.; Feng, Z. Analysis on the Reactivity of Five Subunits of Antigen B Family in Serodiagnosis of Echinococcosis. Exp. Parasitol. 2012, 131, 85–91. [Google Scholar] [CrossRef]
- Manzano-Román, R.; Sánchez-Ovejero, C.; Hernández-González, A.; Casulli, A.; Siles-Lucas, M. Serological Diagnosis and Follow-Up of Human Cystic Echinococcosis: A New Hope for the Future? Biomed. Res. Int. 2015, 2015, 428205. [Google Scholar] [CrossRef]
- Petrone, L.; Vanini, V.; Amicosante, M.; Corpolongo, A.; Gomez Morales, M.A.; Ludovisi, A.; Ippolito, G.; Pozio, E.; Teggi, A.; Goletti, D. A T-Cell Diagnostic Test for Cystic Echinococcosis Based on Antigen B Peptides. Parasite Immunol. 2017, 39, e12499. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Vanini, V.; Petruccioli, E.; Ettorre, G.M.; Busi Rizzi, E.; Schininà, V.; Girardi, E.; Ludovisi, A.; Gómez-Morales, M.Á.; Pozio, E.; et al. IL-4 Specific-Response in Whole Blood Associates with Human Cystic Echinococcosis and Cyst Activity. J. Infect. 2015, 70, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Tamarozzi, F.; Vola, A.; Gomez Morales, M.A.; Ludovisi, A.; Najafi Fard, S.; Mariconti, M.; Brunetti, E.; Goletti, D. Accuracy of an Experimental Whole-Blood Test for Detecting Reactivation of Echinococcal Cysts. PLoS Negl. Trop. Dis. 2021, 15, e0009648. [Google Scholar] [CrossRef] [PubMed]
- Pagnozzi, D.; Tamarozzi, F.; Roggio, A.M.; Tedde, V.; Addis, M.F.; Pisanu, S.; Masu, G.; Santucciu, C.; Vola, A.; Casulli, A.; et al. Structural and Immunodiagnostic Characterization of Synthetic Antigen B Subunits from Echinococcus granulosus and Their Evaluation as Target Antigens for Cyst Viability Assessment. Clin. Infect. Dis. 2018, 66, 1342–1351. [Google Scholar] [CrossRef]
- Monteiro, K.M.; Scapin, S.M.N.; Navarro, M.V.A.S.; Zanchin, N.I.T.; Cardoso, M.B.; da Silveira, N.P.; Gonçalves, P.F.B.; Stassen, H.K.; Zaha, A.; Ferreira, H.B. Self-Assembly and Structural Characterization of Echinococcus granulosus Antigen B Recombinant Subunit Oligomers. Biochim. Biophys. Acta 2007, 1774, 278–285. [Google Scholar] [CrossRef]
- Lundström, W.; Fewkes, N.M.; Mackall, C.L. IL-7 in Human Health and Disease. Semin. Immunol. 2012, 24, 218–224. [Google Scholar] [CrossRef]
- Tiberti, N.; Buonfrate, D.; Carbone, C.; Piro, G.; Bisoffi, Z.; Piubelli, C. Systemic Profile of Immune Factors in an Elderly Italian Population Affected by Chronic Strongyloidiasis. Parasit. Vectors 2020, 13, 515. [Google Scholar] [CrossRef]
- Petrone, L.; Najafi-Fard, S.; Falasca, L.; Sbarra, S.; Teggi, A.; Nicastri, E.; Grillo, L.R.; Burocchi, M.; Ettorre, G.M.; Ludovisi, A.; et al. Evaluation of the Local and Peripheral Immune Responses in Patients with Cystic Echinococcosis. Pathogens 2024, 13, 477. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Riganò, R.; Buttari, B.; De Falco, E.; Profumo, E.; Ortona, E.; Margutti, P.; Scottà, C.; Teggi, A.; Siracusano, A. Echinococcus granulosus-Specific T-Cell Lines Derived from Patients at Various Clinical Stages of Cystic Echinococcosis. Parasite Immunol. 2004, 26, 45–52. [Google Scholar] [CrossRef]
- Petrone, L.; Vanini, V.; Petruccioli, E.; Ettorre, G.M.; Schininà, V.; Busi Rizzi, E.; Ludovisi, A.; Corpolongo, A.; Ippolito, G.; Pozio, E.; et al. Polyfunctional Specific Response to Echinococcus granulosus Associates to the Biological Activity of the Cysts. PLoS Negl. Trop. Dis. 2015, 9, e0004209. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, Z.J.; Shenoy, A.; Chen, A.; Heller, N.M.; Spangler, J.B. Engineering the IL-4/IL-13 Axis for Targeted Immune Modulation. Immunol. Rev. 2023, 320, 29–57. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Meroni, V.; Genco, F.; Tamarozzi, F.; Tinelli, C.; Filice, C.; Brunetti, E. Serum Cytokine Profile by ELISA in Patients with Echinococcal Cysts of the Liver: A Stage-Specific Approach to Assess Their Biological Activity. Clin. Dev. Immunol. 2012, 2012, 483935. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Tamarozzi, F.; Macchia, G.; Bertuccini, L.; Mariconti, M.; Birago, C.; Iriarte, A.; Brunetti, E.; Cretu, C.M.; Akhan, O.; et al. Proteomic Analysis of Plasma Exosomes from Cystic Echinococcosis Patients Provides in Vivo Support for Distinct Immune Response Profiles in Active vs Inactive Infection and Suggests Potential Biomarkers. PLoS Negl. Trop. Dis. 2020, 14, e0008586. [Google Scholar] [CrossRef]
- Siracusano, A.; Delunardo, F.; Teggi, A.; Ortona, E. Cystic Echinococcosis: Aspects of Immune Response, Immunopathogenesis and Immune Evasion from the Human Host. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 16–23. [Google Scholar] [CrossRef]
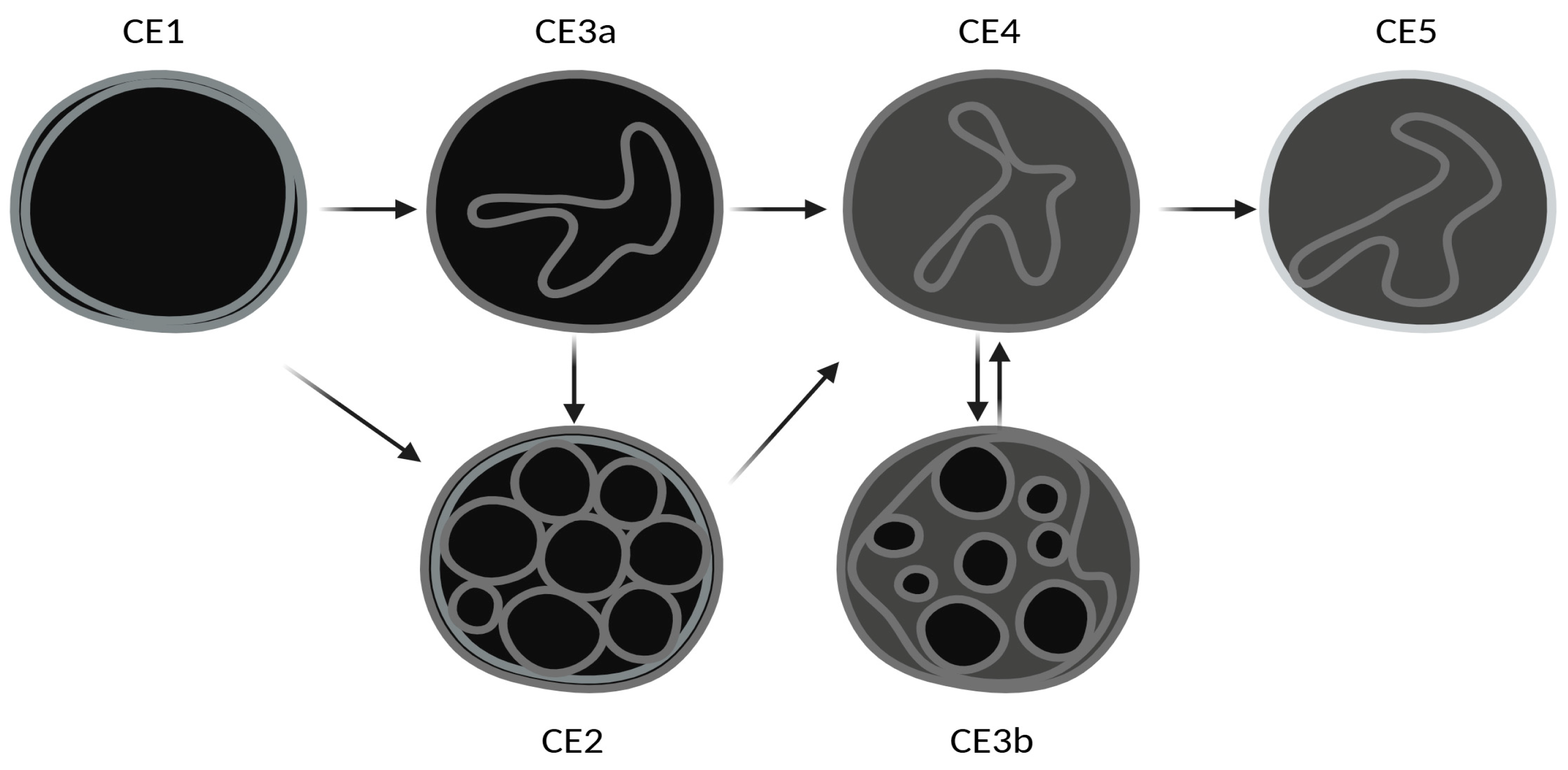
| CE | No CE | p | |||
|---|---|---|---|---|---|
| CE3a | CE3b | CE4-CE5 | |||
| N (%) | 3 | 6 | 15 | 14 | |
| Median age in years (IQR) | 36 (23–43) | 51 (35–71) | 51 (33–61) | 65 (57–68) | 0.0716 * |
| Female sex N (%) | 3 (100) | 1 (17) | 9 (60) | 12 (86) | 0.0136 # |
| Serology positive results N (%) ° | 2 (67) | 5 (83) | 7 (47) | 0 (0) | 0.0014 |
| Previous albendazole treatment N (%) | 2 (67) | 3 (50) | 4 (27) | 1 (7) | 0.0748 # |
| Current pharmacological treatment N (%) | 1 (33) | 2 (33) | 0 (0) | 1 (7) | 0.0750 |
| Cyst number/patient range | 1–4 | 1–1 | 1–5 | 1–3 § | 0.3868 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbarra, S.; Vola, A.; Tamarozzi, F.; Najafi-Fard, S.; Ludovisi, A.; Teggi, A.; Nicastri, E.; Albarello, F.; Brunetti, E.; Goletti, D.; et al. Stage-Specific Immune Responses to AgB T-Peptides in Patients with Cystic Echinococcosis. Infect. Dis. Rep. 2025, 17, 51. https://doi.org/10.3390/idr17030051
Sbarra S, Vola A, Tamarozzi F, Najafi-Fard S, Ludovisi A, Teggi A, Nicastri E, Albarello F, Brunetti E, Goletti D, et al. Stage-Specific Immune Responses to AgB T-Peptides in Patients with Cystic Echinococcosis. Infectious Disease Reports. 2025; 17(3):51. https://doi.org/10.3390/idr17030051
Chicago/Turabian StyleSbarra, Settimia, Ambra Vola, Francesca Tamarozzi, Saeid Najafi-Fard, Alessandra Ludovisi, Antonella Teggi, Emanuele Nicastri, Fabrizio Albarello, Enrico Brunetti, Delia Goletti, and et al. 2025. "Stage-Specific Immune Responses to AgB T-Peptides in Patients with Cystic Echinococcosis" Infectious Disease Reports 17, no. 3: 51. https://doi.org/10.3390/idr17030051
APA StyleSbarra, S., Vola, A., Tamarozzi, F., Najafi-Fard, S., Ludovisi, A., Teggi, A., Nicastri, E., Albarello, F., Brunetti, E., Goletti, D., & Petrone, L. (2025). Stage-Specific Immune Responses to AgB T-Peptides in Patients with Cystic Echinococcosis. Infectious Disease Reports, 17(3), 51. https://doi.org/10.3390/idr17030051







