Streptococcus intermedius Brain Abscess with Lung Abscess and Aortic Valve Endocarditis: A Case Report and Literature Review
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shinzato, T.; Saito, A. The Streptococcus milleri group as a cause of pulmonary infections. Clin. Infect. Dis. 1995, 21 (Suppl. S3), S238–S243. [Google Scholar]
- Noguchi, S.; Yatera, K.; Kawanami, T.; Yamasaki, K.; Naito, K.; Akata, K.; Shimabukuro, I.; Ishimoto, H.; Yoshii, C.; Mukae, H. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm. Med. 2015, 15, 133. [Google Scholar] [CrossRef]
- Melo, J.C.; Raff, M.J. Brain abscess due to Streptococcus MG-intermedius (Streptococcus milleri). J. Clin. Microbiol. 1978, 7, 529–532. [Google Scholar] [CrossRef]
- Honnorat, E.; Seng, P.; Riberi, A.; Habib, G.; Stein, A. Late infectious endocarditis of surgical patch closure of atrial septal defects diagnosed by 18F-fluorodeoxyglucose gated cardiac computed tomography (18F-FDG-PET/CT): A case report. BMC Res. Notes 2016, 9, 416. [Google Scholar] [CrossRef]
- Syros, G.; Briasoulis, A.; Psevdos, G. Suspicious seizures: Uncommon complication of PFO/ASA. J. Cardiol. Cases 2011, 3, e170–e172. [Google Scholar]
- Nakaya, M.; Okimoto, M.; Abe, H.; Sato, A.; Watanabe, Y.; Nakajima, N. A mitral valve reconstruction of infective endocarditis with brain abscess and intracranial mycotic aneurysm. Jpn. J. Thorac. Cardiovasc. Surg. 1998, 46, 647–650. [Google Scholar]
- Tran, M.P.; Caldwell-McMillan, M.; Khalife, W.; Young, V.B. Streptococcus intermedius causing infective endocarditis and abscesses: A report of three cases and review of the literature. BMC Infect. Dis. 2008, 8, 154. [Google Scholar]
- Carrena, O.; Oluoha, O.; Wahba, A.; Eshun, D.; Endsley, M.; Okafor, H. Complicated Infective Endocarditis Limited to a Chiari Network. Case Rep. Cardiol. 2018, 2018, 3837825. [Google Scholar]
- Bueno, C.O.P.; Trillos, S.J.G.; Rosales, D.J.C.; García, E.A.B. Lung abscess due to Streptococcus intermedius associated with SARS-CoV-2 infection in pregnancy: Unusual presentation and complication of a commensal bacteria during pregnancy. Clin. Case Rep. 2023, 11, e6763. [Google Scholar]
- Thwaites, G.E.; Nguyen, D.B.; Nguyen, H.D.; Hoang, T.Q.; Do, T.T.; Nguyen, T.C.; Nguyen, Q.H.; Nguyen, T.T.; Nguyen, N.H.; Nguyen, T.N.; et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 2004, 351, 1741–1751. [Google Scholar]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; DiBernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-ISCVID Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023, ciad271. [Google Scholar] [CrossRef]
- Erne, B.V.; Exner, C.; Frauenfelder, T.; Schmid, S.; Weder, W. A curious case of convulsion. Lancet 2010, 375, 2050. [Google Scholar] [CrossRef]
- Jerng, J.S.; Hsueh, P.R.; Teng, L.J.; Lee, L.N.; Yang, P.C.; Luh, K.T. Empyema thoracis and lung abscess caused by viridans streptococci. Am. J. Respir. Crit. Care Med. 1997, 156, 1508–1514. [Google Scholar] [CrossRef]
- Chandy, B.; Todd, J.; Stucker, F.J.; Nathan, C.O. Pott’s puffy tumor and epidural abscess arising from dental sepsis: A case report. Laryngoscope 2001, 111, 1732–1734. [Google Scholar] [CrossRef]
- May, A.M.; Riede, F.N.; Riede, U.N. Acute subepicardial infarction associated with severe septic shock--insight in myocardial perfusion. Pathol. Res. Pract. 2010, 206, 401–404. [Google Scholar] [CrossRef]
- Van Laren, M.; van Walree, N.C.; Kluytmans, J.A. Multiple lung abscesses secondary to a uterine empyema caused by an intrauterine device. Infection 2011, 39, 385–387. [Google Scholar] [CrossRef]
- De Kruif, M.D.; van Gorp, E.C.; Bel, E.H.; Gerlag, D.M.; Kunst, P.W. Streptococcal lung abscesses from a dental focus following tocilizumab: A case report. Clin. Exp. Rheumatol. 2012, 30, 951–953. [Google Scholar]
- Trabue, C.; Pearman, R.; Doering, T. Pyogenic brain and lung abscesses due to Streptococcus intermedius. J. Gen. Intern. Med. 2014, 29, 407. [Google Scholar] [CrossRef]
- Maeda, S.; Sado, T.; Sakurada, A.; Okada, Y.; Kondo, T. Successful closure of an open-window thoracostomy wound by negative-pressure wound therapy: Report of a case. Surg. Today 2012, 42, 295–298. [Google Scholar] [CrossRef]
- Armendariz-Guezala, M.; Undabeitia-Huertas, J.; Samprón-Lebed, N.; Michan-Mendez, M.; Ruiz-Diaz, I.; Úrculo-Bareño, E. Actinomycotic brain abscess in immunocompetent patient. Cir. Cir. 2017, 85 (Suppl. S1), 103–107. [Google Scholar]
- Sakurai, M.; Nagasawa, H.; Takeuchi, I.; Yanagawa, Y. A Case of an 80-Year-Old Man with Empyema and Psoas Abscess. Case Rep. Emerg. Med. 2020, 2020, 8895785. [Google Scholar]
- Fujihara, T.; Itoh, N.; Yamamoto, S.; Kurai, H. Lateral thoracic artery aneurysm with lung abscess and empyema caused by Streptococcus intermedius. J. Gen. Fam. Med. 2021, 22, 296–297. [Google Scholar] [CrossRef]
- Tasleem, A.; Mahmood, A.; Sharma, R. Streptococcus intermedius Pleuropulmonary Disease: A Not So Commonly Seen Radiological Picture. Cureus 2021, 13, 17385. [Google Scholar] [CrossRef]
- Manasrah, N.; Nanja Reddy, S.; Al Sbihi, A.; Hafeez, W. Streptococcus intermedius: Unusual presentation and complication of lung abscess. BMJ Case Rep. 2021, 14, e245675. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Otake, S.; Oue, T.; Ryu, H.; Kasai, M. Case of infant invasive Streptococcus intermedius infection suggesting the need for anaerobic cultures. J. Infect. Chemother. 2022, 28, 437–439. [Google Scholar] [CrossRef]
- Christensen, P.J.; Kutty, K.; Adlam, R.T.; Taft, T.A.; Kampschroer, B.H. Septic pulmonary embolism due to periodontal disease. Chest 1993, 104, 1927–1929. [Google Scholar]
- Patail, H.; Patail, H.; Ahmad, S. A Man in His 30s Presenting with Shortness of Breath and Productive Cough After a Recent Pneumonia. Chest 2020, 157, e91–e93. [Google Scholar] [CrossRef]
- Takahashi, S.; Ishitsuka, T.; Namatame, K.; Nakamura, M. A false-positive pneumococcal rapid urinary antigen test in Streptococcus intermedius infection. Respirol. Case Rep. 2019, 7, e00466. [Google Scholar]
- Dyrhovden, R.; Nygaard, R.M.; Patel, R.; Ulvestad, E.; Kommedal, Ø. The bacterial aetiology of pleural empyema. A descriptive and comparative metagenomic study. Clin. Microbiol. Infect. 2019, 25, 981–986. [Google Scholar] [CrossRef]
- Cobo, F.; Sampedro, A.; Rodríguez-Granger, J.; Aliaga-Martínez, L.; Navarro-Marí, J.M. Clinical and microbiologic characteristics of pleuro-pulmonary infection due to Streptococcus intermedius. Rev. Esp. Quimioter. 2018, 31, 146–151. [Google Scholar]
- Crespo Valadés, E.; Fernández Blanco, J.M.; Troya García, J.; Malmierca Corral, M. Primary left subphrenic abscess due to Streptococcus intermedius. Ann. Med. Intern. 2005, 22, 202–203. [Google Scholar]
- Noguchi, S.; Yatera, K.; Kawanami, T.; Yamasaki, K.; Fukuda, K.; Naito, K.; Akata, K.; Nagata, S.; Ishimoto, H.; Taniguchi, H.; et al. Pneumonia and empyema caused by Streptococcus intermedius that shows the diagnostic importance of evaluating the microbiota in the lower respiratory tract. Intern. Med. 2014, 53, 47–50. [Google Scholar] [CrossRef]
- Hannoodi, F.; Ali, I.; Sabbagh, H.; Kumar, S. Streptococcus intermedius Causing Necrotizing Pneumonia in an Immune Competent Female: A Case Report and Literature Review. Case Rep. Pulmonol. 2016, 2016, 7452161. [Google Scholar] [PubMed]
- Mautner, G.H.; Lu, I.; Ort, R.J.; Grossman, M.E. Transverse leukonychia with systemic infection. Cutis 2000, 65, 318–320. [Google Scholar] [PubMed]
- Huang, M.; Li, S.; Wu, X.; Xu, D.; Tang, L.; Chen, Z. An isolated pulmonary nodule secondary to Streptococcus intermedius infection in an otherwise healthy 10-year-old boy: A case report and literature review. Front. Pediatr. 2022, 10, 921258. [Google Scholar] [CrossRef] [PubMed]
- Kurkowski, S.C.; Thimmesch, M.J.; Jha, P.; Abdelgadir, Y.H. Streptococcus intermedius Bacteremia and Pyogenic Liver Abscess in a Patient with No Risk Factors. Cureus 2022, 14, e26786. [Google Scholar] [CrossRef]
- Jud, P.; Fink-Neuboeck, N.; Lindenmann, J. Massive ventilator-associated pleural empyema. Korean J. Intern. Med. 2019, 34, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Lescan, M.; Lepski, G.; Steger, V.; Schlensak, C.; Walker, T. Rapidly progressive paraplegia and pleural empyema: How does that correlate? Gen. Thorac. Cardiovasc. Surg. 2013, 61, 640–642. [Google Scholar]
- Stelzmueller, I.; Berger, N.; Wiesmayr, S.; Eller, M.; Tabarelli, W.; Fille, M.; Margreiter, R.; Bonatti, H. Group milleri streptococci: Significant pathogens in solid organ recipients. Transpl. Int. 2007, 20, 51–56. [Google Scholar] [CrossRef]
- Iskandar, S.B.; Al Hasan, M.A.; Roy, T.M.; Byrd, R.P., Jr. Streptococcus intermedius: An unusual cause of a primary empyema. Tenn. Med. 2006, 99, 37–39. [Google Scholar]
- Lau, S.K.; Woo, P.C.; Chan, B.Y.; Fung, A.M.; Que, T.L.; Yuen, K.Y. Haemophilus segnis polymicrobial and monomicrobial bacteraemia identified by 16S ribosomal RNA gene sequencing. J. Med. Microbiol. 2002, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, T.; Fukuda, K.; Yatera, K.; Kido, M.; Mukae, H.; Taniguchi, H. A higher significance of anaerobes: The clone library analysis of bacterial pleurisy. Chest 2011, 139, 600–608. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kawanami, T.; Yatera, K.; Fukuda, K.; Noguchi, S.; Nagata, S.; Nishida, C.; Kido, T.; Ishimoto, H.; Taniguchi, H.; et al. Significance of anaerobes and oral bacteria in community-acquired pneumonia. PLoS ONE 2013, 8, e63103. [Google Scholar] [CrossRef]
- Teramoto, S.; Fukuchi, Y.; Sasaki, H.; Sato, K.; Sekizawa, K.; Matsuse, T.; Japanese Study Group on Aspiration Pulmonary Disease. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: A multicenter, prospective study in Japan. J. Am. Geriatr. Soc. 2008, 56, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Issa, E.; Salloum, T.; Tokajian, S. From Normal Flora to Brain Abscesses: A Review of Streptococcus intermedius. Front. Microbiol. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
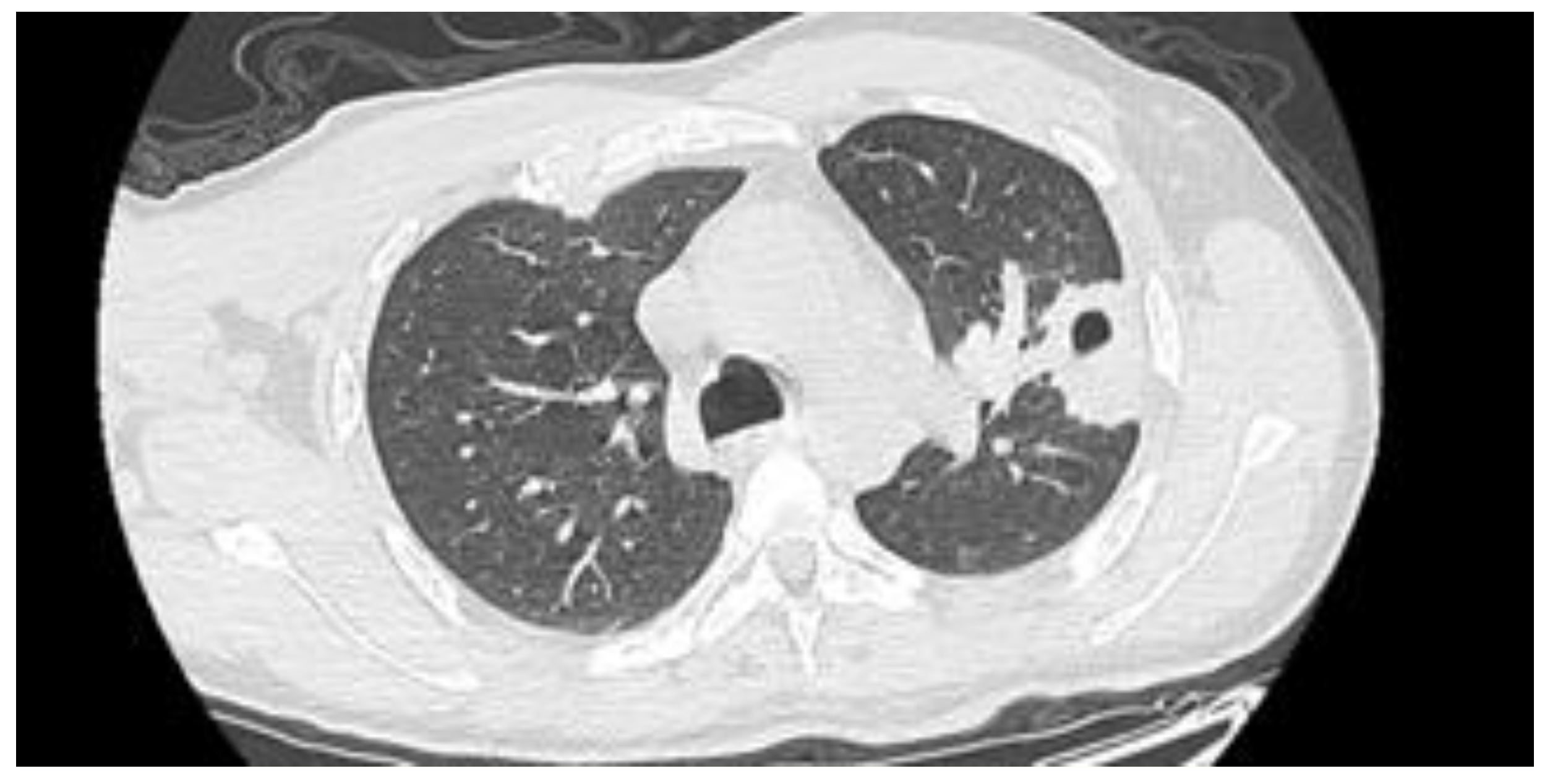
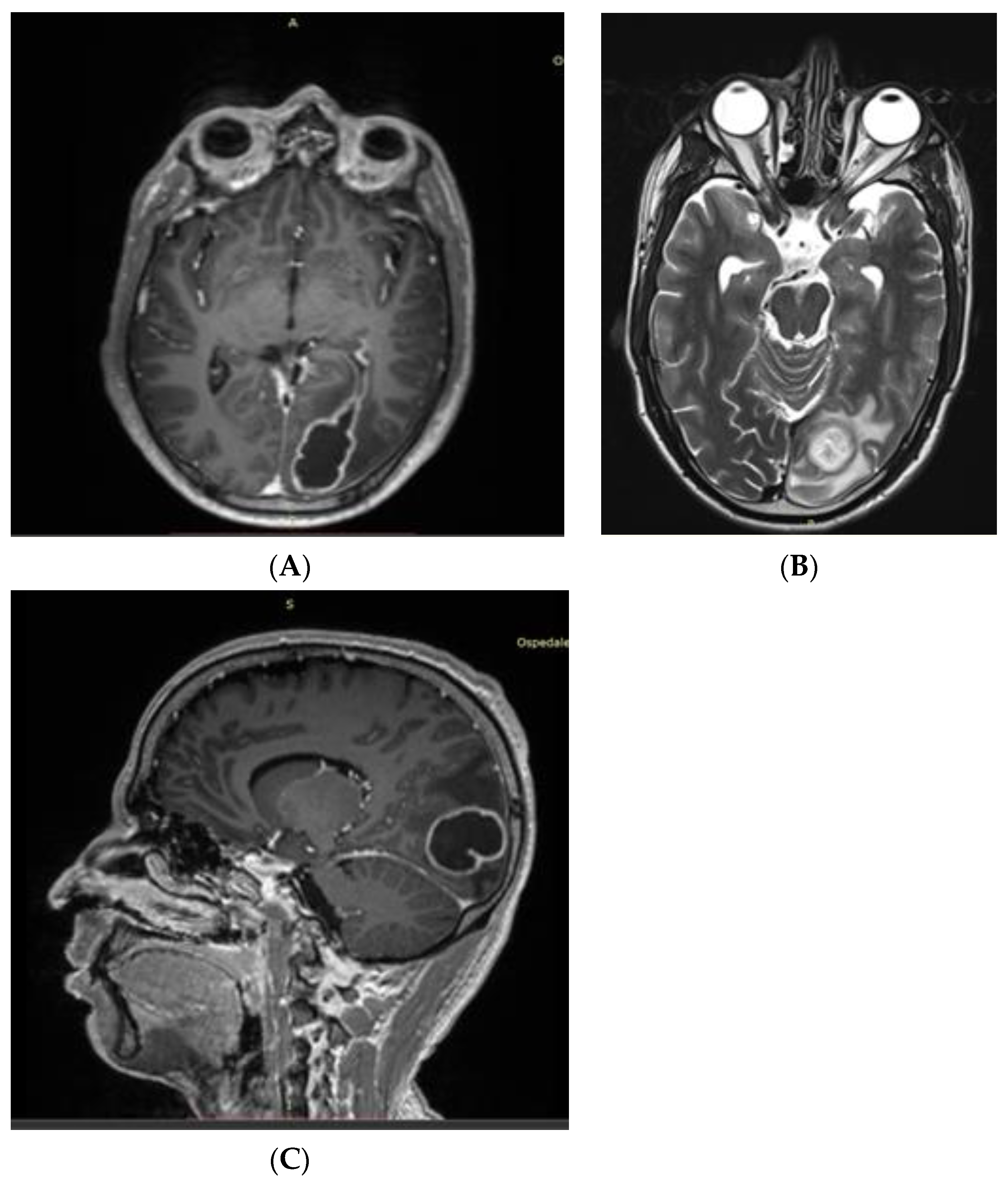
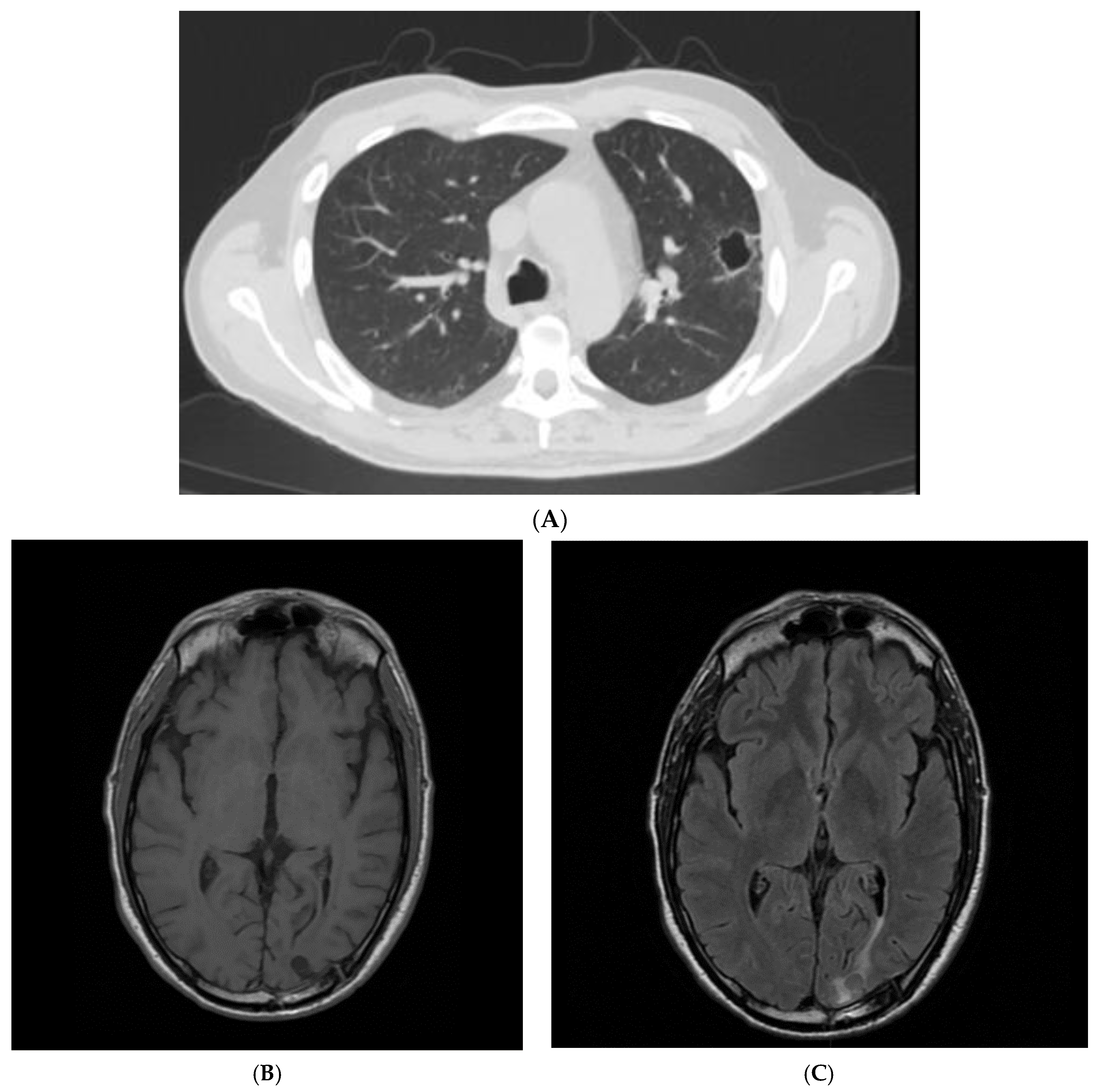
| Article | n. of Patients | Sex | Age (y) | Infection Site | Outcome |
|---|---|---|---|---|---|
| Carrena et al., 2018 [8] | 1 | M | 61 | Lung, brain, Chiari network endocarditis | Recovered |
| Honnorat et al., 2016 [4] | 1 | M | 56 | Brain, endocarditis on surgical patch | Recovered |
| Syros et al., 2011 [5] | 1 | M | 20 | Brain; concomitant PFO | Recovered |
| Nakaya et al., 1998 [6] | 1 | M | 60 | Brain, mitral valve endocarditis | Recovered |
| Melo et al., 1978 [3] | 1 | M | 69 | Liver, brain and presumptive endocarditis | Death |
| Article | n. of Patients | Sex | Age (y) | Infection Site | Outcome |
|---|---|---|---|---|---|
| Shinzato et al., 1995 [1] | 9 | M = 8 F = 1 | Mean 61.3 | Lung, pleura | NA |
| Noguchi et al., 2015 [2] | 14 | M = 10 F = 4 | Mean 77.3 | Lung/pleura | Recovered = 12 Death = 2 |
| Bueno et al., 2023 [9] | 1 | F | 25 | Lung | Recovered |
| Erne et al., 2010 [12] | 1 | M | 61 | Lung, brain | Recovered |
| Jerng et al., 1997 [13] | 17 | NA | NA | Lung/pleura | NA |
| Chandy et al., 2001 [14] | 1 | M | 21 | Blood, lung, frontal sinus, epidural abscess | Recovered |
| May et al., 2010 [15] | 1 | M | 53 | Lung | Death |
| Van Laren et al., 2011 [16] | 1 | F | 29 | Blood, lung, genital | Recovered |
| De Cruif et al., 2012 [17] | 1 | F | 63 | Lung, dental abscess | Recovered |
| Trabue et al., 2014 [18] | 1 | M | 36 | Lung, brain | NA |
| Maeda et al., 2012 [19] | 1 | F | 46 | Chest wall abscess, pleura | Recovered |
| Armendariz-Guezala et al., 2017 [20] | 1 | M | 33 | Brain, lung | Recovered |
| Sakurai et al., 2020 [21] | 1 | M | 80 | Pleura, iliopsoas abscess | Recovered |
| Carrena et al., 2018 [8] | 1 | M | 61 | Lung, brain, Chiari network endocarditis | Recovered |
| Fujihara et al., 2021 [22] | 1 | M | 64 | Lung, pleura | Death |
| Tasleem et al., 2021 [23] | 1 | M | 54 | Lung, pleura | Death |
| Manasrah et al., 2021 [24] | 1 | M | 54 | Lung, pleura, vertebrae, and discitis | Recovered |
| Nakagawa et al., 2022 [25] | 1 | M | 6 mo | Lung, pleura | Recovered |
| Christensen et al., 1993 [26] | 1 | M | 56 | Lung | Recovered |
| Patail et al., 2020 [27] | 1 | M | 30 | Lung, pleura | NA |
| Takahashi et al., 2019 [28] | 1 | F | 83 | Pleura | Recovered |
| Dyrhovden et al., 2019 [29] | 16 | NA | NA | Pleura | NA |
| Cobo et al., 2018 [30] | 9 | M = 7 F = 2 | Mean 63.9 | Lung, pleura | Recovered = 8 Death = 1 |
| Crespo Valades et al., 2005 [31] | 1 | M | 60 | Pleural effusion, subphrenic abscess | Recovered |
| Noguchi et al., 2014 [32] | 1 | M | 79 | Lung, pleura | Recovered |
| Hannoodi et al., 2016 [33] | 1 | F | 52 | Lung, pleura | Recovered |
| Mautner et al., 2000 [34] | 1 | M | 80 | Lung, pleura | Recovered |
| Huang et al., 2022 [35] | 1 | M | 10 | Lung, pleura | Recovered |
| Kurkowski et al., 2022 [36] | 1 | M | 39 | Liver, pleura, blood | Recovered |
| Jud et al., 2019 [37] | 1 | M | 59 | Lung, pleura | Recovered |
| Lescan et al., 2013 [38] | 1 | M | 74 | Lung, pleura, epidural abscess | Recovered |
| Stelzmueller et al., 2006 [39] | 2 | NA | NA | Pleural empyema | Recovered |
| Iskandar et al., 2006 [40] | 1 | NA | NA | Pleural empyema | NA |
| Lau et al., 2002 [41] | 1 | M | 32 | Pleural empyema, blood | Recovered |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavaruzzi, F.; Chinello, P.; Cucinotta, G.; Oliva, G.; Capone, A.; Granata, G.; Al Moghazi, S.; Caraffa, E.; Taglietti, F. Streptococcus intermedius Brain Abscess with Lung Abscess and Aortic Valve Endocarditis: A Case Report and Literature Review. Infect. Dis. Rep. 2023, 15, 445-453. https://doi.org/10.3390/idr15040045
Gavaruzzi F, Chinello P, Cucinotta G, Oliva G, Capone A, Granata G, Al Moghazi S, Caraffa E, Taglietti F. Streptococcus intermedius Brain Abscess with Lung Abscess and Aortic Valve Endocarditis: A Case Report and Literature Review. Infectious Disease Reports. 2023; 15(4):445-453. https://doi.org/10.3390/idr15040045
Chicago/Turabian StyleGavaruzzi, Francesca, Pierangelo Chinello, Giuseppe Cucinotta, Gianluigi Oliva, Alessandro Capone, Guido Granata, Samir Al Moghazi, Emanuela Caraffa, and Fabrizio Taglietti. 2023. "Streptococcus intermedius Brain Abscess with Lung Abscess and Aortic Valve Endocarditis: A Case Report and Literature Review" Infectious Disease Reports 15, no. 4: 445-453. https://doi.org/10.3390/idr15040045
APA StyleGavaruzzi, F., Chinello, P., Cucinotta, G., Oliva, G., Capone, A., Granata, G., Al Moghazi, S., Caraffa, E., & Taglietti, F. (2023). Streptococcus intermedius Brain Abscess with Lung Abscess and Aortic Valve Endocarditis: A Case Report and Literature Review. Infectious Disease Reports, 15(4), 445-453. https://doi.org/10.3390/idr15040045







