Humoral and Cell-Mediated Immunity Against SARS-CoV-2 in Healthcare Personnel Who Received Multiple mRNA Vaccines: A 4-Year Observational Study
Abstract
1. Introduction
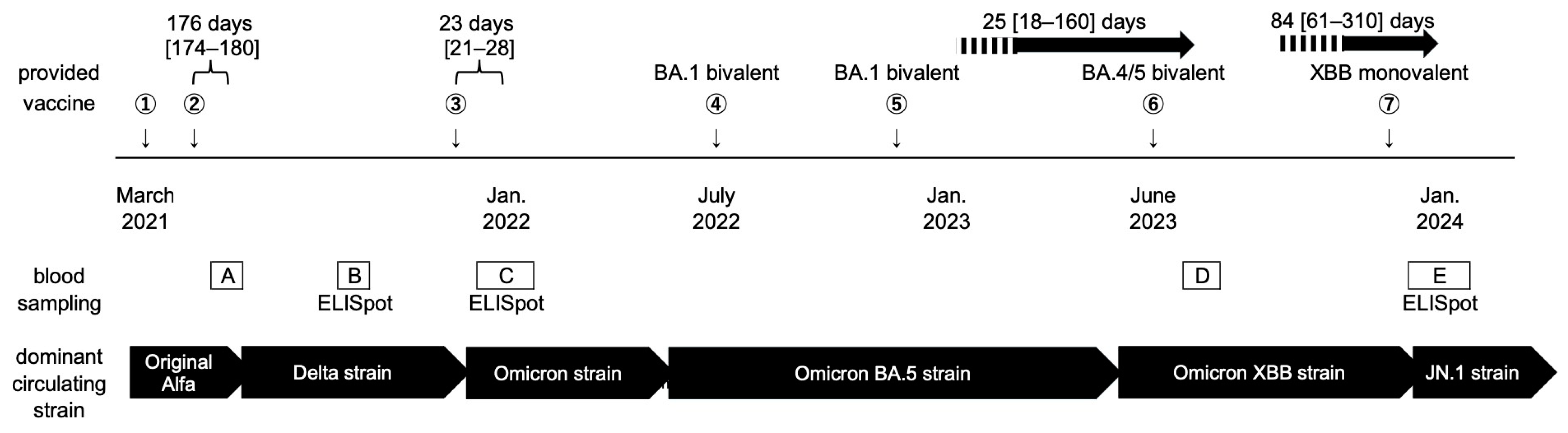
2. Materials and Methods
3. Results
3.1. Subjects’ Characteristics
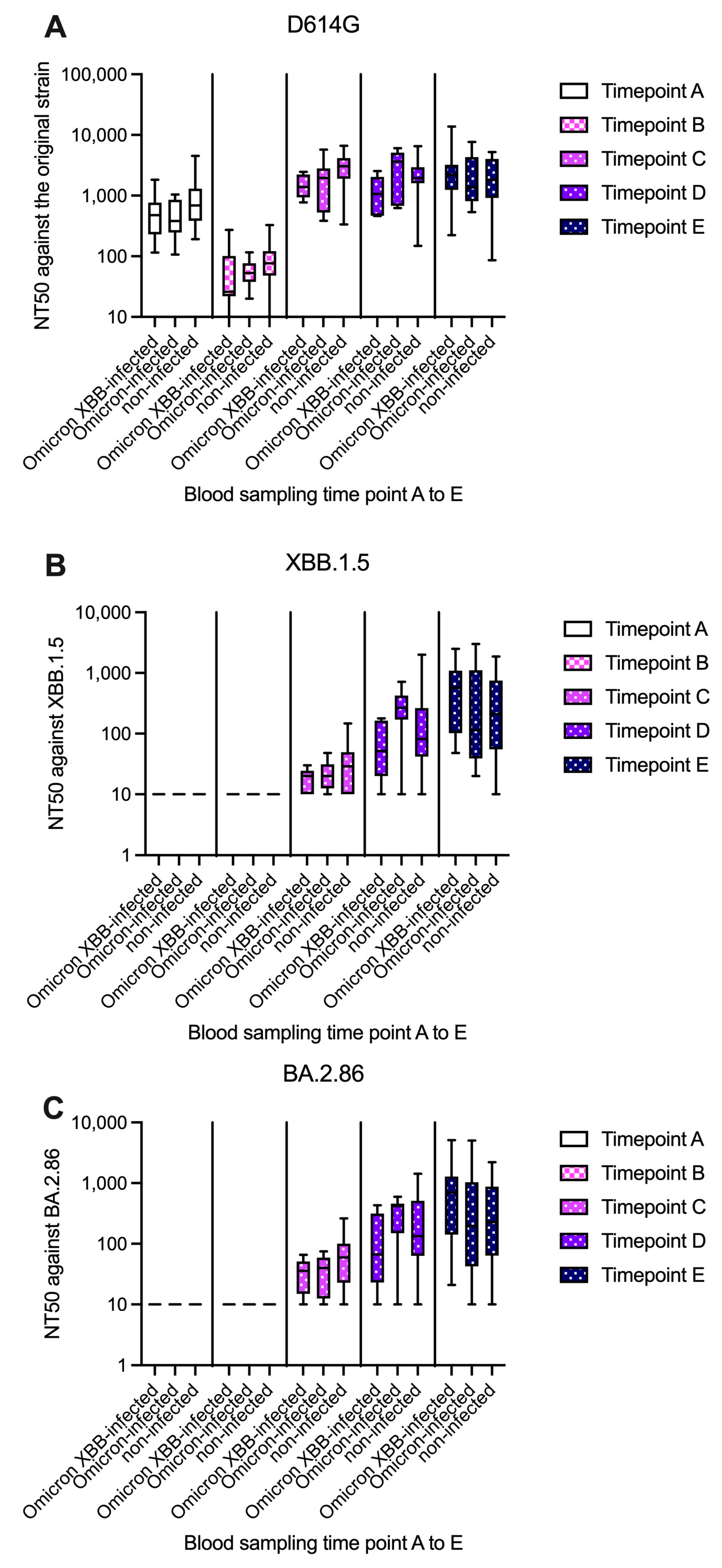
3.2. Analysis of Humoral Immunity
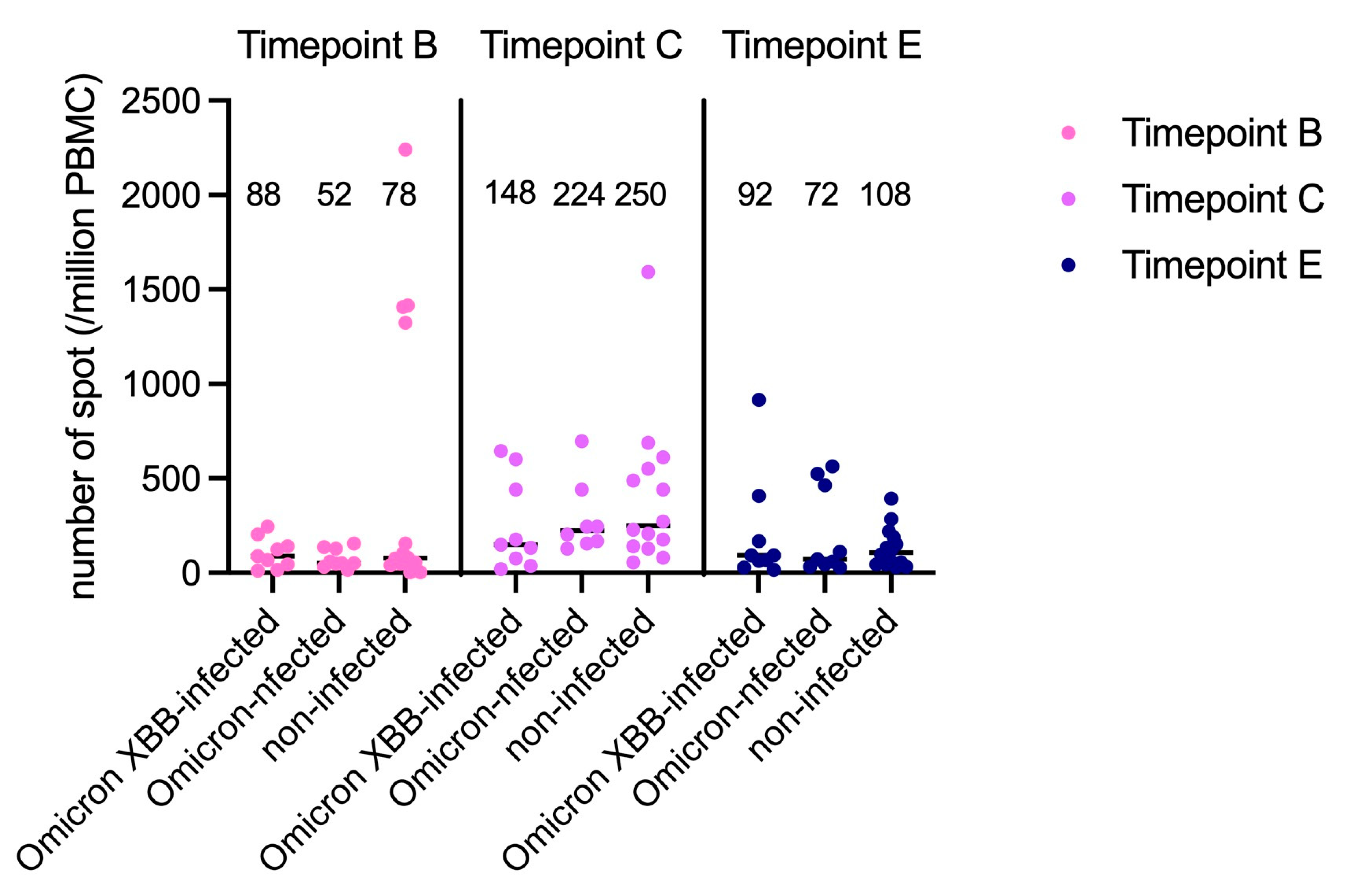
3.3. Analysis of Cell-Mediated Immunity
3.4. Factors Affecting Humoral Immunity in the Subjects Received Multiple Vaccination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| GMT | Geometric mean titer |
| PBMC | Peripheral blood mononuclear cells |
| SFC | Spot-forming cells |
References
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Regev-Yochay, G. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Shen, X. Boosting immunity to omicron. Nat. Med. 2022, 28, 445–446. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Miyakawa, K.; Ohtake, N.; Yamaoka, Y.; Yajima, S.; Yamazaki, E.; Shimada, T.; Goto, A.; Nakajima, H.; Ryo, A. Vaccine-induced humoral response against SARS-CoV-2 dramatically declined but cellular immunity possibly remained at 6 months post BNT162b2 vaccination. Vaccine 2022, 40, 2652–2655. [Google Scholar] [CrossRef]
- Furió, V.; Moya, A.; Sanjuán, R. The cost of replication fidelity in an RNA virus. Proc. Natl. Acad. Sci. USA 2005, 102, 10233–10237. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; Robertson, D.L.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Staying Up to Date with COVID-19 Vaccines. Available online: https://www.cdc.gov/covid/vaccines/stay-up-to-date.html (accessed on 15 March 2025).
- Tamura, T.; Irie, T.; Deguchi, S.; Yajima, H.; Tsuda, M.; Nasser, H.; Mizuma, K.; Plianchaisuk, A.; Suzuki, S.; Uriu, K.; et al. Virological characteristics of the SARS-CoV-2 omicron XBB.1.5 variant. Nat. Commun. 2024, 15, 1176. [Google Scholar] [CrossRef]
- Tamura, T.; Mizuma, K.; Nasser, H.; Deguchi, S.; Padilla-Blanco, M.; Oda, Y.; Uriu, K.; Tolentino, J.E.M.; Tsujino, S.; Suzuki, R.; et al. Virological characteristics of the SARS-CoV-2 BA.2.86 variant. Cell Host Microbe 2024, 32, 170–180. [Google Scholar] [CrossRef]
- Kruse, M.; Dark, C.; Aspden, M.; Cochrane, D.; Competiello, R.; Peltz, M.; Torres, L.; Wrighton-Smith, P.; Dudek, M. Performance of the T-SPOT®. COVID test for detecting SARS-CoV-2-responsive T cells. Int. J. Infect. Dis. 2021, 113, 155–161. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef]
- Faas, M.R.; Mak, W.A.; Markus, H.Y.; van der Zwan, E.M.; van der Vliet, M.; Koeleman, J.G.M.; Ong, D.S.Y. Dynamics of antibody and T cell immunity against SARS-CoV-2 variants of concern and the impact of booster vaccinations in previously infected and infection-naïve individuals. Vaccines 2022, 10, 2132. [Google Scholar] [CrossRef]
- Aoki, H.; Kitabatake, M.; Abe, H.; Xu, P.; Tsunoda, M.; Shichino, S.; Hara, A.; Ouji-Sageshima, N.; Motozono, C.; Ito, T.; et al. CD8+ T cell memory induced by successive SARS-CoV-2 mRNA vaccinations is characterized by shifts in clonal dominance. Cell Rep. 2024, 43, 113887. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Addetia, A.; Seo, A.J.; Brown, J.; Sprouse, K.; Logue, J.; Clark, E.; Franko, N.; Chu, H.; Veesler, D. Persistent immune imprinting occurs after vaccination with the COVID-19 XBB.1.5 mRNA booster in humans. Immunity 2024, 57, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Addetia, A.; Piccoli, L.; Case, J.B.; Park, Y.; Beltramello, M.; Guarino, B.; Clark, E.; Franko, N.; Chu, H.; Veesler, D.; et al. Neutralization, effector function and immune imprinting of omicron variants. Nature 2023, 621, 592–601. [Google Scholar] [CrossRef]
- Kotaki, R.; Moriyama, S.; Oishi, S.; Onodera, T.; Adachi, Y.; Sasaki, E.; Ishino, K.; Morikawa, M.; Takei, H.; Takahashi, H.; et al. Repeated omicron exposures redirect SARS-CoV-2-specific memory B cell evolution toward the latest variants. Sci. Transl. Med. 2024, 16, eadp9927. [Google Scholar] [CrossRef] [PubMed]
- Alsoussi, W.B.; Malladi, S.K.; Zhou, J.Q.; Liu, Z.; Ying, B.; Kim, W.; Schmitz, A.J.; Lei, T.; Horvath, S.C.; Sturtz, A.J.; et al. SARS-CoV-2 omicron boosting induces de novo B cell response in humans. Nature 2023, 617, 592–598. [Google Scholar] [CrossRef]
- Inoue, T.; Shinnakasu, R.; Kawai, C.; Yamamoto, H.; Sakakibara, S.; Ono, C.; Itoh, Y.; Terooatea, T.; Yamashita, K.; Okamoto, T.; et al. Antibody feedback contributes to facilitating the development of omicron-reactive memory B cells in SARS-CoV-2 mRNA vaccinees. J. Exp. Med. 2023, 220, e20221786. [Google Scholar] [CrossRef]
- Yisimayi, A.; Song, W.; Wang, J.; Jian, F.; Yu, Y.; Chen, X.; Xu, Y.; Yang, S.; Niu, X.; Xiao, T.; et al. Repeated omicron exposures override ancestral SARS-CoV-2 immune imprinting. Nature 2024, 625, 148–156. [Google Scholar] [CrossRef]
- Arashiro, T.; Arima, Y.; Muraoka, H.; Sato, A.; Oba, K.; Uehara, Y.; Arioka, H.; Yanai, H.; Kuramochi, J.; Ihara, G.; et al. Coronavirus disease 19 (COVID-19) vaccine effectiveness against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during delta-dominant and omicron-dominant periods in japan: A multicenter prospective case-control study. Clin. Infect. Dis. 2023, 76, e108–e115. [Google Scholar] [CrossRef]
- Kato, H.; Kurosawa, T.; Horikawa, K.; Kimura, Y.; Miyakawa, K.; Ryo, A.; Goto, A. Humoral response against spike protein enhanced by fifth and sixth COVID-19 mRNA vaccine in the uninfected and infected subjects. Hum. Vaccin. Immunother. 2023, 19, 2278376. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Moody, M.A.; Haynes, B.F. Antigen-specific B cell detection reagents: Use and quality control. Cytom. Part A J. Int. Soc. Anal. Cytol. 2008, 73, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A.J.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024, 24, e82. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Marshall, J.L.; Mawer, A.; Lopez-Ramon, R.; Harris, S.A.; Satti, I.; Hughes, E.; Preston-Jones, H.; Puig, I.C.; Longet, S.; et al. Safety, tolerability, viral kinetics, and immune correlates of protection in healthy, seropositive UK adults inoculated with SARS-CoV-2: A single-centre, open-label, phase 1 controlled human infection study. Lancet Microbe 2024, 5, 655–668. [Google Scholar] [CrossRef]
- Ishii, T.; Hamada, K.; Jubishi, D.; Hashimoto, H.; Okamoto, K.; Hisasue, N.; Sunohara, M.; Saito, M.; Shinohara, T.; Yamashita, M.; et al. Waning cellular immune responses and predictive factors in maintaining cellular immunity against SARS-CoV-2 six months after BNT162b2 mRNA vaccination. Sci. Rep. 2023, 13, 9607. [Google Scholar] [CrossRef]
- Juhl, A.K.; Loksø Dietz, L.; Schmeltz Søgaard, O.; Reekie, J.; Nielsen, H.; Johansen, S.; Benfield, T.; Wiese, L.; Stærke, N.B.; Jensen, T.Ø.; et al. Longitudinal evaluation of SARS-CoV-2 T cell immunity over 2 years following vaccination and infection. J. Infect. Dis. 2024, 230, e605–e615. [Google Scholar] [CrossRef]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- Peeters, M.; Verbruggen, L.; Teuwen, L.; Vanhoutte, G.; Vande Kerckhove, S.; Peeters, B.; Raats, S.; Van der Massen, I.; De Keersmaecker, S.; Debie, Y.; et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021, 6, 100274. [Google Scholar] [CrossRef]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transpl. 2021, 21, 2719–2726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, H.; Sano, K.; Miyakawa, K.; Kurosawa, T.; Horikawa, K.; Kimura, Y.; Goto, A.; Ryo, A. Humoral and Cell-Mediated Immunity Against SARS-CoV-2 in Healthcare Personnel Who Received Multiple mRNA Vaccines: A 4-Year Observational Study. Infect. Dis. Rep. 2025, 17, 42. https://doi.org/10.3390/idr17030042
Kato H, Sano K, Miyakawa K, Kurosawa T, Horikawa K, Kimura Y, Goto A, Ryo A. Humoral and Cell-Mediated Immunity Against SARS-CoV-2 in Healthcare Personnel Who Received Multiple mRNA Vaccines: A 4-Year Observational Study. Infectious Disease Reports. 2025; 17(3):42. https://doi.org/10.3390/idr17030042
Chicago/Turabian StyleKato, Hideaki, Kaori Sano, Kei Miyakawa, Takayuki Kurosawa, Kazuo Horikawa, Yayoi Kimura, Atsushi Goto, and Akihide Ryo. 2025. "Humoral and Cell-Mediated Immunity Against SARS-CoV-2 in Healthcare Personnel Who Received Multiple mRNA Vaccines: A 4-Year Observational Study" Infectious Disease Reports 17, no. 3: 42. https://doi.org/10.3390/idr17030042
APA StyleKato, H., Sano, K., Miyakawa, K., Kurosawa, T., Horikawa, K., Kimura, Y., Goto, A., & Ryo, A. (2025). Humoral and Cell-Mediated Immunity Against SARS-CoV-2 in Healthcare Personnel Who Received Multiple mRNA Vaccines: A 4-Year Observational Study. Infectious Disease Reports, 17(3), 42. https://doi.org/10.3390/idr17030042


_Rachiotis.png)


