SARS-CoV-2 Serology Testing in an Asymptomatic, At-Risk Population: Methods, Results, Pitfalls
Abstract
:1. Introduction
2. Materials and Methods
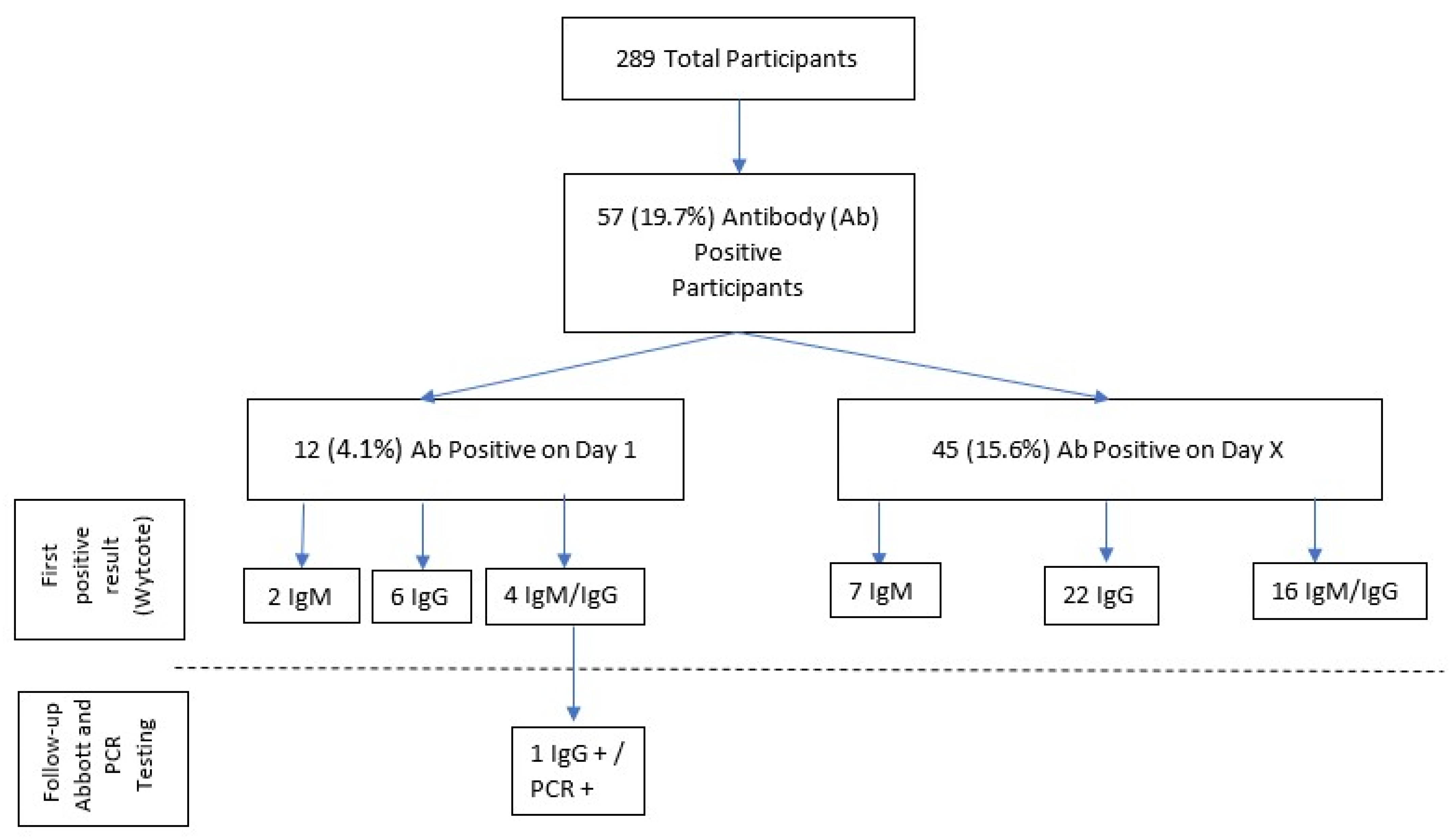
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Johns Hopkins Coronavirus Research Center. Published 2021. Available online: https://coronavirus.jhu.edu/ (accessed on 10 January 2021).
- Føns, S.; Krogfelt, K.A. How can we interpret SARS-CoV-2 antibody test results? Pathog. Dis. 2021, 79. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.L.; Whitman, J.D.; Lacanienta, N.P.; Beckerdite, E.W.; Kastner, S.A.; Shy, B.R.; Goldgof, G.M.; Levine, A.G.; Bapat, S.P.; Stramer, S.L.; et al. Magnitude and kinetics of anti-severe acute respiratory syndrome Coronavirus 2 antibody responses and their relationship to disease severity. Clin. Infect. Dis. 2021, 72, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.; McLawhon, R.W.; Kurian, S.; Fitzgerald, R.L.; Case, J.; Marsh, C.; Quigley, M. Healthcare worker seroconversion for SARS-CoV-2 at two large health systems in San Diego. Am. J. Infect. Control 2021, 49, 506–507. [Google Scholar] [CrossRef]
- Goldblatt, D.; Johnson, M.; Falup-Pecurariu, O.; Ivaskeviciene, I.; Spoulou, V.; Tamm, E.; Wagner, M.; Zar, H.J.; Bleotu, L.; Ivaskevicius, R.; et al. Cross-Sectional Prevalence of SARS-CoV-2 Antibodies in healthcare workers in paediatric facilities in eight countries. J. Hosp. Infect. 2021, 110, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Tuckerman, J.; Lee, L.-Y.; Wurzel, D.; Tosif, S.; Clifford, V.; McMinn, A.; O’Donaghue, K.; Rautenbacher, K.; Licciardi, P.V.; Toh, R.; et al. Seroprevalence of SARS-CoV-2 Antibodies in health-care workers at a tertiary paediatric hospital. J. Paediatr. Child. Health 2021, 57, 1136–1139. [Google Scholar] [CrossRef]
- Tatsi, E.-B.; Dellis, C.; Petridou, E.; Banou, K.; Zachariadou, L.; Syriopoulou, V.; Michos, A. SARS-CoV-2 Seroepidemiological study in healthcare workers and discordant results using seven different diagnostic methods. Infection 2021, 1–6. [Google Scholar] [CrossRef]
- Kasztelewicz, B.; Janiszewska, K.; Burzyńska, J.; Szydłowska, E.; Migdał, M.; Dzierżanowska-Fangrat, K. Prevalence of IgG antibodies against SARS-CoV-2 among healthcare workers in a tertiary pediatric hospital in Poland. PLoS ONE 2021, 16, e0249550. [Google Scholar] [CrossRef]
- Madhusudan, M.; Sankar, J.; Dhanalakshmi, K.; Putlibai, S.; Balasubramanian, S. Seroprevalence to SARS-CoV-2 Among healthcare workers in an exclusive pediatric hospital. Indian Pediatr. 2021, 58, 279–280. [Google Scholar] [CrossRef]
- Brant-Zawadzki, M.; Fridman, D.; Robinson, P.A.; Zahn, M.; Chau, C.; German, R.; Breit, M.; Bock, J.R.; Hara, J. SARS-CoV-2 Antibody prevalence in health care workers: Preliminary report of a single center study. PLoS ONE 2020, 15, e0240006. [Google Scholar] [CrossRef]
- COVID-19 Case Counts and Testing Figures. OC Healthcare Agency. Published 2021. Available online: https://occovid19.ochealthinfo.com/coronavirus-in-oc (accessed on 10 January 2021).
- Laursen, J.; Petersen, J.; Didriksen, M.; Iversen, K.; Ullum, H. Prevalence of SARS-CoV-2 IgG/IgM antibodies among danish and swedish falck emergency and non-emergency healthcare workers. Int. J. Environ. Res. Public Health 2021, 18, 923. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Botwin, G.J.; Albert, C.M.; Alotaibi, M.; Arditi, M.; Berg, A.H.; Binek, A.; Botting, P.; Fert-Bober, J.; Figueiredo, J.C.; et al. Seroprevalence of antibodies to SARS-CoV-2 in healthcare workers: A cross-sectional study. BMJ Open 2021, 11, e043584. [Google Scholar] [CrossRef]
- Thomas, S.N.; Altawallbeh, G.; Zaun, C.P.; Pape, K.A.; Peters, J.M.; Titcombe, P.J.; Dileepan, T.; Rapp, M.J.; Bold, T.D.; Schacker, T.W.; et al. Initial determination of COVID-19 seroprevalence among outpatients and healthcare workers in Minnesota using a novel SARS-CoV-2 total antibody ELISA. Clin. Biochem. 2021, 90, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Leven, E.; Muellers, K.; Stone, K.; Mendu, D.R.; Wajnberg, A. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J. Gen. Intern. Med. 2020, 35, 2485–2486. [Google Scholar] [CrossRef]
- Steensels, D.; Oris, E.; Coninx, L.; Nuyens, D.; Delforge, M.-L.; Vermeersch, P.; Heylen, L. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA 2020, 324, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, J.; Nie, S.; Li, H.; Kong, Y.; Liang, M.; Hou, J.; Huang, X.; Li, D.; Ma, T.; et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat. Med. 2020, 26, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Korth, J.; Wilde, B.; Dolff, S.; Anastasiou, O.E.; Krawczyk, A.; Jahn, M.; Cordes, S.; Ross, B.; Esser, S.; Lindemann, M.; et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020, 128, 104437. [Google Scholar] [CrossRef] [PubMed]
- Insúa, C.; Stedile, G.; Figueroa, V.; Hernández, C.; Svartz, A.; Ferrero, F.; Ossorio, M.F.; Brunetto, O. Seroprevalence of SARS-CoV-2 antibodies among physicians from a children’s hospital. Arch. Argent. Pediatr. 2020, 118, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Amendola, A.; Tanzi, E.; Folgori, L.; Barcellini, L.; Bianchi, S.; Gori, M.; Cammi, G.; Albani, E.; Zuccotti, G.V. Low seroprevalence of SARS-CoV-2 infection among healthcare workers of the largest children hospital in Milan during the pandemic wave. Infect. Control Hosp. Epidemiol. 2020, 41, 1468–1469. [Google Scholar] [CrossRef] [PubMed]
- Dacosta-Urbieta, A.; Rivero-Calle, I.; Pardo-Seco, J.; Redondo-Collazo, L.; Salas, A.; Gómez-Rial, J.; Martinón-Torres, F. Seroprevalence of SARS-CoV-2 among pediatric healthcare workers in Spain. Front. Pediatr. 2020, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.; Pepper, G.; Wener, M.H.; Fink, S.L.; Morishima, C.; Chaudhary, A.; Jerome, K.R.; Mathias, P.C.; Greninger, A.L. Performance characteristics of the abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J. Clin. Microbiol. 2020, 58, e00941-20. [Google Scholar] [CrossRef]
- Manalac, J.; Yee, J.; Calayag, K.; Nguyen, L.; Patel, P.M.; Zhou, D.; Shi, R.-Z. Evaluation of Abbott Anti-SARS-CoV-2 CMIA IgG and Euroimmun ELISA IgG/IgA assays in a clinical lab. Clin. Chim. Acta 2020, 510, 687–690. [Google Scholar] [CrossRef]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Najm, A.; Alunno, A.; Mariette, X.; Terrier, B.; De Marco, G.; Emmel, J.; Mason, L.; McGonagle, D.G.; Machado, P.M. Pathophysiology of acute respiratory syndrome Coronavirus 2 infection: A systematic literature review to Inform EULAR points to consider. RMD Open 2021, 7, e001549. [Google Scholar] [CrossRef] [PubMed]
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and Meta-analysis. PLoS Med. 2020, 17, e1003346. [Google Scholar] [CrossRef]
- Furukawa, K.; Arii, J.; Nishimura, M.; Tjan, L.H.; Lystia Poetranto, A.; Ren, Z.; Aktar, S.; Huang, J.R.; Sutandhio, S.; Kurahashi, Y.; et al. Seroepidemiological survey of the antibody for severe acute respiratory syndrome Coronavirus 2 with neutralizing activity at hospitals: A cross-sectional study in Hyogo Prefecture, Japan. JMAJ 2021, 4, 41–49. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; Fernández de Larrea, N.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Kalish, H.; Klumpp-Thomas, C.; Hunsberger, S.; Baus, H.A.; Fay, M.P.; Siripong, N.; Wang, J.; Hicks, J.; Mehalko, J.; Travers, J.; et al. Mapping a pandemic: SARS-CoV-2 seropositivity in the United States. medRxiv 2021. [Google Scholar] [CrossRef]
- Vogelzang, E.H.; Loeff, F.C.; Derksen, N.I.L.; Kruithof, S.; Ooijevaar-de Heer, P.; van Mierlo, G.; Linty, F.; Mok, J.Y.; van Esch, W.; de Bruin, S.; et al. Development of a SARS-CoV-2 total antibody assay and the dynamics of antibody response over time in hospitalized and nonhospitalized patients with COVID-19. J. Immunol. 2020, 205, 3491–3499. [Google Scholar] [CrossRef]
- Pradenas, E.; Trinité, B.; Urrea, V.; Marfil, S.; Ávila-Nieto, C.; de la Concepción, M.L.R.; Tarrés-Freixas, F.; Pérez-Yanes, S.; Rovirosa, C.; Ainsua-Enrich, E.; et al. Stable neutralizing antibody levels 6 months after mild and severe COVID-19 episodes. Medcine 2021, 2, 313.e4–320.e4. [Google Scholar] [CrossRef]
- Marot, S.; Malet, I.; Leducq, V.; Zafilaza, K.; Sterlin, D.; Planas, D.; Gothland, A.; Jary, A.; Dorgham, K.; Bruel, T.; et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat. Commun. 2021, 12, 844. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Carlson, N.E.; Slifka, M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007, 357, 1903–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, D.A.; Reses, H.E.; Cool, A.J.; Shapiro, C.N.; Hsu, J.; Boehmer, T.K.; Cornwell, C.R.; Gray, E.B.; Henley, S.J.; Lochner, K.; et al. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents aged 0-17 years—United States, August 2020–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1249–1254. [Google Scholar] [CrossRef]
- Hanson, K.E.; Caliendo, A.M.; Arias, C.A.; Englund, J.A.; Hayden, M.K.; Lee, M.J.; Loeb, M.; Patel, R.; Altayar, O.; El Alayli, A.; et al. Guidelines on the Diagnosis of COVID-19: Serologic Testing. Infectious Diseases Society of America. 2020. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-serology/ (accessed on 10 January 2021).
| 18–30 | 27.68% |
| 31–40 | 32.87% | |
| 41–50 | 24.57% | |
| Over 50 | 14.88% | |
| Male | 25.61% |
| Female | 74.39% | |
| American Indian or Alaska Native | 0.35% |
| Asian or Asian American | 15.57% | |
| Black or African American | 1.38% | |
| Hispanic/Latino | 19.38% | |
| Multiracial | 5.19% | |
| Other | 2.77% | |
| Pacific Islander | 1.38% | |
| White or Caucasian | 53.98% | |
| Range | 1–52 years |
| Mean | 13.21 | |
| Full Time | 74.39% |
| Part Time | 22.15% | |
| Per Diem | 3.46% | |
| Physician | 20.07% |
| PA/NP 1 | 2.77% | |
| Registered Nurse | 51.21% | |
| Technician | 15.22% | |
| Monitor Tech/Unit Secretary/Administration/Other | 10.73% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyming, T.; Bacon, K.; Lara, B.; Knudsen-Robbins, C.; Tongol, A.; Sanger, T. SARS-CoV-2 Serology Testing in an Asymptomatic, At-Risk Population: Methods, Results, Pitfalls. Infect. Dis. Rep. 2021, 13, 910-916. https://doi.org/10.3390/idr13040082
Heyming T, Bacon K, Lara B, Knudsen-Robbins C, Tongol A, Sanger T. SARS-CoV-2 Serology Testing in an Asymptomatic, At-Risk Population: Methods, Results, Pitfalls. Infectious Disease Reports. 2021; 13(4):910-916. https://doi.org/10.3390/idr13040082
Chicago/Turabian StyleHeyming, Theodore, Kellie Bacon, Bryan Lara, Chloe Knudsen-Robbins, Aprille Tongol, and Terence Sanger. 2021. "SARS-CoV-2 Serology Testing in an Asymptomatic, At-Risk Population: Methods, Results, Pitfalls" Infectious Disease Reports 13, no. 4: 910-916. https://doi.org/10.3390/idr13040082
APA StyleHeyming, T., Bacon, K., Lara, B., Knudsen-Robbins, C., Tongol, A., & Sanger, T. (2021). SARS-CoV-2 Serology Testing in an Asymptomatic, At-Risk Population: Methods, Results, Pitfalls. Infectious Disease Reports, 13(4), 910-916. https://doi.org/10.3390/idr13040082





