Bone Marrow Infection by Pneumocystis jirovecii in a Patient with AIDS: A Case Report and Literature Review
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired immunodeficiency syndrome |
| CMV | Cytomegalovirus |
| DNA | Deoxyribonucleic acid |
| GMS | Grocott–Gömöri’s methenamine silver |
| H-E | Hematoxylin and eosin |
| HHV-8 | Human herpesvirus 8 |
| HIV | Human immunodeficiency virus |
| HLH | Hemophagocytic lymphohistiocytosis |
| KS | Kaposi’s sarcoma |
| LDH | Lactate dehydrogenase |
| MCD | Multicentric Castleman disease |
| Msg | Major surface glycoprotein |
| nPCR | Nested Polymerase chain reaction |
| PAS | Periodic Acid Schiff |
| PCP | Pneumocystis jirovecii pneumonia |
| PCR | Polymerase chain reaction |
| P. Jirovecii | Pneumocystis jirovecii |
| SIRS | Systemic inflammatory response syndrome |
| qPCR | Quantitative Polymerase chain reaction |
| VDRL | Venereal Disease Research Laboratory |
References
- Alanio, A.; Bretagne, S. Pneumocystis jirovecii detection in asymptomatic patients: What does its natural history tell us? F1000Research 2017, 6, 739. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, S.; Tokuda, H. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2012, 18, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Telzak, E.E.; Cote, R.J.; Gold, J.W.M.; Campbell, S.W.; Armstrong, D. Extrapulmonary Pneumocystis carinii Infections. Clin. Infect. Dis. 1990, 12, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Grier, D.D.; Lewis, Z.; Palavecino, E.L. Bone marrow involvement by Pneumocystis jiroveci. Br. J. Haematol. 2009, 145, 149. [Google Scholar] [CrossRef]
- Heyman, M.R.; Rasmussen, P. Pneumocystis carinii Involvement of the Bone Marrow in Acquired Immunodeficiency Syndrome. Am. J. Clin. Pathol. 1987, 87, 780–783. [Google Scholar] [CrossRef]
- Rossi, J.F. Pneumocystis carinii Infection of Bone Marrow in Patients With Malignant Lymphoma and Acquired Immunodeficiency Syndrome: Original Report of Three Cases. Arch. Intern. Med. 1990, 150, 450. [Google Scholar] [CrossRef]
- Rossi, J.F. Pneumocystis carinii in Bone Marrow. Ann. Intern. Med. 1985, 102, 868. [Google Scholar] [CrossRef]
- Unger, P.D.; Rosenblum, M.; Krown, S.E. Disseminated Pneumocystis carinii infection in a patient with acquired immunodeficiency syndrome. Hum. Pathol. 1988, 19, 113–116. [Google Scholar] [CrossRef]
- Lubat, E.; Megibow, A.J.; Balthazar, E.J.; Goldenberg, A.S.; Birnbaum, B.A.; Bosniak, M.A. Extrapulmonary Pneumocystis carinii infection in AIDS: CT findings. Radiology 1990, 174, 157–160. [Google Scholar] [CrossRef]
- Amin, M.B.; Abrash, M.P.; Mezger, E.; Sekerak, G.F. Systemic Dissemination of Pneumocystis Carinii in a Patient with Acquired Immunodeficiency Syndrome. Henry Ford Hosp. Med. J. 1990, 38, 68–71. [Google Scholar]
- Momose, H. Pneumocystis carinii as Foamy Exudate in Bone Marrow. JAMA J. Am. Med. Assoc. 1991, 265, 1672. [Google Scholar] [CrossRef]
- Lerdlamyong, K.; Sukpanichnant, S.; Rattanaumpawan, P. Bone marrow infection by Pneumocystis jirovecii. Br. J. Haematol. 2017, 176, 849. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.L.; Yajko, D.M.; Hadley, W.K. Extrapulmonary pneumocystosis. Clin. Microbiol. Rev. 1997, 10, 401–418. [Google Scholar] [CrossRef]
- Dumic, I.; Radovanovic, M.; Igandan, O.; Savic, I.; Nordstrom, C.W.; Jevtic, D.; Subramanian, A.; Ramanan, P. A Fatal Case of Kaposi Sarcoma Immune Reconstitution Syndrome (KS-IRIS) Complicated by Kaposi Sarcoma Inflammatory Cytokine Syndrome (KICS) or Multicentric Castleman Disease (MCD): A Case Report and Review. Am. J. Case Rep. 2020, 21, e926433-1–e926433-7. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Chattaraj, A.; Iqbal, Q.; Anjum, A.; Rehman, M.E.U.; Aijaz, Z.; Nasir, F.; Ansar, S.; Zangeneh, T.T.; Iftikhar, A. Pneumocystis jiroveci Pneumonia: A Review of Management in Human Immunodeficiency Virus (HIV) and Non-HIV Immunocompromised Patients. Avicenna J. Med. 2023, 13, 23–34. [Google Scholar] [CrossRef]
- Charpentier, E.; Ménard, S.; Marques, C.; Berry, A.; Iriart, X. Immune Response in Pneumocystis Infections According to the Host Immune System Status. J. Fungi 2021, 7, 625. [Google Scholar] [CrossRef]
- Castro, J.; Morrison-Bryant, M. Management of Pneumocystis Jirovecii pneumonia in HIV infected patients: Current options, challenges and future directions. HIVAIDS-Res. Palliat. Care 2010, 2, 123–134. [Google Scholar] [CrossRef]
- Fujii, T.; Iwamoto, A.; Nakamura, T.; Iwamoto, A.; Iwamoto, A.; Iwamoto, A. Pneumocystis pneumonia in patients with HIV infection: Clinical manifestations, laboratory findings, and radiological features. J. Infect. Chemother. 2007, 13, 1–7. [Google Scholar] [CrossRef]
- Weyant, R.B.; Kabbani, D.; Doucette, K.; Lau, C.; Cervera, C. Pneumocystis jirovecii: A review with a focus on prevention and treatment. Expert Opin. Pharmacother. 2021, 22, 1579–1592. [Google Scholar] [CrossRef]
- White, P.L.; Price, J.S.; Backx, M. Pneumocystis jirovecii Pneumonia: Epidemiology, Clinical Manifestation and Diagnosis. Curr. Fungal Infect. Rep. 2019, 13, 260–273. [Google Scholar] [CrossRef]
- Cruciani, M.; Marcati, P.; Malena, M.; Bosco, O.; Serpelloni, G.; Mengoli, C. Meta-analysis of diagnostic procedures for Pneumocystis carinii pneumonia in HIV-1-infected patients. Eur. Respir. J. 2002, 20, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Norris, K.A. Colonization by Pneumocystis jirovecii and Its Role in Disease. Clin. Microbiol. Rev. 2012, 25, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J.F.; Taylor, M.S.; Kocjan, G.; Evans, H.E.; Morris-Jones, S.; Gant, V.; Novak, T.; Costello, A.M.; Zumla, A.; Miller, R.F. Development and evaluation of a real-time PCR assay for detection of Pneumocystis jirovecii DNA in bronchoalveolar lavage fluid of HIV-infected patients. Thorax 2007, 63, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Larsen, H.H.; Huang, L.; Kovacs, J.A.; Crothers, K.; Silcott, V.A.; Morris, A.; Turner, J.R.; Beard, C.B.; Masur, H.; Fisher, S.H. A Prospective, Blinded Study of Quantitative Touch-Down Polymerase Chain Reaction Using Oral-Wash Samples for Diagnosis of Pneumocystis Pneumonia in HIV-Infected Patients. J. Infect. Dis. 2004, 189, 1679–1683. [Google Scholar] [CrossRef]
- Brown, L.; Rautemaa-Richardson, R.; Mengoli, C.; Alanio, A.; Barnes, R.A.; Bretagne, S.; Chen, S.C.; Cordonnier, C.; Donnelly, J.P.; Heinz, W.J.; et al. Polymerase Chain Reaction on Respiratory Tract Specimens of Immunocompromised Patients to Diagnose Pneumocystis Pneumonia: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2024, 79, 161–168. [Google Scholar] [CrossRef]
- Vohra, S.; Dhaliwal, H.S. Miliary Tuberculosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK562300/ (accessed on 15 September 2024).
- Tabaja, H.; Kanj, A.; El Zein, S.; Comba, I.Y.; Chehab, O.; Mahmood, M. A Review of Hemophagocytic Lymphohistiocytosis in Patients with HIV. Open Forum Infect. Dis. 2022, 9, ofac071. [Google Scholar] [CrossRef]

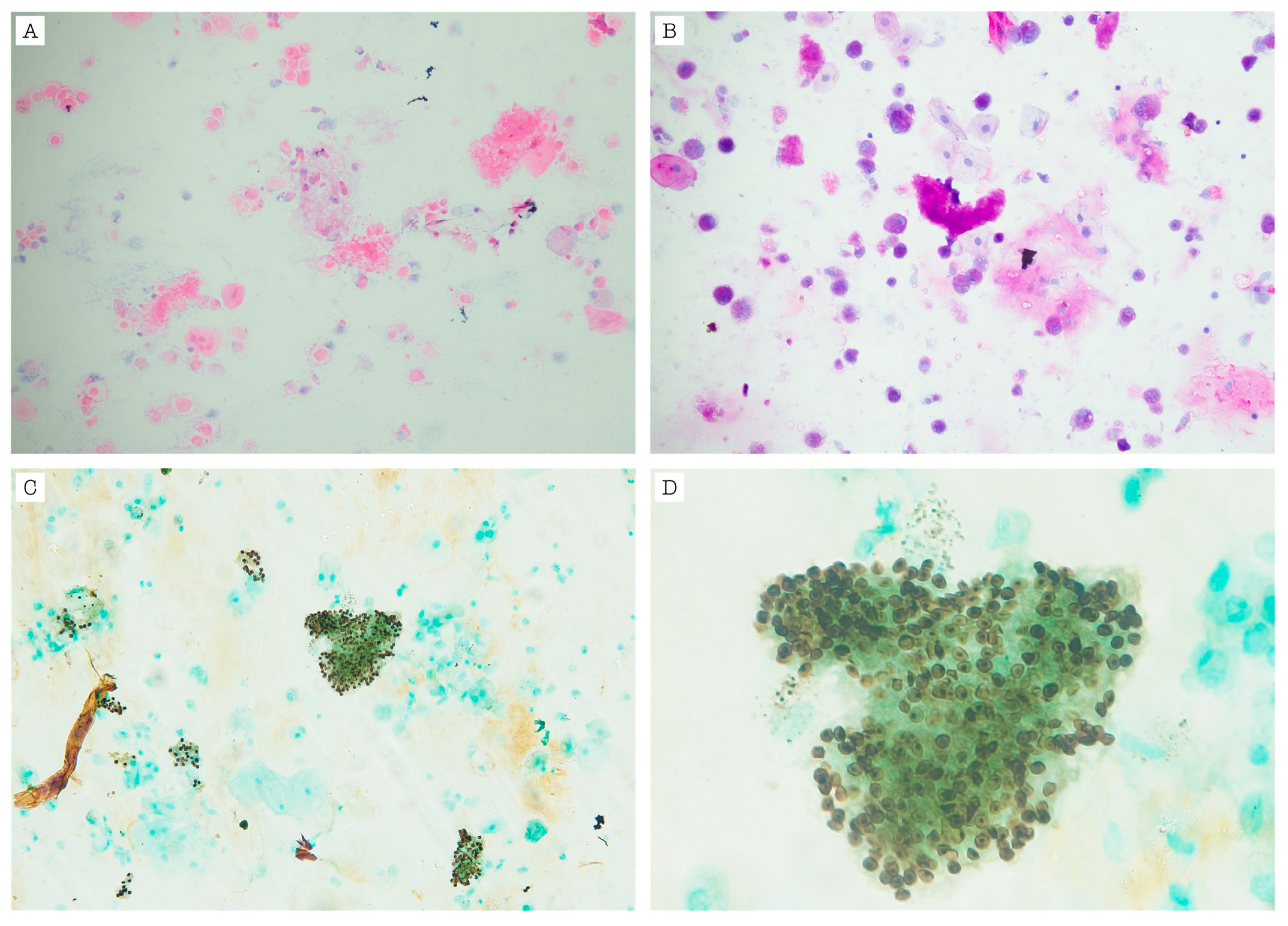
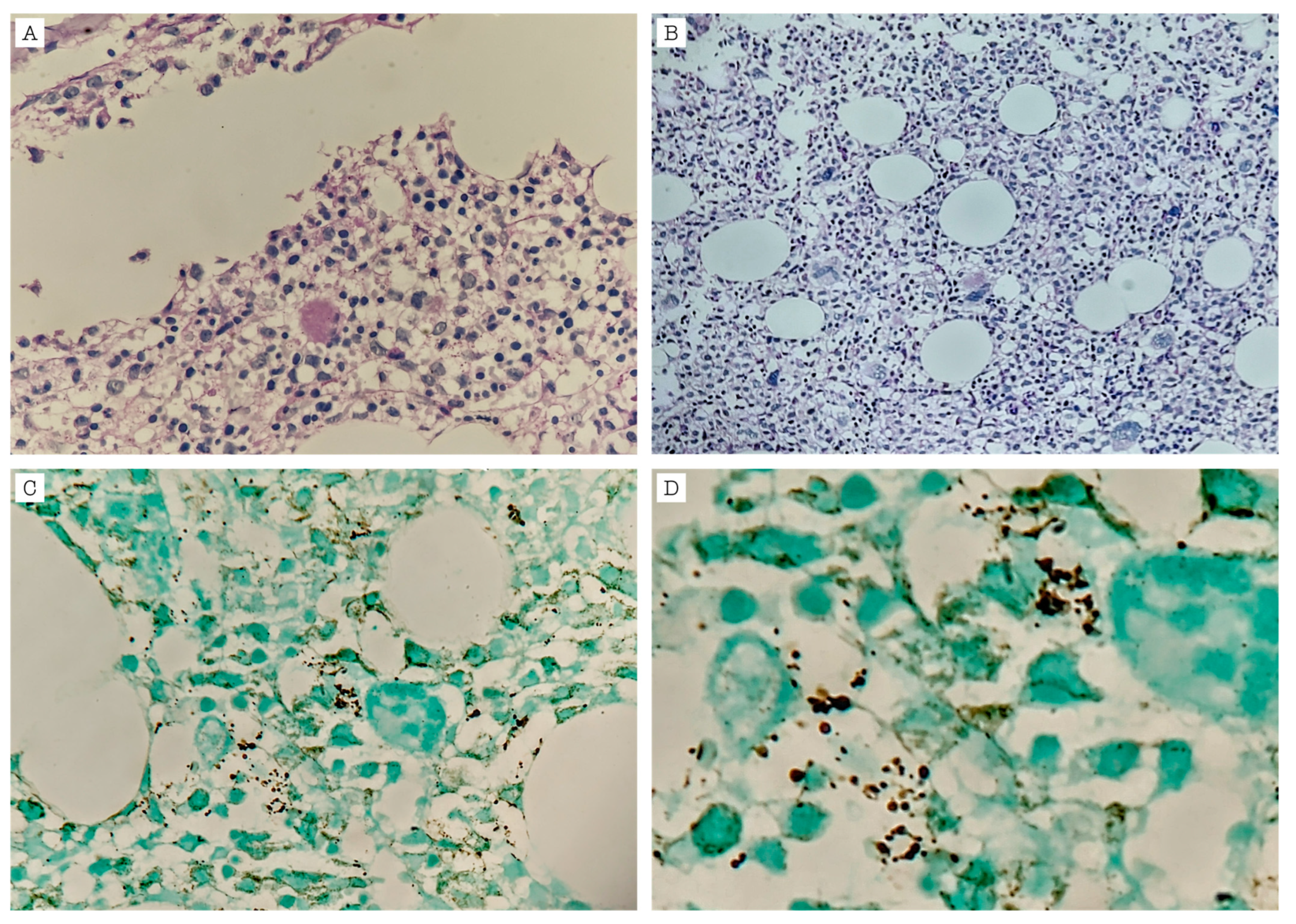
| Laboratory | Reference Values | Admission | Readmission (5 Days After Discharge) | Second Discharge (38 Days After Readmission) |
|---|---|---|---|---|
| White blood cell count (×103/μL) | 4.8–10.00 | 5.1 | 11.65 | 7.3 |
| Neutrophils (×103/μL) | 1.40–6.50 | 3.03 | 9.84 | 5.4 |
| Lymphocytes (×103/μL) | 0.80–4.00 | 1.54 | 1.13 | 1.37 |
| Monocytes (×103/μL) | 0.00–0.70 | 0.36 | 0.66 | 0.47 |
| Eosinophils (×103/μL) | 0.00–2.00 | 0.15 | 0 | 0.03 |
| Hematocrit (%) | 45.0–54.0 | 48.5 | 50.2 | 33.5 |
| Hemoglobin (g/dL) | 14.0–18.0 | 16.5 | 17 | 11.2 |
| Platelets (×103/μL) | 150–450 | 141 | 70 | 303 |
| C-reactive Protein (mg/L) | 0.00–10.00 | 22.4 | 76.06 | |
| Lactate Dehydrogenase (U/L) | 98.00–192.00 | 331 | ||
| Total Bilirubin (mg/dL) | 0.30–1.20 | 15.3 | 2.54 | |
| Direct bilirubin (mg/dL) | 0.10–0.50 | 10.2 | 1.17 | |
| Alanine aminotransferase (U/L) | 17.00–63.00 | 75.5 | 31.7 | |
| Aspartate aminotransferase (U/L) | 15.00–41.00 | 104.12 | 44.3 | |
| Blood urea nitrogen (mg/dL) | 6.00–20.00 | 8.74 | 17.4 | 25.2 |
| Creatinine (mg/dL) | 0.61–1.24 | 0.39 | 0.92 | 0.85 |
| pH | 7.350–7.450 | 7.45 | 7.51 | |
| SaO2 (%) | 80.0–100.0 | 92.6 | 93 | |
| PaO2 (mmHg) | 75.2 | 66 | ||
| PCO2 (mmHg) | 26.0–50.0 | 31 | 26 | |
| HCO3 (mmol/L) | 18.0–23.0 | 21.4 | 21 | |
| FiO2 (%) | 40 | 21 | ||
| PaO2/FiO2 | 188 | 314 | ||
| Alveolar–arterial gradient | 91.25 | 9.23 |
| Laboratory | Specimen | Results |
|---|---|---|
| Aerobic and anaerobic cultures | Blood | Negative |
| Treponema pallidum (VDRL) | Blood | Positive (1:4 dilutions) |
| HIV antibodies | Blood | Positive |
| Human immunodeficiency virus viral load (copies/mL) | Blood | Positive (2,095,776) |
| Lymphocytes CD4 (cells/mm3) | Blood | 33 |
| Lymphocytes CD45 (cells/mm3) | Blood | 524 |
| Lymphocytes CD3 (cells/mm3) | Blood | 368 |
| Lymphocytes CD8 (cells/mm3) | Blood | 305 |
| Hepatitis A antibodies | Blood | Negative |
| Hepatitis B antigen | Blood | Negative |
| Hepatitis C antibodies | Blood | Negative |
| Cytomegalovirus IgG antibodies | Blood | Positive (30.3) |
| Cytomegalovirus IgM antibodies | Blood | Negative |
| Typhoid O Antibodies (latex agglutination) | Blood | Negative |
| Typhoid H Antibodies (latex agglutination) | Blood | Negative |
| Brucella abortus Antibodies (latex agglutination) | Blood | Negative |
| Aspergillus Galactomannan Antigen (lateral flow assay) | Blood | Negative |
| Mycobacterium tuberculosis PCR (GeneXpert Ultra) | Sputum | Negative |
| Leptospira IgM Antibodies | Blood | Negative |
| Histoplasma Antigen | Urine | Negative |
| Cryptococcus Antigen (latex agglutination) | Blood | Negative |
| Epstein Barr IgG Antibodies | Blood | Positive |
| Epstein Barr IgM Antibodies | Blood | Negative |
| Ziehl-Neelsen | Bronchoalveolar lavage | Negative |
| Mycobacterium tuberculosis PCR (GeneXpert Ultra) | Bronchoalveolar lavage | Negative |
| Direct Fungal Microscopy | Bronchoalveolar lavage | Negative |
| Aspergillus Galactomannan Antigen | Bronchoalveolar lavage | Negative |
| Mycobacteria Growth Indicator Tube | Bronchoalveolar lavage | Negative |
| Fungal Culture | Bronchoalveolar lavage | Negative |
| Common Microorganisms Culture | Bronchoalveolar lavage | Negative |
| Author and Year | Sex and Age | Clinical Manifestations | Hematological Manifestations | Medical Records | Extramedullary Infection | Concomitant Conditions | Diagnosis | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Rossi, J.F., et al. (1985) [7] | F/75 | Cervical mass, signs and symptoms of hypercalcemia | Pancytopenia | Lymphoplasmacytic lymphoma in chemotherapy | PCP (postmortem findings) | Candida albicans septicemia | Bone marrow biopsy (G with amorphous eosinophilic material and some organisms with a central red nucleus and pale blue cytoplasm were seen, GMS with PJ cystic walls) | ND | Death |
| M/36 | Respiratory failure | Anemia, severe thrombocytopenia | Hodgkin’s disease in chemotherapy and radiotherapy, Hip osteonecrosis, Pulmonary fibrosis | PCP (first) | Bacteroides melaninogenica infection in hip | Bone marrow biopsy (G with amorphous eosinophilic material and some organisms with a central red nucleus and pale blue cytoplasm were seen, GMS with PJ cystic walls) | ND | Death | |
| Heyman, M.R., et al. (1987) [5] | M/34 | Weight loss, dyspnea, arthralgias, myalgias, malaise, anorexia, hair loss, fever (38.2 °C), night sweats and decreasing visual acuity | Pancytopenia | AIDS (CD4 count and viral load unknown), diabetes | PCP (first) | CMV retinitis, oral candidiasis, Mycobacterium avium intracellulare infection in bone marrow and lungs; CMV infection in lungs and liver (postmortem findings), Mycobacterium infection in liver, colon and lymph nodes (postmortem findings) | Bone marrow biopsy (GMS with numerous extracellular PJ organisms) | Cotrimoxazole | Death |
| Unger, P.D., et al. (1988) [8] | M/40 | Decreasing visual acuity, progressive confusion and withdrawal, decreased urinary output, and severe dyspnea | Anemia | AIDS (CD4 count and viral load unknown), KS | PCP (first), PJ infection of spleen, lymph nodes, adrenal gland, Virchow–Robin spaces (postmortem findings) | Staphylococcus aureus sepsis, KS | Post-mortem histologic examination of bone marrow (GMS with PJ cysts in characteristic ovoid, crescentic, and helmet-shaped configurations, MC stain was negative) | ND | Death |
| Lubat, E., et al. (1990) [9] | ND | ND | ND | ND | ND | ND | Bone marrow biopsy | ND | ND |
| Amin, M.B., et al. (1990) [10] | M/29 | Dyspnea, weakness, progressive cough, fever, cervical adenopathy | Pancytopenia | AIDS (CD4 count and viral load unknown) | PCP (first), PJ infection of lymph nodes, liver, spleen and kidneys (postmortem findings) | None | Post-mortem histologic examination of bone marrow (GMS with PJ cysts and amorphous exudates) | Dapsone, azidothymidine, aerosolized pentamidine, and aspirin (cotrimoxazole allergy) | Death |
| Telzak, E.E., et al. (1990) [3] | M/33 | Fever, chest pain, lethargy | Pancytopenia | AIDS (CD4 count and viral load unknown), KS, Candida esophagitis, Herpes zoster of the neck and jaw, CMV retinitis | PCP (first), PJ infection in liver, spleen, adrenals, kidneys and pituitary (postmortem findings) | KS involving the skin, trachea, lungs, duodenum, lymph nodes, and bone marrow; Mycobacterium avium intracellulare infection in lungs, lymph nodes and spleen; CMV infection in adrenal medulla (postmortem findings) | Post-mortem histologic examination of bone marrow (H-E with fluffy eosinophilic material and GMS with complete replacement of hematopoietic elements by dark round structures suggestive of PJ cysts) | Broad-spectrum antibiotics | Death |
| Rossi, J.F., et al. (1990) [6] | M/74 | Respiratory failure | ND | Alcoholic cirrhosis, in situ gastric neoplasm, AIDS (T CD4/CD8 0.7 ratio) | PCP (first) | ND | Bone marrow biopsy (G with foamy eosinophilic material, GMS with PJ cystic walls) | ND | Death |
| Momose, H., et al. (1991) [11] | M/35 | Fatigue and dyspnea | ND | AIDS (CD4 count and viral load unknown), CMV retinitis, KS | PCP (first) | CMV retinitis, KS | Bone marrow biopsy (H-E stain with a focus of foamy eosinophilic exudate with otherwise unremarkable hematopoietic elements; GMS showed PJ cysts; Immunoperoxidase stain positive for PJ on the eosinophilic material) | Intravenous pentamidine switched to oral cotrimoxazole | Death |
| Grier, D.D., et al. (2009) [4] | F/34 | Shortness of breath, abdominal distention, and lower extremity edema | Pancytopenia | Cutaneous T cell lymphoma | PJ infection of peritoneum and spleen | Disseminated Candida and CMV infections | Bone marrow biopsy (H-E stain with focal aggregates of foamy eosinophilic material; GMS stain demonstrated numerous cup-shaped organisms consistent with PJ) | ND | Death |
| Lerdlamyong, K., et al. (2017) [12] | M/45 | Fever, weight loss | Anemia, leukopenia | AIDS (CD4 count 0.01 × 109/L, viral load undetectable) | PCP (first) | Cryptococcal meningitis, CMV retinitis | Bone marrow biopsy (H-E stain, multiple small clusters of histiocytes containing several small yeast cells; GMS, numerous intracellular cup-shaped yeast cells without mucinous capsule, with lack of budding; MC staining was negative) | Clindamycin and primaquine for 3 weeks, then dapsone for secondary prophylaxis (cotrimoxazole allergy) | Symptoms and hematologic compromise improved on treatment. |
| this work | M/34 | Fatigue, diaphoresis, weight loss, dry cough, diarrhea, dyspnea | Thrombocytopenia with progression to pancytopenia | AIDS (CD4 Count: 33 cells/mL3. Viral load: 2,095,776 copies/mL) | PCP (first) | Syphilis, Oral candidiasis, KS | Bone marrow biopsy (H-E stain with foamy amorphic exudates; GMS with dark round small structures with no budding within myeloid and erythroid cells suggestive of PJ yeasts) | Cotrimoxazole for 21 days | Clinical improvement, died two months later |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubides-Diaz, D.A.; Negrette-Lazaro, V.; Poveda-Hurtado, V.; López-Salazar, J.P.; Calderón-Vargas, C.M.; Álvarez-Moreno, C.A. Bone Marrow Infection by Pneumocystis jirovecii in a Patient with AIDS: A Case Report and Literature Review. Infect. Dis. Rep. 2025, 17, 47. https://doi.org/10.3390/idr17030047
Cubides-Diaz DA, Negrette-Lazaro V, Poveda-Hurtado V, López-Salazar JP, Calderón-Vargas CM, Álvarez-Moreno CA. Bone Marrow Infection by Pneumocystis jirovecii in a Patient with AIDS: A Case Report and Literature Review. Infectious Disease Reports. 2025; 17(3):47. https://doi.org/10.3390/idr17030047
Chicago/Turabian StyleCubides-Diaz, Diego Alejandro, Valentina Negrette-Lazaro, Viviana Poveda-Hurtado, Juan Pablo López-Salazar, Carlos Mauricio Calderón-Vargas, and Carlos Arturo Álvarez-Moreno. 2025. "Bone Marrow Infection by Pneumocystis jirovecii in a Patient with AIDS: A Case Report and Literature Review" Infectious Disease Reports 17, no. 3: 47. https://doi.org/10.3390/idr17030047
APA StyleCubides-Diaz, D. A., Negrette-Lazaro, V., Poveda-Hurtado, V., López-Salazar, J. P., Calderón-Vargas, C. M., & Álvarez-Moreno, C. A. (2025). Bone Marrow Infection by Pneumocystis jirovecii in a Patient with AIDS: A Case Report and Literature Review. Infectious Disease Reports, 17(3), 47. https://doi.org/10.3390/idr17030047






