Update on the Epidemiology of Macrolide-Resistant Mycoplasma pneumoniae in Europe: A Systematic Review
Abstract
1. Introduction
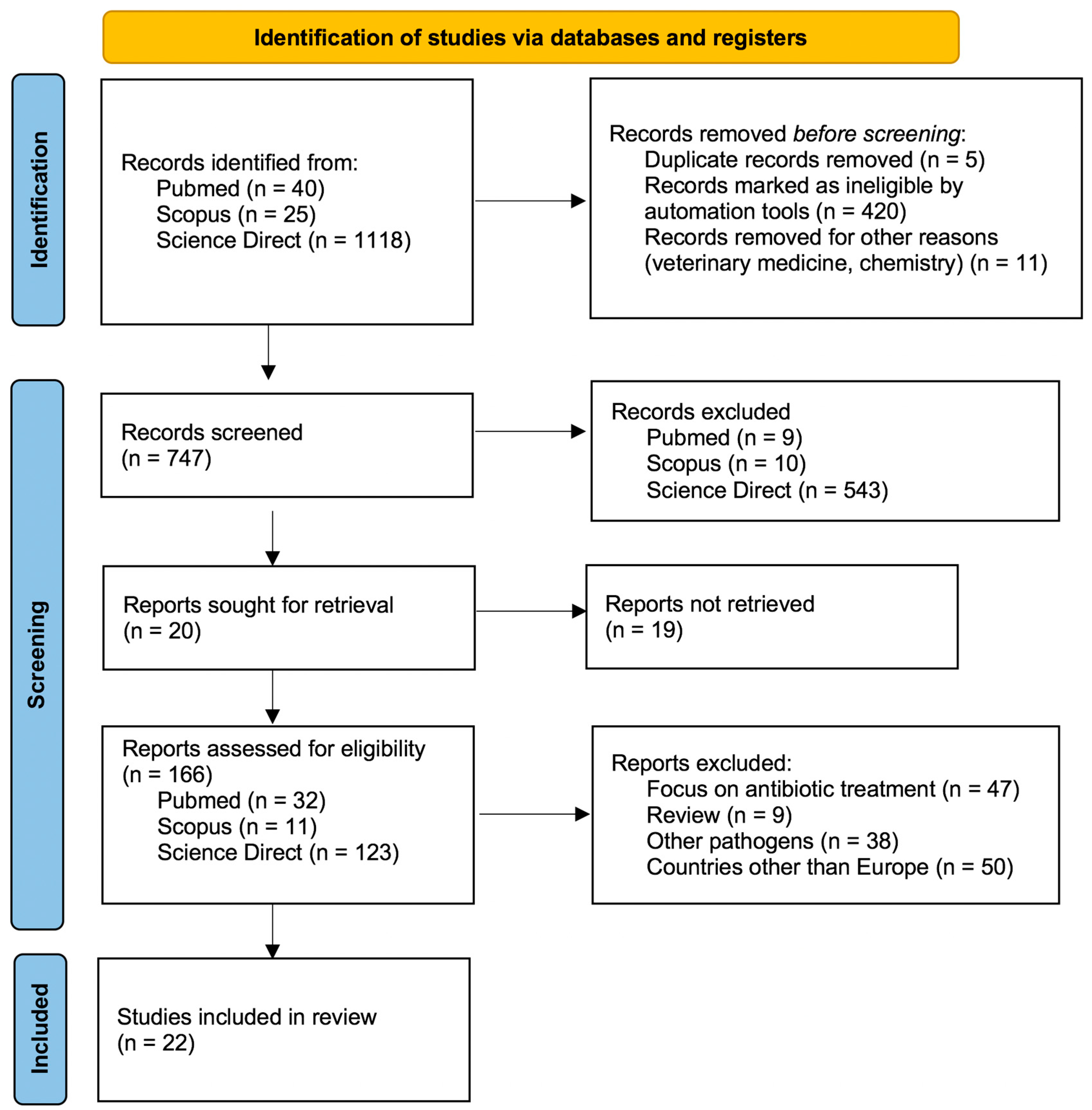
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, S.; Williams, D.J.; Arnold, S.R.; Ampofo, K.; Bramley, A.M.; Reed, C.; Stockmann, C.; Anderson, E.J.; Grijalva, C.G.; Self, W.H.; et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015, 372, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Kim, Y.; Jeong, J.Y.; Jung, Y.M.; Lee, M.H.; Chung, E.H. The changes of prevalence and etiology of pediatric pneumonia from National Emergency Department Information System in Korea, between 2007 and 2014. Korean J. Pediatr. 2018, 61, 291–300. [Google Scholar] [CrossRef]
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Macrolide-resistant Mycoplasma pneumoniae: Its role in respiratory infection. J. Antimicrob. Chemother. 2013, 68, 506–511. [Google Scholar] [CrossRef]
- Narita, M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J. Infect. Chemother. 2010, 16, 162–169. [Google Scholar] [CrossRef] [PubMed]
- de Groot, R.C.A.; Meyer Sauteur, P.M.; Unger, W.W.J.; van Rossum, A.M.C. Things that could be Mycoplasma pneumoniae. J. Infect. 2017, 74, S95–S100. [Google Scholar] [CrossRef]
- Dumke, R.; Catrein, I.; Herrmann, R.; Jacobs, E. Preference, adaptation and survival of Mycoplasma pneumoniae subtypes in an animal model. Int. J. Med. Microbiol. 2004, 294, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.P.; Balish, M.F.; Waites, K.B. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol. Rev. 2008, 32, 956–973. [Google Scholar] [CrossRef]
- Rasmussen, J.N.; Voldstedlund, M.; Andersen, R.L.; Ellermann-Eriksen, S.; Jensen, T.G.; Johansen, H.K.; Kolmos, B.; Mølvadgaard, M.; Nielsen, S.S.; Olsen, E.; et al. Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010. Eur. Surveill. 2010, 15, 19708, Erratum in Euro Surveill. 2010, 25, 15. [Google Scholar] [CrossRef]
- Kenri, T.; Okazaki, N.; Yamazaki, T.; Narita, M.; Izumikawa, K.; Matsuoka, M.; Suzuki, S.; Horino, A.; Sasaki, T. Genotyping analysis of Mycoplasma pneumoniae clinical strains in Japan between 1995 and 2005: Type shift phenomenon of M. pneumoniae clinical strains. J. Med. Microbiol. 2008, 57, 469–475. [Google Scholar] [CrossRef]
- von Baum, H.; Welte, T.; Marre, R.; Suttorp, N.; LuÃàck, C.; Ewig, S. Mycoplasma pneumoniae pneumonia revisited within the German Competence Network for Community-acquired pneu- monia (C.A.P.N.E.T.Z). BMC Infect. Dis. 2009, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect. Dis. 2001, 1, 334–344. [Google Scholar] [CrossRef]
- Oishi, T.; Takahashi, K.; Wakabayashi, S.; Nakamura, Y.; Ono, S.; Kono, M.; Kato, A.; Saito, A.; Kondo, E.; Tanaka, Y.; et al. Comparing Antimicrobial Susceptibilities among Mycoplasma pneumoniae Isolates from Pediatric Patients in Japan between Two Recent Epidemic Periods. Antimicrob. Agents Chemother. 2019, 63, e02517–e02518. [Google Scholar] [CrossRef]
- Okazaki, N.; Narita, M.; Yamada, S.; Izumikawa, K.; Umetsu, M.; Kenri, T.; Sasaki, Y.; Arakawa, Y.; Sasaki, T. Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol. Immunol. 2001, 45, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, M.; Takahashi, T.; Ubukata, K. Macrolide-resistant Mycoplasma pneumoniae: Characteristics of isolates and clinical aspects of community-acquired pneumonia. J. Infect. Chemother. 2010, 16, 78–86. [Google Scholar] [CrossRef]
- Chironna, M.; Sallustio, A.; Esposito, S.; Perulli, M.; Chinellato, I.; Di Bari, C.; Quarto, M.; Cardinale, F. Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J. Antimicrob. Chemother. 2011, 66, 734–737. [Google Scholar] [CrossRef]
- Miyashita, N.; Maruyama, T.; Kobayashi, T.; Kobayashi, H.; Taguchi, O.; Kawai, Y.; Yamaguchi, T.; Ouchi, K.; Oka, M. Community-acquired macrolide-resistant Mycoplasma pneumoniae pneumonia in patients more than 18 years of age. J. Infect. Chemother. 2011, 17, 114–118. [Google Scholar] [CrossRef]
- Li, S.L.; Sun, H.M.; Zhu, B.L.; Liu, F.; Zhao, H.Q. Whole Genome Analysis Reveals New Insights into Macrolide Resistance in Mycoplasma pneumoniae. Biomed Environ. Sci. 2017, 30, 343–350. [Google Scholar]
- Cardinale, F.; Chironna, M.; Chinellato, I.; Principi, N.; Esposito, S. Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J. Clin. Microbiol. 2013, 51, 723–724. [Google Scholar] [CrossRef]
- Pereyre, S.; Goret, J.; Bébéar, C. Mycoplasma pneumoniae: Current Knowledge on Macrolide Resistance and Treatment. Front. Microbiol. 2016, 22, 974. [Google Scholar] [CrossRef]
- Dorigo-Zetsma, J.W.; de Wit, M.; Szabo, J.N.; Schneeberger, P.M. Epidemie van luchtweginfecties door Mycoplasma pneumoniae in een instelling voor verstandelijk gehandicapten onderzocht met polymerasekettingreactie op keeluitstrijk [Epidemic of respiratory tract infections by Mycoplasma pneumoniae in an institute for mentally disabled, investigated with polymerase chain reaction of a throat swab specimen]. Ned. Tijdschr. Geneeskd. 1999, 143, 1261–1265. [Google Scholar]
- Chironna, M.; Loconsole, D.; De Robertis, A.L.; Morea, A.; Scalini, E.; Quarto, M.; Tafuri, S.; Germinario, C.; Manzionna, M. Clonal Spread of a Unique Strain of Macrolide-Resistant Mycoplasma Pneumoniae Within a Single Family in Italy. Medicine 2016, 95, e3160. [Google Scholar] [CrossRef]
- Gullsby, K.; Olsen, B.; Bondeson, K. Molecular Typing of Mycoplasma pneumoniae Strains in Sweden from 1996 to 2017 and the Emergence of a New P1 Cytadhesin Gene, Variant 2e. J. Clin. Microbiol. 2019, 57, e00049-19. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Deng, H.; Zhang, J.; Zhu, Y.; Rong, Q.; Quan, Y.; Tang, H.; Zhao, D. Mutations in domain V of Mycoplasma pneumoniae 23S rR.N.A and clinical characteristics of pediatric M. pneumoniae pneumonia in Nanjing, China. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef] [PubMed]
- Dumke, R.; Lück, C.; Jacobs, E. Low rate of macrolide resistance in Mycoplasma pneumoniae strains in Germany between 2009 and 2012. Antimicrob. Agents Chemother. 2013, 57, 3460. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dumke, R.; Schnee, C.; Pletz, M.W.; Rupp, J.; Jacobs, E.; Sachse, K.; Rohde, G.; Capnetz Study Group. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011–2012. Emerg. Infect. Dis. 2015, 21, 426–434. [Google Scholar] [CrossRef]
- Dumke, R.; Ziegler, T. Long-Term Low Rate of Macrolide-Resistant Mycoplasma pneumoniae Strains in Germany. Antimicrob. Agents Chemother. 2019, 63, e00455-19. [Google Scholar] [CrossRef]
- Dumke, R.; Stolz, S.; Jacobs, E.; Juretzek, T. Molecular characterization of macrolide resistance of a Mycoplasma pneumoniae strain that developed during therapy of a patient with pneumonia. Int. J. Infect. Dis. 2014, 29, 197–199. [Google Scholar] [CrossRef]
- Loconsole, D.; De Robertis, A.L.; Mallamaci, R.; Sallustio, A.; Morea, A.; Prato, R.; Quarto, M.; Martinelli, D.; Chironna, M. First Description of Macrolide-Resistant Mycoplasma pneumoniae in Adults with Community-Acquired Pneumonia in Italy. Biomed. Res. Int. 2019, 2019, 7168949. [Google Scholar] [CrossRef]
- Pereyre, S.; Charron, A.; Hidalgo-Grass, C.; Touati, A.; Moses, A.E.; Nir-Paz, R.; Bébéar, C. The spread of Mycoplasma pneumoniae is polyclonal in both an endemic setting in France and in an epidemic setting in Israel. PLoS ONE 2012, 7, e38585. [Google Scholar] [CrossRef]
- Pereyre, S.; Touati, A.; Petitjean-Lecherbonnier, J.; Charron, A.; Vabret, A.; Bébéar, C. The increased incidence of Mycoplasma pneumoniae in France in 2011 was polyclonal, mainly involving M. pneumoniae type 1 strains. Clin. Microbiol. Infect. 2013, 19, E212–E217. [Google Scholar] [CrossRef] [PubMed]
- Caballero Jde, D.; del Campo, R.; del Carmen Mafé, M.; Gálvez, M.; Rodríguez-Domínguez, M.; Cantón, R.; Meseguer, M.A.; Hermida, J.M. First report of macrolide resistance in a Mycoplasma pneumoniae isolate causing community-acquired pneumonia in Spain. Antimicrob. Agents Chemother. 2014, 58, 1265–1266. [Google Scholar] [CrossRef][Green Version]
- Rivaya, B.; Jordana-Lluch, E.; Fernández-Rivas, G.; Molinos, S.; Campos, R.; Méndez-Hernández, M.; Matas, L. Macrolide resistance and molecular typing of Mycoplasma pneumoniae infections during a 4 year period in Spain. J. Antimicrob. Chemother. 2020, 75, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.D.; Gadsby, N.J.; Henderson, S.S.; Hardie, A.; Kalima, P.; Morris, A.C.; Hill, A.T.; Cunningham, S.; Templeton, K.E. Clinical outcomes and macrolide resistance in Mycoplasma pneumoniae infection in Scotland, U.K. J. Med. Microbiol. 2013, 62, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Macfarlane-Smith, L.; Phillips, S.; Chalker, V.J. Detection of macrolide resistant Mycoplasma pneumoniae in England, September 2014 to September 2015. Eur. Surveill. 2015, 20, 30078. [Google Scholar] [CrossRef]
- Kogoj, R.; Mrvic, T.; Praprotnik, M.; Kese, D. Prevalence, genotyping and macrolide resistance of Mycoplasma pneumoniae among isolates of patients with respiratory tract infections, Central Slovenia, 2006 to 2014. Eur. Surveill. 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Kogoj, R.; Praprotnik, M.; Mrviƒç, T.; Korva, M.; Keše, D. Genetic diversity and macrolide resistance of Mycoplasma pneumoniae isolates from two consecutive epidemics in Slovenia. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 99–107. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Jensen, J.S.; Björkman, P.; Persson, K. Development of macrolide resistance in Mycoplasma pneumoniae-infected Swedish patients treated with macrolides. Scand. J. Infect. Dis. 2014, 46, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Uldum, S.A.; Bangsborg, J.M.; Gahrn-Hansen, B.; Ljung, R.; Mølvadgaard, M.; Petersen, R.F.; Svarrer, C.W. Epidemic of Mycoplasma pneumoniae infection in Denmark, 2010 and 2011. Eur. Surveill. 2012, 17, 20073. [Google Scholar] [CrossRef]
- Meyer Sauteur, P.M.; Bleisch, B.; Voit, A.; Maurer, F.P.; Relly, C.; Berger, C.; Nadal, D.; Bloemberg, G.V. Survey of macrolide-resistant Mycoplasma pneumoniae in children with community-acquired pneumonia in Switzerland. Swiss Med. Wkly. 2014, 144, w14041. [Google Scholar] [CrossRef] [PubMed]
- Kurkela, S.; Puolakkainen, M.; Hokynar, K.; Nieminen, T.; Saxen, H.; Mannonen, L.; Pietikäine, R. Mycoplasma pneumoniae outbreak, Southeastern Finland, 2017–2018: Molecular epidemiology and laboratory diagnostic lessons. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1867–1871. [Google Scholar] [CrossRef]
- Spuesens, E.B.; Meijer, A.; Bierschenk, D.; Hoogenboezem, T.; Donker, G.A.; Hartwig, N.G.; Koopmans, M.P.; Vink, C.; van Rossum, A.M. Macrolide resistance determination and molecular typing of Mycoplasma pneumoniae in respiratory specimens collected between 1997 and 2008 in The Netherlands. J. Clin. Microbiol. 2012, 50, 1999–2004. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, B.; Wu, X.; Yin, Q.; Wang, Y.; Li, J.; Jiao, W.; Quan, S.; Sun, L.; Wang, Y.; et al. Increased Macrolide Resistance Rate of M3562 Mycoplasma pneumoniae Correlated with Macrolide Usage and Genotype Shifting. Front. Cell. Infect. Microbiol. 2021, 11, 675466. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xue, G.; Yan, C.; Li, S.; Zhao, H.; Feng, Y.; Wang, L. Changes in Molecular Characteristics of Mycoplasma pneumoniae in Clinical Specimens from Children in Beijing between 2003 and 2015. PLoS ONE 2017, 12, e0170253. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xue, G.; Zhao, H.; Feng, Y.; Li, S.; Cui, J.; Ni, S.; Sun, H. Molecular and clinical characteristics of severe Mycoplasma pneumoniae pneumonia in children. Pediatr. Pulmonol. 2019, 54, 1012–1021. [Google Scholar] [CrossRef]
- Nakamura, Y.; Oishi, T.; Kaneko, K.; Kenri, T.; Tanaka, T.; Wakabayashi, S.; Kono, M.; Ono, S.; Kato, A.; Kondo, E.; et al. Recent acute reduction in macrolide-resistant Mycoplasma pneumoniae infections among Japanese children. J. Infect. Chemother. 2021, 27, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Morozumi, M.; Tajima, T.; Hasegawa, M.; Sakata, H.; Ohnari, S.; Chiba, N.; Iwata, S.; Ubukata, K. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin. Infect. Dis. 2012, 55, 1642–1649. [Google Scholar] [CrossRef]
- Morozumi, M.; Tajima, T.; Sakuma, M.; Shouji, M.; Meguro, H.; Saito, K.; Iwata, S.; Ubukata, K. Sequence Type Changes Associated with Decreasing Macrolide-Resistant Mycoplasma pneumoniae, Japan. Emerg. Infect. Dis. 2020, 26, 2210–2213. [Google Scholar] [CrossRef]
- Xue, G.; Li, M.; Wang, N.; Zhao, J.; Wang, B.; Ren, Z.; Yan, C.; Wu, C.; Liu, Y.; Sun, H.; et al. Comparison of the molecular characteristics of Mycoplasma pneumoniae from children across different regions of China. PLoS ONE 2018, 13, e0198557. [Google Scholar] [CrossRef]
- Zhao, F.; Li, J.; Liu, J.; Guan, X.; Gong, J.; Liu, L.; He, L.; Meng, F.; Zhang, J. Antimicrobial susceptibility and molecular characteristics of Mycoplasma pneumoniae isolates across different regions of China. Antimicrob. Resist. Infect. Control. 2019, 8, 143. [Google Scholar] [CrossRef]
- Lenglet, A.; Herrador, Z.; Magiorakos, A.P.; Leitmeyer, K.; Coulombier, D. European Working Group on Mycoplasma pneumoniae surveillance. Surveillance status and recent data for Mycoplasma pneumoniae infections in the European Union and European Economic Area, January 2012. Eur. Surveill. 2012, 17, 20075. [Google Scholar] [CrossRef]
- Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; Molenberghs, G.; et al. European Surveillance of Antimicrobial Consumption (ESA: Outpatient macrolide, lincosamide and streptogramin (MLS) use in Europe (1997–2009). J. Antimicrob. Chemother. 2011, 66, vi37–vi45. [Google Scholar] [CrossRef] [PubMed]
- Pereyre, S.; Guyot, C.; Renaudin, H.; Charron, A.; Bébéar, C.; Bébéar, C.M. In vitro selection and characterization of resistance to macrolides and related antibiotics in Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, D.; Hidalgo-Grass, C.; Moses, A.E.; Engelhard, D.; Nir-Paz, R. Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerg. Infect. Dis. 2011, 17, 1079–1082. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; He, L.; Meng, F.; Zhang, J.; Zhao, F. First report of macrolide-resistant and -susceptible Mycoplasma pneumoniae clinical strains isolated from a single case. J. Glob. Antimicrob. Resist. 2021, 24, 228–232. [Google Scholar] [CrossRef]
- Yu, J.L.; Song, Q.F.; Xie, Z.W.; Jiang, W.H.; Chen, J.H.; Fan, H.F.; Xie, Y.P.; Lu, G. iTRAQ-based Quantitative Proteomics Study in Patients with Refractory Mycoplasma pneumoniae Pneumonia. Jpn. J. Infect. Dis. 2017, 70, 571–578. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Sheng, Y.; Zhang, L.; Shen, Z.; Chen, Z. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1034–1038. [Google Scholar] [CrossRef]
- Spuesens, E.B.; Meyer Sauteur, P.M.; Vink, C.; van Rossum, A.M. Mycoplasma pneumoniae infections--does treatment help? J. Infect. 2014, 69, S42–S46. [Google Scholar] [CrossRef]
- Luo, Z.; Luo, J.; Liu, E.; Xu, X.; Liu, Y.; Zeng, F.; Li, S.; Fu, Z. Effects of prednisolone on refractory mycoplasma pneumoniae pneu-monia in children. Pediatr. Pulmonol. 2014, 49, 377. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19, Interim Guidance. 27 May 2020. Available online: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed on 8 July 2021).
- Oliva, A.; Siccardi, G.; Migliarini, A.; Cancelli, F.; Carnevalini, M.; D’Andria, M.; Attilia, I.; Danese, V.C.; Cecchetti, V.; Romiti, R.; et al. Co-infection of SARS.-CoV-2 with Chlamydia or Mycoplasma pneumoniae: A case series and review of the literature. Infection 2020, 48, 871–877. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, M.A.; Poiesi, C.; Gargiulo, F.; Bonfanti, C.; Pollara, P.; Fiorentini, S.; Caccuri, F.; Carta, V.; Mangeri, L.; Pellizzeri, S.; et al. Co-infection of chlamydia pneumoniae and mycoplasma pneumoniae with SARS-CoV-2 is associated with more severe features. J. Infect. 2021, 82, e4–e7. [Google Scholar] [CrossRef] [PubMed]
| N. | Authors | Year of Study | Journal | Country | Type of Study | Population (Number of MP Positive Samples) | Diagnostic Methods for MP Identification | Main Findings |
|---|---|---|---|---|---|---|---|---|
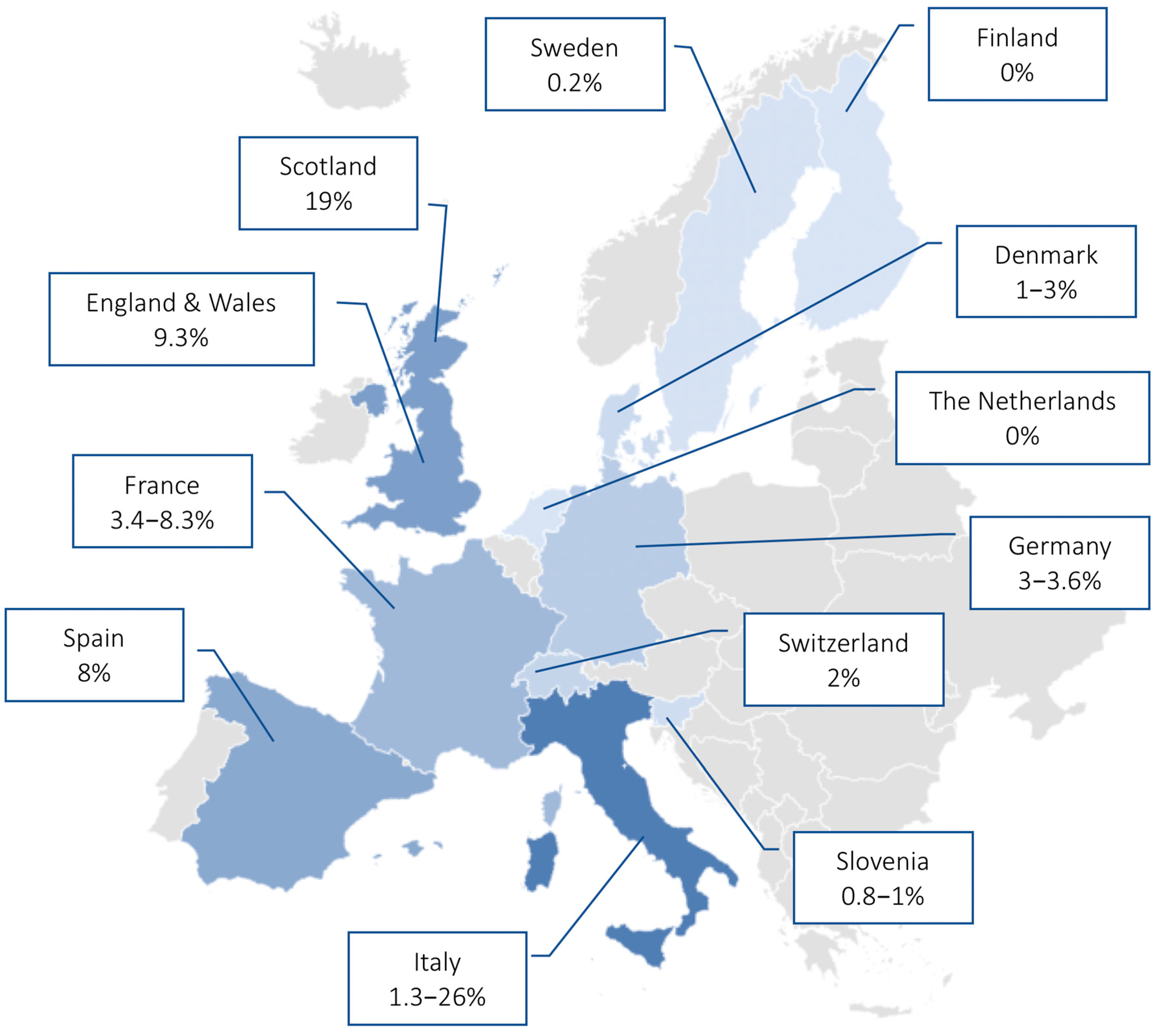
| 1 | Dumke et al. [25] | 2013 | Antimicrobial Agents and Chemotherapy | Germany | Prospective study of out- and inpatients | Children and adults (n = 84) | Real-time PCR | Prevalence of MR-MP was 3.6% during an epidemic period |
| 2 | Dumke et al. [26] | 2015 | Emerging Infectious Diseases | Germany | Prospective study | Adults (n = 96) | Real-time PCR | Low levels of macrolide resistance (3.1%) during 2011–2012 period |
| 3 | Dumke et al. [27] | 2019 | Antimicrobial Agents and Chemotherapy | Germany | Retrospective study | Children and adults (n = 166) | Real-time PCR | Low rate of MR-MP infection (3%) during the 2016–2018 period |
| 4 | Dumke et al. [28] | International Journal of Infectious Diseases | Germany | Case report | 15-year-old boy | Real-time PCR | Development of macrolide resistance after macrolide treatment. Presence of a mixture of wild-type and macrolide-resistant strains | |
| 5 | Chironna et al. [16] | 2011 | Journal of Antimicrobial Chemotherapy | Italy | Prospective study of hospitalized patients | Children (n = 43) | Real-time PCR | MR-MP prevalence of 26% in children hospitalized for LRTI |
| 6 | Cardinale et al. [19] | 2013 | Journal of Clinical Microbiology | Italy | Prospective study of hospitalized patients | Children (n = 46) | Real-time PCR | The presence of MR-MP does not change the clinical presentation of CAP; however, children with MR-MP infection were more likely to show longer duration of fever, cough, and hospitalization |
| 7 | Chironna et al. [22] | 2016 | Medicine | Italy | Outbreak investigation | Children and adults (n = 8) | Real-time PCR | High transmission rates of MR-MP infection/carriage within a single family |
| 8 | Loconsole et al. [29] | 2019 | BioMed Research International | Italy | Retrospective study | Adults (n = 15) | Real-time PCR | 1.3% of adults with CAP had a MR-MP infection |
| 9 | Pereyre et al. [30] | 2012 | PLOS One | France | Retrospective study | Children and adults (n = 34) | Real-time PCR | MR-MP prevalence of 3.4% in the 2007–2010 endemic period |
| 10 | Pereyre et al. [31] | 2013 | Clinical Microbiology and Infection | France | Retrospective study in hospitalized patients | Children and adults (n = 94) | Real-time PCR, culture, serology | MR-MP prevalence of 8.3% in the 2011 epidemic period |
| 11 | Caballero et al. [32] | 2014 | Antimicrobial Agents and Chemotherapy | Spain | Case report | 23-year-old female | Real-time PCR, culture, serology | First description of MR-MP in a Chinese 23-year-old female coming back from China 13 days before CAP onset |
| 12 | Rivaya et al. [33] | 2020 | Journal of Antimicrobial Chemotherapy | Spain | Retrospective study | Children and adults (n = 138) | Real-time PCR, serology | MR-MP detected in 8% of infections in the 2013–2017 period |
| 13 | Ferguson et al. [34] | 2013 | Journal of Medical Microbiology | Scotland | Retrospective study | Children and adults (n = 32) | Real-time PCR | MR-MP prevalence of 19% in the 2010–2011 period, which was associated with a significant burden on local hospital admissions |
| 14 | Brown et al. [35] | 2015 | Euro Surveillance | England and Wales | Retrospective study | Children and adults (n = 43) | qPCR | MR-MP detected in 9.3% of collected samples |
| 15 | Kogoy et al. [36] | 2015 | Euro Surveillance | Slovenia | Retrospective study in out- and inpatients | Children and adults (n = 1255) | Real-time PCR, culture | Sporadic identification by MR-MP in the 2006–2014 period covering two nationwide epidemics |
| 16 | Kogoy et al. [37] | 2018 | European Journal of Clinical Microbiology & Infectious Disease | Slovenia | Retrospective study in out- and inpatients | Children and adults (n = 1477) | qPCR, culture | 0.8% of MP isolated in the 2006–2016 period showed macrolide resistance |
| 17 | Nilsson et al. [38] | 2014 | Scandinavian Journal of Infectious Diseases | Sweden | Prospective study | Children and adults (n = 38) | Real-time PCR | 1/38 cases developed MR mutations during macrolide treatment |
| 18 | Gullsby et al. [23] | 2019 | Journal of Clinical Microbiology | Sweden | Retrospective study | Children and adults (n = 578) | Real-time PCR | Very low prevalence (0.2%) of macrolide resistance in the 1996–2017 period |
| 19 | Uldum et al. [39] | 2012 | Euro Surveillance | Denmark | Data from MP national surveillance system | Children and adults (n = 140) | Real-time PCR | Low rate of MR-MP infections (1–3%) during the three waves in the 2010–2011 epidemic |
| 20 | Meyer Sauteur et al. [40] | 2014 | Swiss Medical Weekly | Switzerland | Prospective study in out- and inpatients | Children (n = 50) | Real-time PCR | Low level of macrolide resistance (1/50) during a 3 year period (2011–2013) |
| 21 | Kurkela et al. [41] | 2019 | European Journal of Clinical Microbiology & Infectious Disease | Finland | Outbreak investigation | Children and adults (n = 535) | Real-time PCR, serology | No MR-MP infections identified in the 2017 outbreak in Kimenlaakso |
| 22 | Spuesens et al. [42] | 2012 | Journal of Clinical Microbiology | The Netherlands | Retrospective study | Children and adults (n = 96) | Nested-PCR | MR-MP genotypes not found in 114 MP positive samples collected during the 1997–2008 period |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loconsole, D.; De Robertis, A.L.; Sallustio, A.; Centrone, F.; Morcavallo, C.; Campanella, S.; Accogli, M.; Chironna, M. Update on the Epidemiology of Macrolide-Resistant Mycoplasma pneumoniae in Europe: A Systematic Review. Infect. Dis. Rep. 2021, 13, 811-820. https://doi.org/10.3390/idr13030073
Loconsole D, De Robertis AL, Sallustio A, Centrone F, Morcavallo C, Campanella S, Accogli M, Chironna M. Update on the Epidemiology of Macrolide-Resistant Mycoplasma pneumoniae in Europe: A Systematic Review. Infectious Disease Reports. 2021; 13(3):811-820. https://doi.org/10.3390/idr13030073
Chicago/Turabian StyleLoconsole, Daniela, Anna Lisa De Robertis, Anna Sallustio, Francesca Centrone, Caterina Morcavallo, Silvia Campanella, Marisa Accogli, and Maria Chironna. 2021. "Update on the Epidemiology of Macrolide-Resistant Mycoplasma pneumoniae in Europe: A Systematic Review" Infectious Disease Reports 13, no. 3: 811-820. https://doi.org/10.3390/idr13030073
APA StyleLoconsole, D., De Robertis, A. L., Sallustio, A., Centrone, F., Morcavallo, C., Campanella, S., Accogli, M., & Chironna, M. (2021). Update on the Epidemiology of Macrolide-Resistant Mycoplasma pneumoniae in Europe: A Systematic Review. Infectious Disease Reports, 13(3), 811-820. https://doi.org/10.3390/idr13030073









