Exploring the Antibiotic Potential of a Serine Protease from Solanum trilobatum Against Staphylococcus aureus Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Purification
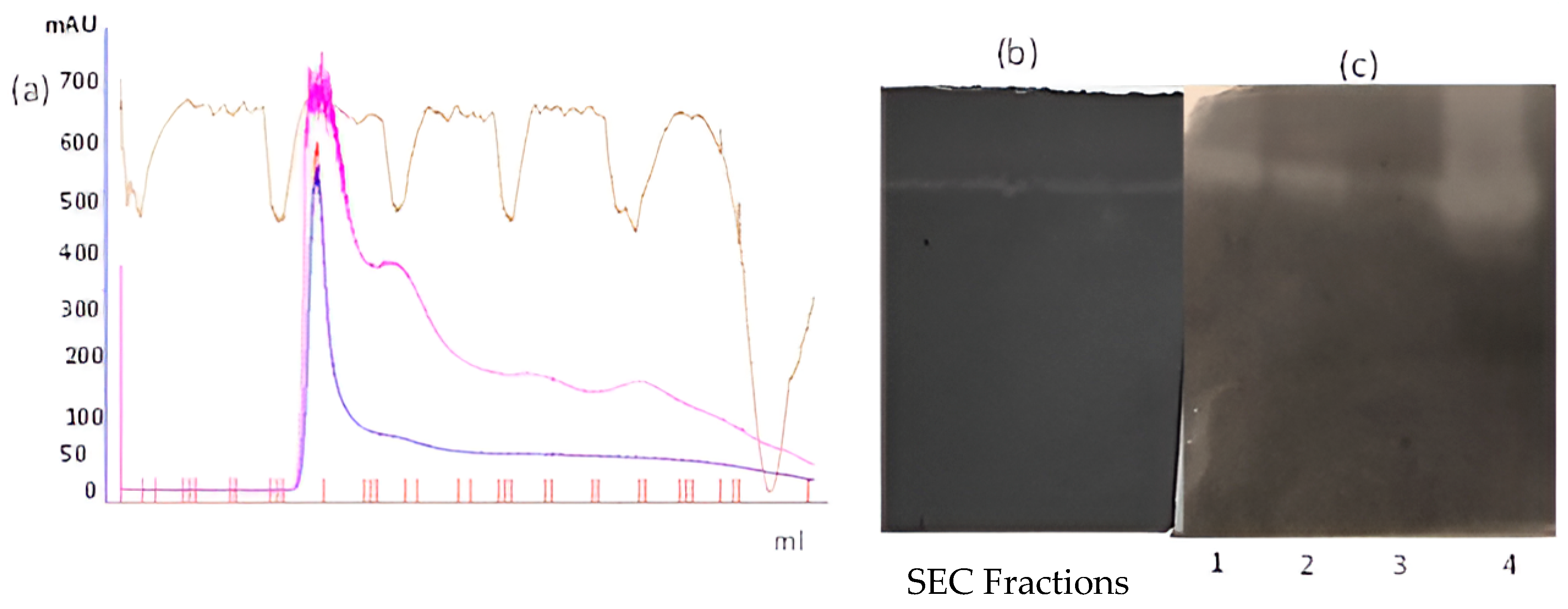
2.2. Purification of Active Fraction via Anionic Exchange Chromatography
2.3. Biofilm Eradication Assay
2.4. Proteolytic Activity Measurement
2.5. Effect of Standard Inhibitors on Protease Activity
2.6. Effect of Detergents, Organic Solvents, and Metal Ions on Proteolytic Activity
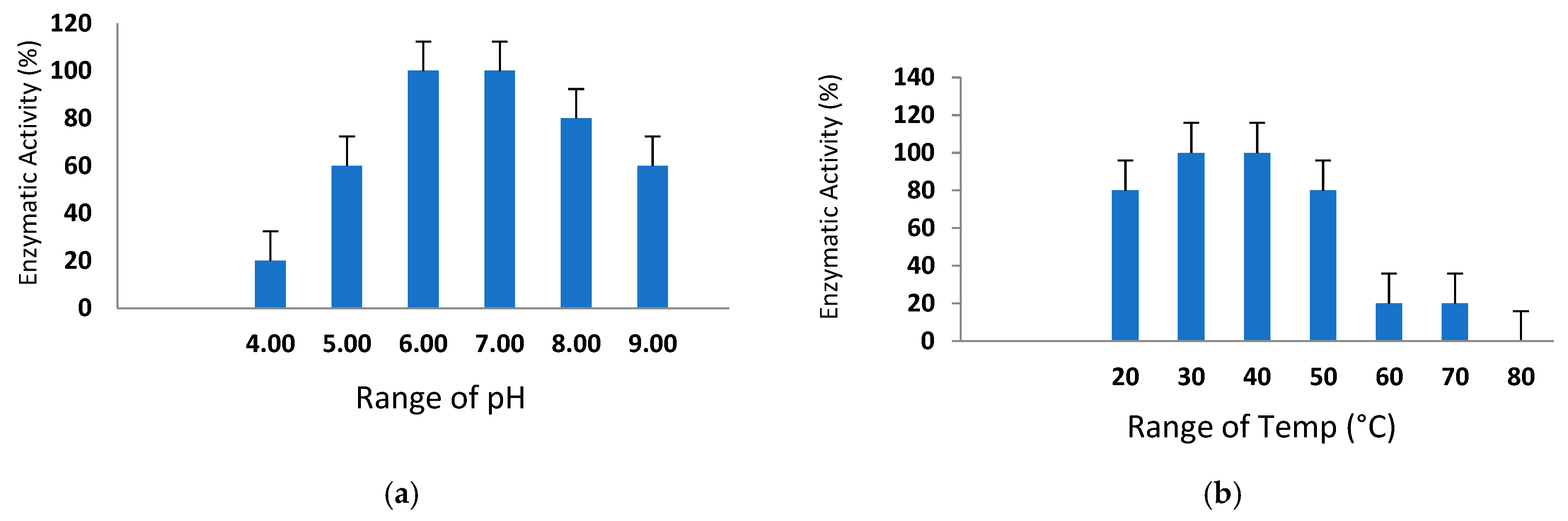
2.7. Effect of pH and Temperature on Proteolytic Activity
2.8. Zymography
2.9. Determination of Vmax and km
3. Results and Discussion
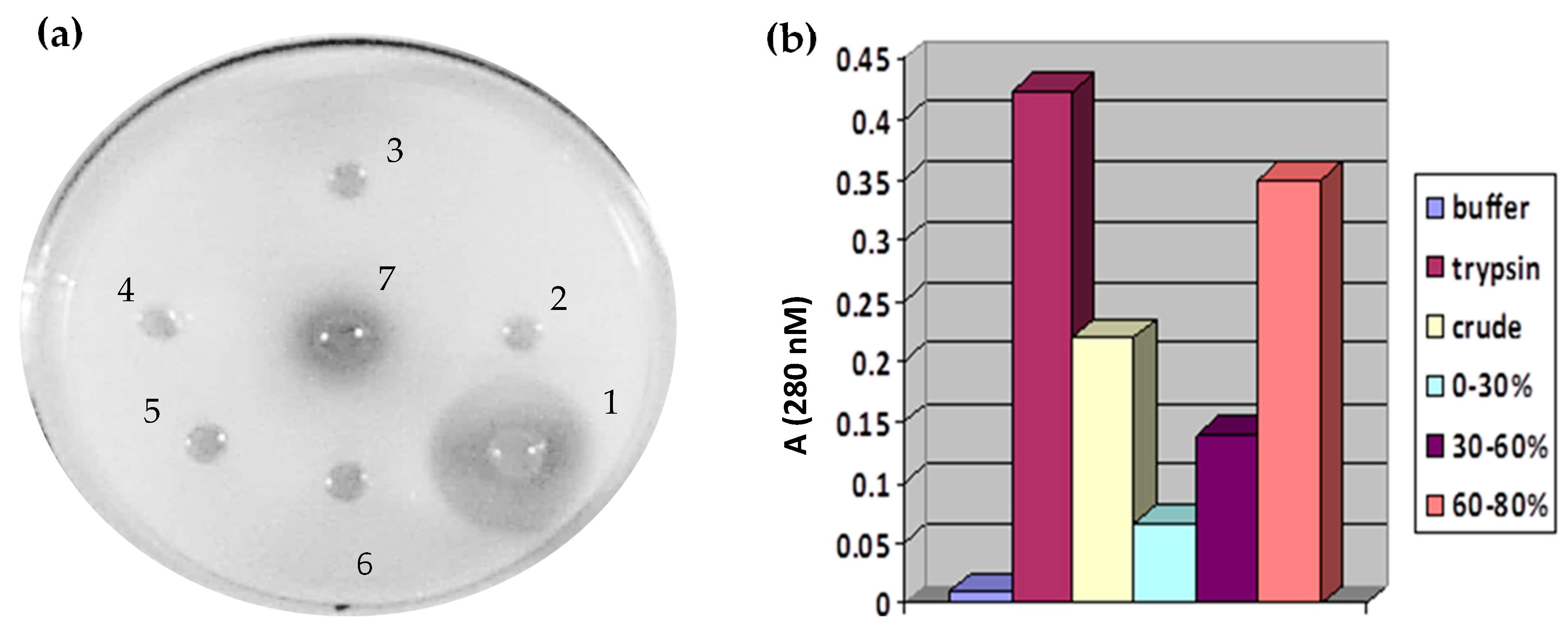
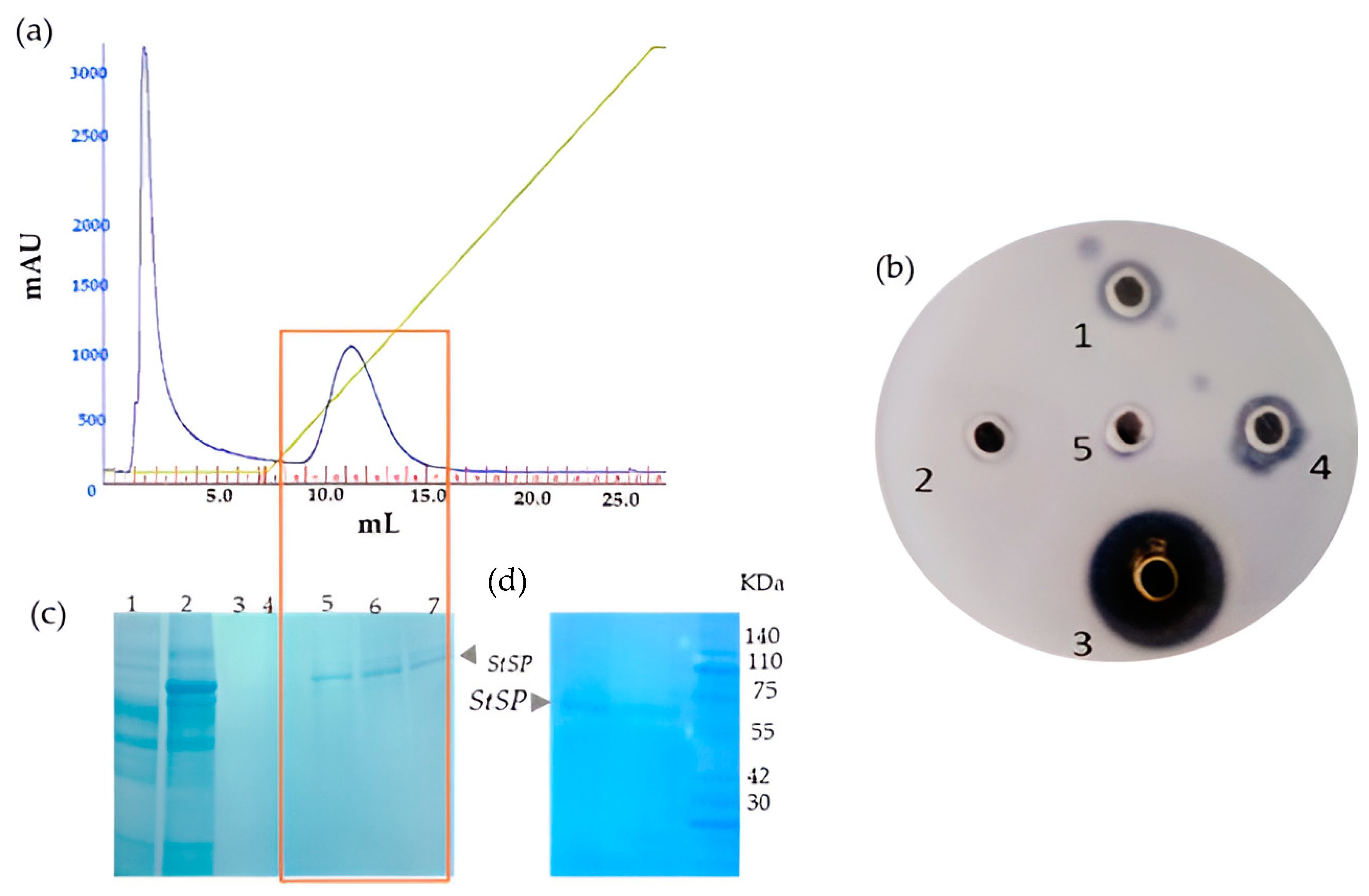
3.1. Isolation and Purification of Protease from Solanum Trilobatum
3.2. Inhibition of Serine Protease by Serine Protease Inhibitor (PMSF)
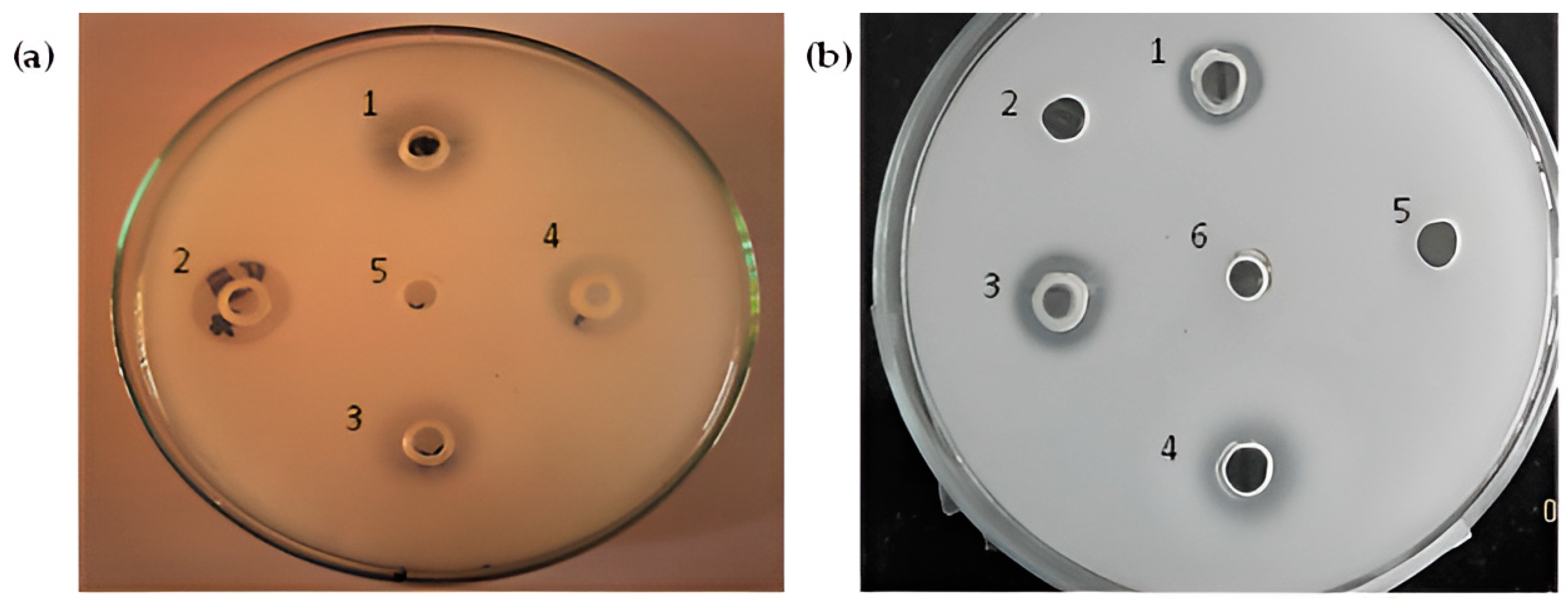
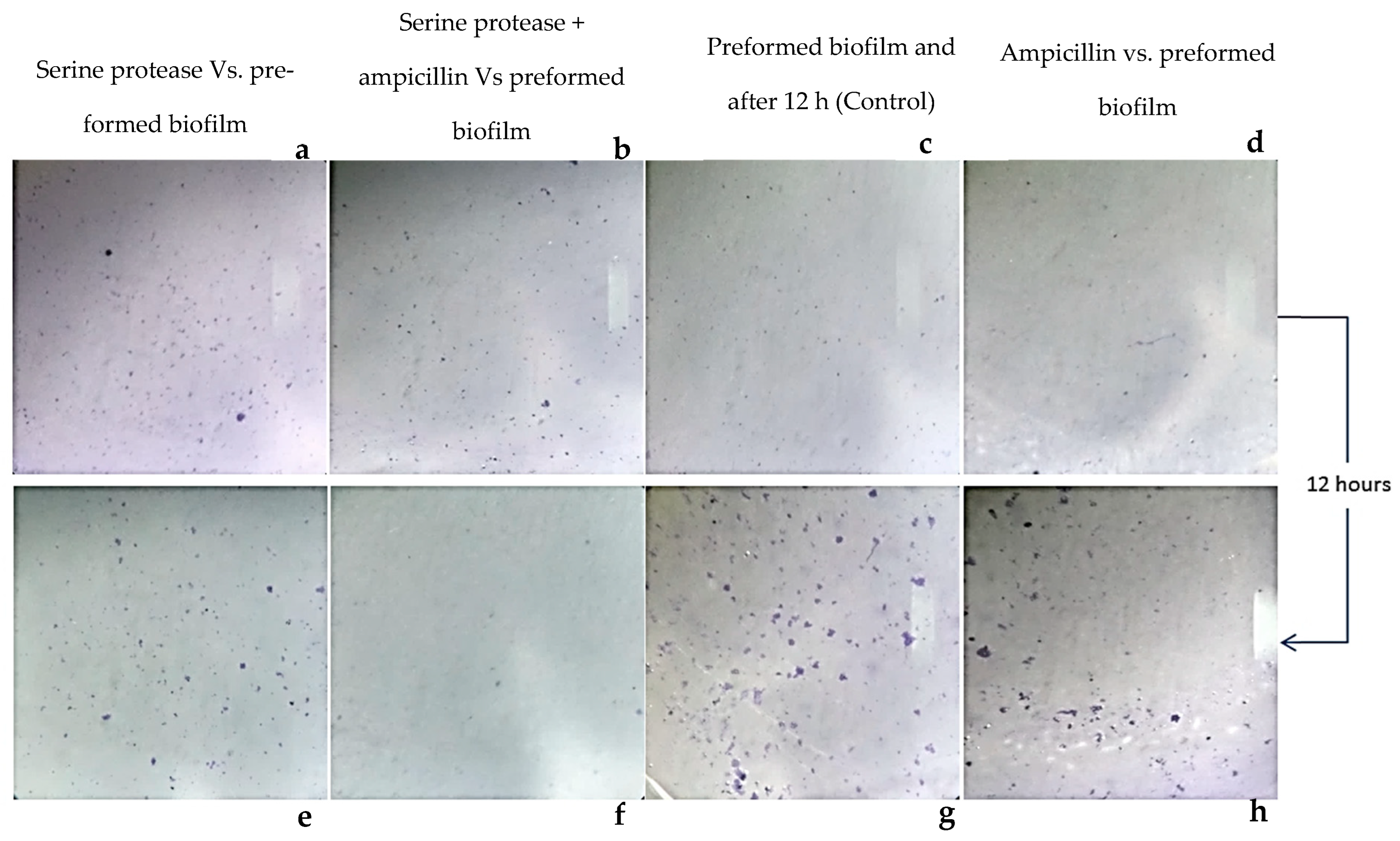
3.3. Staphylococcus aureus-Preformed Biofilm Eradicated by StSP
3.4. Effect of Metal Ions
3.5. Effect of Surfactants
3.6. Effect of Organic Solvents
3.7. Determination of Kinetic Parameters
3.8. Sustainability of Various pH Ranges and Temperatures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ch’ng, J.-H.; Chong, K.K.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by Enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Gunn, J.S.; Bakaletz, L.O.; Wozniak, D.J. What’s on the outside matters: The role of the extracellular polymeric substance of gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J. Biol. Chem. 2016, 291, 12538–12546. [Google Scholar] [CrossRef]
- Jones, E.A.; McGillivary, G.; Bakaletz, L.O. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human defensin-3 and reduces its antimicrobial activity. J. Innate Immun. 2013, 5, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral. Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haaber, J.; Cohn, M.T.; Frees, D.; Andersen, T.J.; Ingmer, H. Planktonic aggregates of Staphylococcus aureus protect against common antibiotics. PLoS ONE 2012, 7, e41075. [Google Scholar] [CrossRef]
- Ciofu, O.; Mandsberg, L.F.; Wang, H.; Hoiby, N. Phenotypes selected during chronic lung infection in cystic fibrosis patients: Implications for the treatment of Pseudomonas aeruginosa biofilm infections. FEMS Immunol. Med. Microbiol. 2012, 65, 215–225. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Salli, K.M.; Ouwehand, A.C. The use of in vitro model systems to study dental biofilms associated with caries: A short review. J. Oral. Microbiol. 2015, 7, 26149. [Google Scholar] [CrossRef]
- Giordani, B.; Costantini, P.E.; Fedi, S.; Cappelletti, M.; Abruzzo, A.; Parolin, C.; Vitali, B. Liposomes containing biosurfactants isolated from Lactobacillus gasseri exert antibiofilm activity against methicillin resistant Staphylococcus aureus strains. Eur. J. Pharm. Biopharm. 2019, 139, 246–252. [Google Scholar] [CrossRef]
- Hogan, S.; Zapotoczna, M.; Stevens, N.T.; Humphreys, H.; O’Gara, J.P.; O’Neill, E. Potential use of targeted enzymatic agents in the treatment of Staphylococcus aureus biofilm-related infections. J. Hosp. Infect. 2017, 96, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Gharieb, R.M.A.; Saad, M.F.; Mohamed, A.S.; Tartor, Y.H. Characterization of two novel lytic bacteriophages for reducing biofilms of zoonotic multidrug-resistant Staphylococcus aureus and controlling their growth in milk. LWT 2020, 124, 109145. [Google Scholar] [CrossRef]
- Manohar, R.; Kutumbarao, N.H.V.; Krishna Nagampalli, R.S.; Velmurugan, D.; Gunasekaran, K. Structural insights and binding of a natural ligand, succinic acid with serine and cysteine proteases. Biochem. Biophys. Res. Commun. 2018, 495, 679–685. [Google Scholar] [CrossRef]
- Smith, A.; Datta, S.P.; Smith, G.H.; Campbell, P.N.; Bentley, R.; McKenzie, H. Oxford Dictionary of Biochemistry and Molecular Biology; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Antão, C.M.; Malcata, F.X. Plant serine proteases: Biochemical, physiological and molecular features. Plant Physiol. Biochem. 2005, 43, 637–650. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Morishima, I.; Babiker, E.E.; Mori, N. Characterisation of partially purified milk-clotting enzyme from Solanum dubium Fresen seeds. Food Chem. 2009, 116, 395–400. [Google Scholar] [CrossRef]
- Govindan, S.; Viswanathan, S.; Vijayasekaran, V.; Alagappan, R. A pilot study on the clinical efficacy of Solanum xanthocarpum and S. trilobatum in bronchial asthma. J. Ethnopharmacol. 1999, 66, 205–210. [Google Scholar] [CrossRef]
- Madhavan, S.; Balu, S. Ethnobotanical studies on S. trilobatum L.—An Indian drug plant. In Ethnobotany and Medicinal Plants of INDIAN Subcontinent; Scientific Publishers: Jodhpur, India, 2000; pp. 43–46. [Google Scholar]
- Mohanan, P.; Devi, K. Cytotoxic potential of the preparations from S. trilobatum and the effect of sobatum on tumour reduction in mice. Cancer Lett. 1996, 110, 71–76. [Google Scholar] [CrossRef]
- Shahjahan, M.; Sabitha, K.; Jainu, M.; Devi, C.S. Effect of S. trilobatum against carbon tetra chloride induced hepatic damage in albino rats. Indian J. Med. Res. 2004, 120, 194–198. [Google Scholar]
- Emmanuel, S.; Ignacimuthu, S.; Perumalsamy, R.; Amalraj, T. Antiinflammatory activity of S. trilobatum. Fitoterapia 2006, 77, 611–612. [Google Scholar] [CrossRef]
- Doss, A.; Mubarack, H.M.; Dhanabalan, R. Antibacterial activity of tannins from the leaves of S. trilobatum Linn. Indian J. Sci. Technol. 2009, 2, 41–43. [Google Scholar] [CrossRef]
- Radhakrishnan, M.; Palayam, M.; Altemimi, A.B.; Karthik, L.; Krishnasamy, G.; Cacciola, F.; Leucine-Rich, P. Anti-Bacterial Protein against Vibrio cholerae, Staphylococcus aureus from Solanum trilobatum Leaves. Molecules 2022, 27, 1167. [Google Scholar] [CrossRef]
- Kannabiran, K.; Thanigaiarassu, R.R. Antibacterial Activity of Saponin Isolated from the Leaves of S. trilobatum Linn. J. Appl. Biol. Sci. 2008, 2, 109–112. [Google Scholar]
- Shahjahan, M.; Vani, G.; Shyamaladevi, C. Effect of S. trilobatum on the antioxidant status during diethyl nitrosamine induced and phenobarbital promoted hepatocarcinogenesis in rat. Chem. Biol. Interact. 2005, 156, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.; Jebanesan, A. Oviposition deterrent and skin repellent activities of S. trilobatum leaf extract against the malarial vector Anopheles stephensi. J. Insect Sci. 2005, 5, 15. [Google Scholar] [CrossRef]
- Thirumalaiandi, R.; Selvaraj, M.G.; Rajasekaran, R.; Subbarayalu, M. Cloning and characterization of resistance gene analogs from under-exploited plant species. Electron. J. Biotechnol. 2008, 11, 4–5. [Google Scholar] [CrossRef]
- Jantorn, P.; Tipmanee, V.; Wanna, W.; Prapasarakul, N.; Visutthi, M.; Sotthibandhu, D.S. Potential natural antimicrobial and antibiofilm properties of Piper betle L. against Staphylococcus pseudintermedius and methicillin-resistant strains. J. Ethnopharmacol. 2023, 317, 116820. [Google Scholar] [CrossRef]
- Gomes, F.; Martins, N.; Ferreira, I.C.; Henriques, M. Anti-biofilm activity of hydromethanolic plant extracts against Staphylococcus aureus isolates from bovine mastitis. Heliyon 2019, 5, e01728. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Ranjith, S.; Veerapandian, R.; Natarajaseenivasan, K.; Chidambaram, P.; Kumarasamy, A. Antibiofilm Activity of Epinecidin-1 and Its Variants Against Drug-Resistant Candida krusei and Candida tropicalis Isolates from Vaginal Candidiasis Patients. Infect. Dis. Rep. 2024, 16, 1214–1229. [Google Scholar] [CrossRef]
- Beynon, R.J.; Bond, J.S. Proteolytic Enzymes: A Practical Approach; IRL Press at Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Nagampalli, R.S.K.; Gunasekaran, K.; Narayanan, R.B.; Peters, A.; Bhaskaran, R. A structural biology approach to understand human lymphatic filarial infection. PLoS Neglected Trop. Dis. 2014, 8, e2662. [Google Scholar] [CrossRef]
- Zanphorlin, L.M.; Cabral, H.; Arantes, E.; Assis, D.; Juliano, L.; Juliano, M.A.; Bonilla-Rodriguez, G.O. Purification and characterization of a new alkaline serine protease from the thermophilic fungus Myceliophthora sp. Process Biochem. 2011, 46, 2137–2143. [Google Scholar] [CrossRef]
- Damare, S.; Mishra, A.; D’Souza-Ticlo-Diniz, D.; Krishnaswamy, A.; Raghukumar, C. A deep-sea hydrogen peroxide-stable alkaline serine protease from Aspergillus flavus. 3 Biotech 2020, 10, 528. [Google Scholar] [CrossRef]
- Alagarasan, C.; Ramya, K.S.; Manohar, R.; Siva, G.V. Partial Purification of Cysteine Protease from Nigella Sativa L. Seeds. Available online: http://www.rjlbpcs.com/article-pdf-downloads/2018/21/367.pdf (accessed on 1 January 2020).
- Karthik, L.; Manohar, R.; Elamparithi, K.; Gunasekaran, K. Purification, characterization and functional analysis of a serine protease inhibitor from the pulps of Cicer arietinum L.(Chick Pea). Indian J. Biochem. Biophys. (IJBB) 2019, 56, 117–124. [Google Scholar]
- Asif-Ullah, M.; Kim, K.-S.; Yu, Y.G. Purification and characterization of a serine protease from Cucumis trigonus Roxburghi. Phytochemistry 2006, 67, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Bykova, N.V.; Rampitsch, C.; Xing, T. Identification and characterization of a serine protease from wheat leaves. Eur. J. Plant Pathol. 2016, 146, 293–304. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Morishima, I.; Babiker, E.E.; Mori, N. Dubiumin, a chymotrypsin-like serine protease from the seeds of Solanum dubium Fresen. Phytochemistry 2009, 70, 483–491. [Google Scholar] [CrossRef]
- Alici, E.H.; Arabaci, G. A novel serine protease from strawberry (Fragaria ananassa): Purification and biochemical characterization. Int. J. Biol. Macromol. 2018, 114, 1295–1304. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Demır, N. Purification and characterization of a novel serine protease compositain from compositae (Scorzonera hispanica L.). Eur. Food Res. Technol. 2012, 234, 945–953. [Google Scholar] [CrossRef]
- Rawaliya, R.K.; Patidar, P.; Sharma, S.; Hajela, K. Purification and biochemical characterization of protease from the seeds of Cyamopsis tetragonoloba. J. Appl. Biol. Biotechnol. 2022, 10, 172–180. [Google Scholar]
- Tomar, R.; Kumar, R.; Jagannadham, M.V. A stable serine protease, wrightin, from the latex of the plant Wrightia tinctoria (Roxb.) R. Br.: Purification and biochemical properties. J. Agric. Food Chem. 2008, 56, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Mehrnoush, A.; Mustafa, S.; Sarker, M.Z.I.; Yazid, A.M.M. Optimization of the conditions for extraction of serine protease from Kesinai Plant (Streblus asper) leaves using response surface methodology. Molecules 2011, 16, 9245–9260. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radhakrishnan, M.; Balu, K.E.; Karthik, L.; Nagampalli, R.S.K.; Nadendla, E.K.; Krishnasamy, G. Exploring the Antibiotic Potential of a Serine Protease from Solanum trilobatum Against Staphylococcus aureus Biofilms. Infect. Dis. Rep. 2025, 17, 50. https://doi.org/10.3390/idr17030050
Radhakrishnan M, Balu KE, Karthik L, Nagampalli RSK, Nadendla EK, Krishnasamy G. Exploring the Antibiotic Potential of a Serine Protease from Solanum trilobatum Against Staphylococcus aureus Biofilms. Infectious Disease Reports. 2025; 17(3):50. https://doi.org/10.3390/idr17030050
Chicago/Turabian StyleRadhakrishnan, Manohar, Kanal Elamparithi Balu, Lakshminarayanan Karthik, Raghavendra Sashi Krishna Nagampalli, Eswar Kumar Nadendla, and Gunasekaran Krishnasamy. 2025. "Exploring the Antibiotic Potential of a Serine Protease from Solanum trilobatum Against Staphylococcus aureus Biofilms" Infectious Disease Reports 17, no. 3: 50. https://doi.org/10.3390/idr17030050
APA StyleRadhakrishnan, M., Balu, K. E., Karthik, L., Nagampalli, R. S. K., Nadendla, E. K., & Krishnasamy, G. (2025). Exploring the Antibiotic Potential of a Serine Protease from Solanum trilobatum Against Staphylococcus aureus Biofilms. Infectious Disease Reports, 17(3), 50. https://doi.org/10.3390/idr17030050






