Prevalence of VZV Reactivation and Effectiveness of Vaccination with Recombinant Adjuvanted Zoster Vaccine in Allogeneic Hematopoietic Stem Cell Recipients—A Single-Center Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Allo-HSCT
2.3. Types and Doses of Vaccine
2.4. Methods
3. Results
3.1. Patients
3.2. Transplantations
3.3. GvHD
3.4. The Prevalence of VZV Reactivation in the Entire Group Before Vaccination
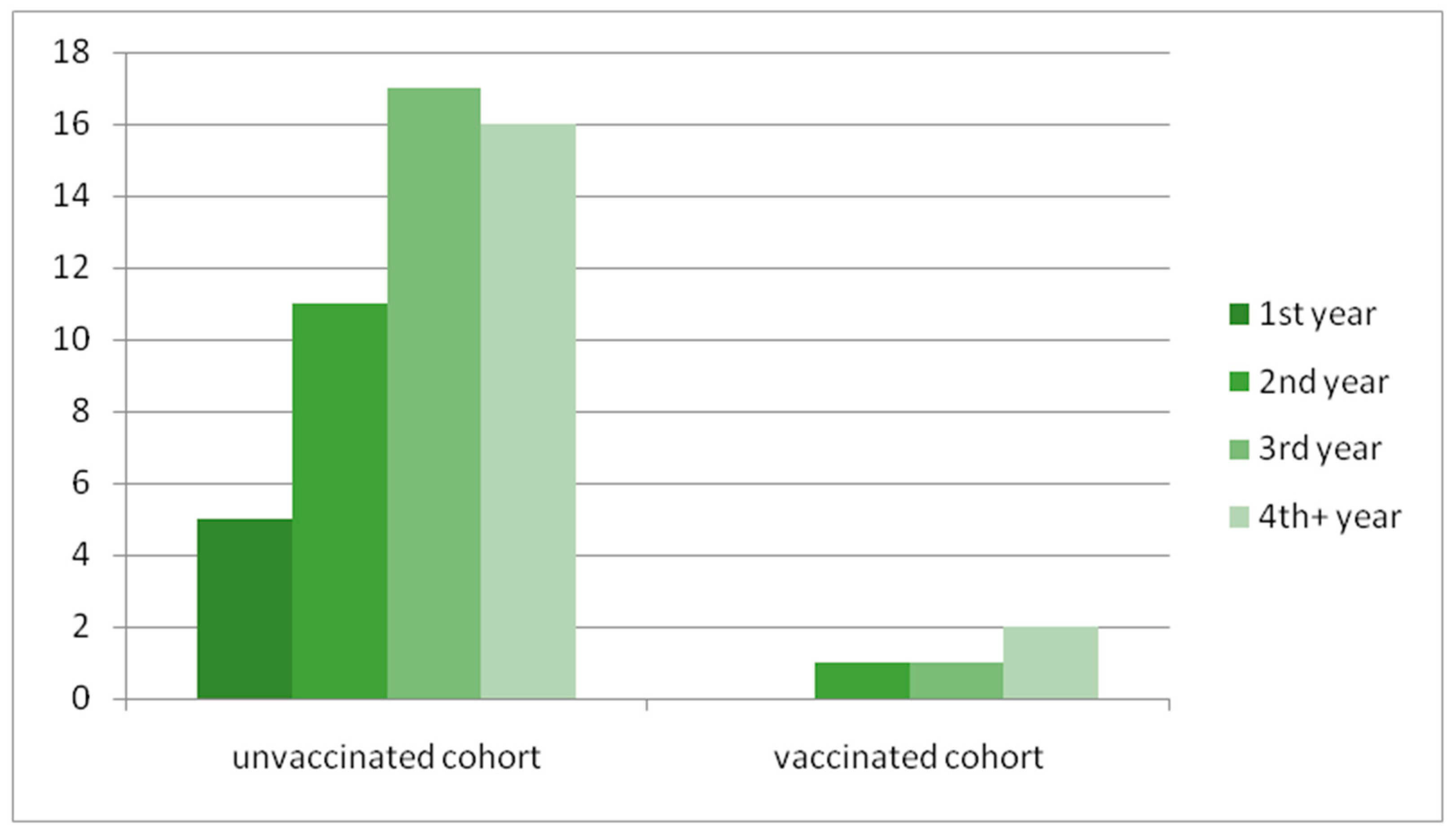
3.5. The Assessment of the Vaccinated Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orenstein, W.A.; Offit, P.A.; Edwards, K.M.; Plotkin, S.A. Plotkin’s Vaccines, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2022; Hardback ISBN: 9780323790581, table Chapter 63 Varicella Vaccines; pp. 1215–1250. [Google Scholar]
- Patel, S.Y.; Carbone, J.; Jolles, S. The Expanding Field of Secondary Antibody Deficiency: Causes, Diagnosis, and Management. Front. Immunol. 2019, 10, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, D.; Como, C.N.; Jing, L.; Blackmon, A.; Neff, C.P.; Krueger, O.; Bubak, A.N.; Palmer, B.E.; Koelle, D.M.; Nagel, M.A. Varicella zoster virus productively infects human peripheral blood mononuclear cells to modulate expression of immunoinhibitory proteins and blocking PD-L1 enhances virus-specific CD8+ T cell effector function. PloS Pathog. 2019, 15, e1007650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.J.; Savani, B.N.; Ljungman, P. Varicella Zoster Virus Reactivation in Adult Survivors of Hematopoietic Cell Transplantation: How Do We Best Protect Our Patients? Biol. Blood Marrow Transplant. 2018, 24, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, A.; de la Serna, J.; El Idrissi, M.; Oostvogels, L.; Quittet, P.; López-Jiménez, J.; Vural, F.; Pohlreich, D.; Zuckerman, T.; Issa, N.C.; et al. ZOE-HSCT Study Group Collaborators. Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster After Autologous Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2019, 322, 123–133, Erratum in JAMA 2019, 322, 785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curran, D.; Matthews, S.; Rowley, S.D.; Young, J.-A.H.; Bastidas, A.; Anagnostopoulos, A.; Barista, I.; Chandrasekar, P.H.; Dickinson, M.; El Idrissi, M.; et al. ZOE-HSCT Study group collaborators. Recombinant Zoster Vaccine Significantly Reduces the Impact on Quality of Life Caused by Herpes Zoster in Adult Autologous Hematopoietic Stem Cell Transplant Recipients: A Randomized Placebo-Controlled Trial (ZOE-HSCT). Biol. Blood Marrow Transplant. 2019, 25, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, M.; Schmidt-Hieber, M.; Sprute, R.; Buchheidt, D.; Hentrich, M.; Karthaus, M.; Penack, O.; Ruhnke, M.; Weissinger, F.; Cornely, O.A.; et al. Prophylaxis, diagnosis and therapy of infections in patients undergoing high-dose chemotherapy and autologous haematopoietic stem cell transplantation. 2020 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2021, 100, 321–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reynolds, G.; Hall, V.G.; Teh, B.W. Vaccine schedule recommendations and updates for patients with hematologic malignancy post-hematopoietic cell transplant or CAR T-cell therapy. Transpl. Infect. Dis. 2023, 25 (Suppl. S1), e14109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, P.; Patel, S.; Skinner, R.; Dignan, F.; Richter, A.; Jeffery, K.; Khan, A.; Heath, P.; Clark, A.; Orchard, K.; et al. Joint consensus statement on the vaccination of adult and paediatrichaematopoietic stem cell transplant recipients: Prepared on behalf of the British society of blood and marrow transplantation and cellular therapy (BSBMTCT), the Children’s cancer and Leukaemia Group (CCLG), and British Infection Association (BIA). J. Infect. 2023, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.C.; Masters, N.B.; Guo, A.; Shepersky, L.; Leidner, A.J.; Lee, G.M.; Kotton, C.N.; Dooling, K.L. Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 80–84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, E200–E212. [Google Scholar] [CrossRef]
- Styczynski, J.; Reusser, P.; Einsele, H.; de la Camara, R.; Cordonnier, C.; Ward, K.N.; Ljungman, P.; Engelhard, D.; Second European Conference on Infections in Leukemia. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: Guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009, 43, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef]
- Cupit-Link, M.C.; Arora, M.; Wood, W.A.; Hashmi, S.K. Relationship between Aging and Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- McGovern, K.E.; Sonar, S.A.; Watanabe, M.; Coplen, C.P.; Bradshaw, C.M.; Nikolich, J.Ž. The aging of the immune system and its implications for transplantation. Geroscience 2023, 45, 1383–1400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dorshkind, K.; Höfer, T.; Montecino-Rodriguez, E.; Pioli, P.D.; Rodewald, H.-R. Do haematopoietic stem cells age? Nat. Rev. Immunol. 2020, 20, 196–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, R.J.; Elias, H.K.; van den Brink, M.R.M. Immune Reconstitution in the Aging Host: Opportunities for Mechanism-Based Therapy in Allogeneic Hematopoietic Cell Transplantation. Front. Immunol. 2021, 12, 674093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerada, C.; Campbell, T.M.; Kennedy, J.J.; McSharry, B.P.; Steain, M.; Slobedman, B.; Abendroth, A. Manipulation of the Innate Immune Response by Varicella Zoster Virus. Front. Immunol. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ljungman, P.; Wilczek, H.; Gahrton, G.; Gustavsson, A.; Lundgren, G.; Lönnqvist, B.; Ringdén, O.; Wahren, B. Long-term acyclovir prophylaxis in bone marrow transplant recipients and lymphocyte proliferation responses to herpes virus antigens in vitro. Bone Marrow Transplant. 1986, 1, 185–192. [Google Scholar] [PubMed]
- Boeckh, M.; Kim, H.W.; Flowers, M.E.D.; Meyers, J.D.; Bowden, R.A. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—A randomized double-blind placebo-controlled study. Blood 2006, 107, 1800–1805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szenborn, L.; Kraszewska-Głomba, B.; Jackowska, T.; Duszczyk, E.; Majda-Stanisławska, E.; Marczyńska, M.; Ołdak, E.; Pawłowska, M.; Służewski, W.; Wysocki, J.; et al. Polish consensus guidelines on the use of acyclovir in the treatment and prevention of VZV and HSV infections. J. Infect. Chemother. 2016, 22, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Fathy, R.A.; McMahon, D.E.; Lee, C.; Chamberlin, G.C.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: A review of 40 cases in an International Dermatology Registry. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e6–e9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castagnoli, R.; Delmonte, O.M.; Calzoni, E.; Notarangelo, L.D. Hematopoietic Stem Cell Transplantation in Primary Immunodeficiency Diseases: Current Status and Future Perspectives. Front. Pediatr. 2019, 7, 295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers. 2015, 1, 15016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, P.G.E.; Gershon, A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumrin, E.; Izaguirre, N.E.; Bausk, B.; Feeley, M.M.; Bay, C.P.; Yang, Q.; Ho, V.T.; Baden, L.R.; Issa, N.C. Safety and reactogenicity of the recombinant zoster vaccine after allogeneic hematopoietic cell transplantation. Blood Adv. 2021, 5, 1585–1593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arvin, A.M. Varicella-Zoster virus: Pathogenesis, immunity, and clinicalmanagement in hematopoietic cell transplant recipients. Biol. Blood Marrow Transplant. 2000, 6, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Yamashita, K.; Mizugishi, K.; Kondo, T.; Kitano, T.; Hishizawa, M.; Kadowaki, N.; Takaori-Kondo, A. Risk factors for hypogammaglobulinemia after allo-SCT. Bone Marrow Transplant. 2014, 49, 859–861. [Google Scholar] [CrossRef]
- Feng, C.-J.; Zhao, P.; Fu, H.-X.; Yan, C.-H.; Wang, C.-C.; Zhu, X.-L.; He, Y.; Wang, F.-R.; Zhang, Y.-Y.; Mo, X.-D.; et al. Clinical characteristics and risk stratification for late-onset herpes zoster following allogeneic hematopoietic stem cell transplantation. Cancer Lett. 2024, 603, 217202. [Google Scholar] [CrossRef] [PubMed]
- de Berranger, E.; Derache, A.; Ramdane, N.; Labreuche, J.; Navarin, P.; Gonzales, F.; Abou-Chahla, W.; Nelken, B.; Bruno, B. VZV Prophylaxis After Allogeneic Hematopoietic Stem Cell Transplantation in Children: When to Stop? Cancer Rep. 2024, 7, e70015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sureda, A.; Corbacioglu, S.; Greco, R.; Kröger, N.; Carreras, E. The EBMT Handbook, 8th ed.; Springer: Cham, Switzerland, 2024; Chapter 38, Viral infections; pp. 331–343. [Google Scholar] [PubMed]
- Marijam, A.; Vroom, N.; Bhavsar, A.; Posiuniene, I.; Lecrenier, N.; Vroling, H. Systematic Literature Review on the Incidence of Herpes Zoster in Populations at Increased Risk of Disease in the EU/EEA, Switzerland, and the UK. Infect. Dis. Ther. 2024, 13, 1083–1104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jamani, K.; MacDonald, J.; Lavoie, M.; Williamson, T.S.; Brown, C.B.; Chaudhry, A.; Jimenez-Zepeda, V.H.; Duggan, P.; Tay, J.; Stewart, D.; et al. Zoster prophylaxis after allogeneic hematopoietic cell transplantation using acyclovir/valacyclovir followed by vaccination. Blood Adv. 2016, 1, 152–159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jotschke, S.; Schulze, S.; Jaekel, N.; Ludwig-Kraus, B.; Engelmann, R.; Kraus, F.B.; Zahn, C.; Nedlitz, N.; Prange-Krex, G.; Mohm, J.; et al. Longitudinal Humoral and Cellular Immune Responses Following SARS-CoV-2 Vaccination in Patients with Myeloid and Lymphoid Neoplasms Compared to a Reference Cohort: Results of a Prospective Trial of the East German Study Group for Hematology and Oncology (OSHO). Cancers 2022, 14, 1544. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients | Share of the Total (%) | Number of Patients Who Experienced HZ | Share of All HZ Patients (%) | Share of HZ Patients of the Total (%) | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 85 | 57 | 30 | 61 | 20 |
| Female | 64 | 43 | 19 | 39 | 12 |
| Age in years | |||||
| 18–40 | 57 | 38 | 24 | 49 | 16 |
| 41–60 | 74 | 49 | 20 | 41 | 13 |
| >=60 | 18 | 13 | 5 | 10 | 3 |
| Diagnosis | |||||
| AML | 82 | 55 | 27 | 55 | 18 |
| MDS | 17 | 11.5 | 7 | 14 | 5 |
| ALL | 17 | 11.5 | 6 | 12 | 4 |
| AA | 6 | 4 | 3 | 6 | 2 |
| MPN | 14 | 9 | 5 | 10 | 3 |
| Lymphoma | 13 | 9 | 1 | 2 | 0.7 |
| Conditioning | |||||
| MAC | 104 | 70 | 37 | 76 | 25 |
| NMA | 5 | 3 | 3 | 6 | 2 |
| RIC | 40 | 27 | 9 | 18 | 6 |
| Donor | |||||
| MRD | 49 | 33 | 15 | 31 | 10 |
| MUD | 83 | 56 | 30 | 61 | 20 |
| MMUD | 12 | 8 | 3 | 6 | 2 |
| Haploidentical | 5 | 3 | 1 | 2 | 0.7 |
| Acute GvHD | |||||
| Grade 1–2 | 40 | 27 | 2 | 4 | 1 |
| Grade 3–4 | 24 | 16 | 6 | 12 | 4 |
| Chronic GvHD | |||||
| Mild | 17 | 11 | 15 | 30 | 20 |
| Moderate | 33 | 22 | 6 | 12 | 4 |
| Severe | 34 | 34 | 4 | 8 | 4 |
| Clinical Manifestation | Number/Share of the Total | Need for Hospitalization |
|---|---|---|
| Intercostal nerves | 40 (81%) | |
| Cranial nerve | 3 (6%) | |
| Ophtalmicus | 2 (4%) | 1 |
| Ulnar | 2 (4%) | |
| Sacral plexus | 1 (2%) | |
| Disseminated form | 1 (2%) | 1 |
| Bacterial complication | 2 (4%) | 2 |
| Age | Gender | Diagnosis | Time to First Vaccine Dose After HSCT (Months) | Ratio Value for VZV IgG Before and After Vaccination (or *Titer in mIU/mL) | Time From the First Vaccination to HZ, Clinical Course | CD4/µL N [309–1139] | CD8/µL N [137–823] | CD19/µL N [70–460] | NK/µL N [80–430] | CD4/CD8 N [1.0–5.0] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||||||
| 1 | 22 | F | AML | 12 | 0.64 | 7.07 | 616 | 535 | 227 | 211 | 1.2 | |
| 2 | 34 | M | AML | 18 | 0.52 | 8.16 | 220 | 642 | 406 | 406 | 0.3 | |
| 3 | 53 | F | CML | 36 | 1.15 | 7.43 | 135 | 450 | 83 | 45 | 0.3 | |
| 4 | 40 | F | MDS | 31 | No data | 2.4 | 6 months, intercostal HZ with bacterial complications | 266 | 931 | 418 | 228 | 0.3 |
| 5 | 33 | F | MDS | 35 | 0.25 | 4.28 | 672 | 496 | 160 | 208 | 1.4 | |
| 6 | 41 | M | DLBCL | 18 | 0.77 | 3.09 | 5 months, disseminated form of HZ | 360 | 1400 | 1240 | 840 | 0.3 |
| 7 | 38 | M | PTCL | 60 | No data | 4.05 | 1161 | 826 | 258 | 258 | 1.4 | |
| 8 | 50 | M | CML | 14 | 142 * | 6.86 | 475 | 1425 | 1536 | 144 | 0.3 | |
| 9 | 54 | F | AML | 84 | No data | 3.44 | 312 | 396 | 408 | 72 | 0.8 | |
| 10 | 67 | F | AML | 61 | No data | 3.16 | 260 | 900 | No data | No data | 0.3 | |
| 11 | 50 | M | AML | 34 | 0.65 | 3.92 | 315 | 1095 | No data | No data | 0.3 | |
| 12 | 67 | M | AA | 48 | No data | 5.91 | 223 | 594 | 127 | 106 | 0.4 | |
| 13 | 22 | F | AML | 35 | 0.98 | 2.38 | 419 | 855 | 346 | 164 | 0.5 | |
| 14 | 36 | F | ALL | 29 | 0.72 | No data | 469 | 2180 | 110 | 83 | 0.2 | |
| 15 | 52 | F | AML | 12 | 0.44 | No data | 658 | 2632 | 799 | 423 | 0.3 | |
| 16 | 22 | F | AML | 17 | 0.7 | 8.3 | 451 | 354 | No data | No data | 1.27 | |
| 17 | 48 | F | AML | 48 | No data | No data | 718 | 800 | 773 | 359 | 0.9 | |
| 18 | 66 | M | MDS | 14 | No data | 1041 * | 654 | 2755 | 607 | 467 | 0.2 | |
| 19 | 23 | M | ALL | 47 | 1.01 | 7.57 | 10 months HZ with cranial nerve involvement | 266 | 714 | 336 | 42 | 0.4 |
| 20 | 26 | F | ALL | 26 | 90 * | 538 * | 9 months, intercostal form of HZ | 783 | 1276 | No data | No data | 0.6 |
| 21 | 66 | F | AML | 39 | 0.86 | 6.39 | 842 | 1427 | 421 | 356 | 0.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakulska-Prystupiuk, E.; Feliksbrot-Bratosiewicz, M.; Król, M.; Tomaszewska, A.; Jędrzejczak, W.W.; Basak, G.W. Prevalence of VZV Reactivation and Effectiveness of Vaccination with Recombinant Adjuvanted Zoster Vaccine in Allogeneic Hematopoietic Stem Cell Recipients—A Single-Center Analysis. Infect. Dis. Rep. 2025, 17, 48. https://doi.org/10.3390/idr17030048
Karakulska-Prystupiuk E, Feliksbrot-Bratosiewicz M, Król M, Tomaszewska A, Jędrzejczak WW, Basak GW. Prevalence of VZV Reactivation and Effectiveness of Vaccination with Recombinant Adjuvanted Zoster Vaccine in Allogeneic Hematopoietic Stem Cell Recipients—A Single-Center Analysis. Infectious Disease Reports. 2025; 17(3):48. https://doi.org/10.3390/idr17030048
Chicago/Turabian StyleKarakulska-Prystupiuk, Ewa, Magdalena Feliksbrot-Bratosiewicz, Maria Król, Agnieszka Tomaszewska, Wiesław Wiktor Jędrzejczak, and Grzegorz Władysław Basak. 2025. "Prevalence of VZV Reactivation and Effectiveness of Vaccination with Recombinant Adjuvanted Zoster Vaccine in Allogeneic Hematopoietic Stem Cell Recipients—A Single-Center Analysis" Infectious Disease Reports 17, no. 3: 48. https://doi.org/10.3390/idr17030048
APA StyleKarakulska-Prystupiuk, E., Feliksbrot-Bratosiewicz, M., Król, M., Tomaszewska, A., Jędrzejczak, W. W., & Basak, G. W. (2025). Prevalence of VZV Reactivation and Effectiveness of Vaccination with Recombinant Adjuvanted Zoster Vaccine in Allogeneic Hematopoietic Stem Cell Recipients—A Single-Center Analysis. Infectious Disease Reports, 17(3), 48. https://doi.org/10.3390/idr17030048






