Impact of Hypoglycemia on Morbidity, Mortality, and Resource Utilization in Gastrointestinal Stromal Tumor: A Nationwide Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Study Population
2.3. Study Outcomes
2.4. Statistical Analysis
2.5. Propensity Score-Matched Sensitivity Analysis
3. Results
3.1. Patient- and Hospital-Level Characteristics
3.2. In-Hospital Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GIST | Gastrointestinal Stromal Tumor |
| IGF | Insulin-like Growth Factor |
| NICTH | Non-Islet Cell Tumor Hypoglycemia |
| IGFBP-3 | Insulin-like Growth Factor Binding Protein-3 |
| ICD-10-CM | International Classification of Diseases, Tenth Revision, Clinical Modification |
| NIS | National Inpatient Sample |
| HCUP | Healthcare Cost and Utilization Project |
| AHRQ | Agency for Healthcare Research and Quality |
| aOR | Adjusted Odds Ratio |
| CI | Confidence Interval |
| USA | United States of America |
Appendix A
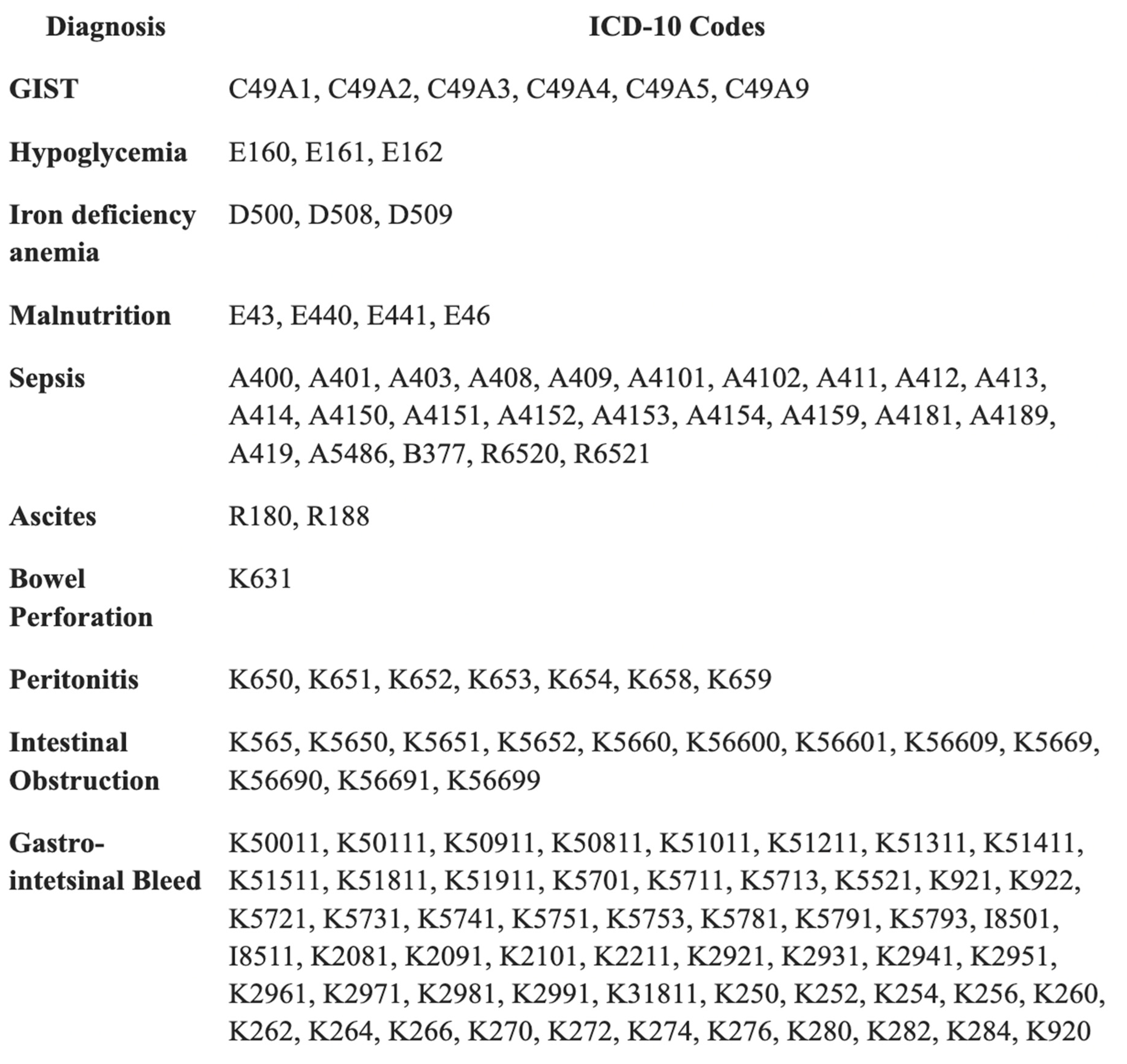
Appendix B
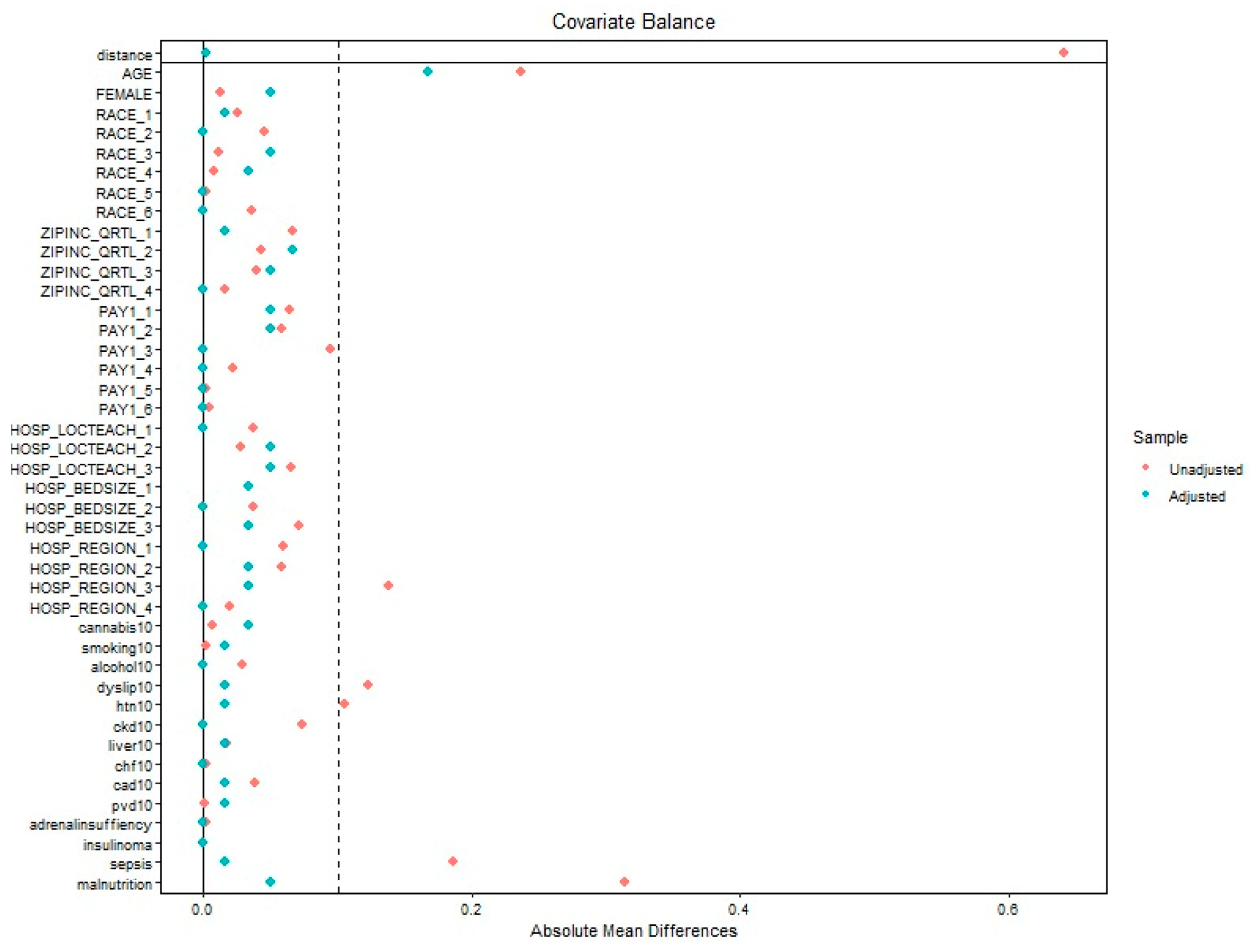
References
- Ginjupalli, M.; Jayakumar, J.; Gujjula, S.R.; Al-bustami, I.; Gaddam, M.; Pulakurthi, Y.S.; Kumar, V.; Bandaru, P.; Chakinala, R.C.; Gayam, V.R. S2302 Is hypoglycemia a reliable indicator of disease severity in gastrointestinal stromal tumors? Revelations from the National Inpatient Sample. Am. J. Gastroenterol. 2024, 119 (Suppl. S10), S1652. [Google Scholar] [CrossRef]
- Escobar, G.A.; Robinson, W.A.; Nydam, T.L.; Heiple, D.C.; Weiss, G.J.; Buckley, L.; Gonzalez, R.; McCarter, M.D. Severe paraneoplastic hypoglycemia in a patient with a gastrointestinal stromal tumor with an exon 9 mutation: A case report. BMC Cancer 2007, 7, 13. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef]
- Eghbali, F.; Karami, R. Refractory hypoglycemia induced by a duodenal wall gastrointestinal stromal tumor: A case report. Casp. J. Intern. Med. 2021, 12 (Suppl. S2), S447–S450. [Google Scholar] [CrossRef]
- Scarpa, M.; Bertin, M.; Ruffolo, C.; Polese, L.; D’Amico, D.F.; Angriman, I. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. J. Surg. Oncol. 2008, 98, 384–392. [Google Scholar] [CrossRef]
- Hizuka, N.; Fukuda, I.; Takano, K.; Okubo, Y.; Asakawa-Yasumoto, K.; Demura, H. Serum insulin-like growth factor II in 44 patients with non-islet cell tumor hypoglycemia. Endocr. J. 1998, 45, S61–S65. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Ginsberg, J.; Cutts, K.; Urban, S. A case of non-islet cell tumor hypoglycemia (NICTH) associated with gastrointestinal stromal tumor (GIST). Am. J. Case Rep. 2017, 18, 984–988. [Google Scholar] [CrossRef]
- Kumar, V.; Gala, D.; Wonders, C.; Marowa, S.; Forlemu, A.; Gayam, V.; Reddy, M. Non-islet cell tumor hypoglycemia associated with gastrointestinal stromal tumor: Case report and review of the literature. Arch. Clin. Cases 2023, 10, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Vu, A.; Chik, C.; Kwong, S. IGF-2-mediated hypoglycemia: A case series and review of the medical therapies for refractory hypoglycemia. Endocrinol. Diabetes Metab. Case Rep. 2024, 2024, 23-0089. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Monson, J.P. Non-islet cell tumour hypoglycaemia. Clin. Endocrinol. 2013, 78, 819–827. [Google Scholar] [CrossRef]
- Iglesias, P.; Díez, J.J. Management of endocrine disease: A clinical update on tumor-induced hypoglycemia. Eur. J. Endocrinol. 2014, 170, R147–R157. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Z.; Taleb, S.; Evans-Molina, C. A case of extragastrointestinal stromal tumor complicated by severe hypoglycemia: A unique presentation of a rare tumor. BMC Cancer 2016, 16, 930. [Google Scholar] [CrossRef]
- Bodnar, T.W.; Acevedo, M.J.; Pietropaolo, M. Management of non–islet-cell tumor hypoglycemia: A clinical review. J. Clin. Endocrinol. Metab. 2014, 99, 713–722. [Google Scholar] [CrossRef]
- Karamanolis, N.N.; Kounatidis, D.; Vallianou, N.G.; Alexandropoulos, K.; Kovlakidi, E.; Kaparou, P.; Karampela, I.; Stratigou, T.; Dalamaga, M. Paraneoplastic hypoglycemia: An overview for optimal clinical guidance. Metab. Open 2024, 23, 100305. [Google Scholar] [CrossRef]
- Looi, E.; Lawler, H.M. Non-diabetic hypoglycemia: Evaluation and management in adults. J. Clin. Med. 2025, 14, 4393. [Google Scholar] [CrossRef] [PubMed]
- Frystyk, J. Free insulin-like growth factors—Measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm. IGF Res. 2004, 14, 337–375. [Google Scholar] [CrossRef]
- Guiteau, J.; Fanucchi, M.; Folpe, A.; Staley, C.A.; Kooby, D.A. Hypoglycemia in the setting of advanced gastrointestinal stromal tumor. Am. Surg. 2006, 72, 1225–1230. [Google Scholar] [CrossRef]
- Hirai, H.; Ogata, E.; Ohki, S.; Fukuda, I.; Tanaka, M.; Watanabe, T.; Satoh, H. Hypoglycemia associated with a gastrointestinal stromal tumor producing high-molecular-weight insulin growth factor II: A case report and literature review. Intern. Med. 2016, 55, 1309–1314. [Google Scholar] [CrossRef][Green Version]
- Kumar, N.; Bhoriwal, S.; Das, P.; Deo, S.V.S. A rare case of paraneoplastic hypoglycemia induced by abdominopelvic gastrointestinal stromal tumor. J. Gastrointest. Cancer 2020, 51, 1065–1069. [Google Scholar] [CrossRef]
- Dimitriadis, G.K.; Gopalakrishnan, K.; Rao, R.; Grammatopoulos, D.K.; Randeva, H.S.; Weickert, M.O.; Murthy, N. Severe paraneoplastic hypoglycemia secondary to a gastrointestinal stromal tumour masquerading as a stroke. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 150062. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, S.H.; Maeng, I.H.; Kim, K.S.; Jang, Y.S.; Kim, H.S.; Lee, J.M.; Park, S.Y.; Kang, S.B. A case of gastrointestinal stromal tumor with recurrent hypoglycemia. Endocrinol. Metab. 2010, 25, 125–129. [Google Scholar] [CrossRef][Green Version]
- Yamasaki, H.; Itawaki, A.; Morita, M.; Miyake, H.; Yamamoto, M.; Sonoyama, H.; Tanaka, S.; Notsu, M.; Yamauchi, M.; Fujii, Y.; et al. A case of insulin-like growth factor 2-producing gastrointestinal stromal tumor with severe hypoglycemia. BMC Endocr. Disord. 2020, 20, 60. [Google Scholar] [CrossRef]
- Alletto, M.; Burgio, A.; Fulco, G.; Geraci, G.; Groppuso, C.; Gruttadauria, G.; Manfrè, E.; Mastrosimone, G.; Salvaggio, S.; Urrico, G.; et al. Severe paraneoplastic hypoglycemia due to a non-islet cell tumor in a patient with an advanced gastrointestinal stromal tumor. Ital. J. Med. 2020, 14, 1276. [Google Scholar] [CrossRef]
- Iqbal, R.; Solipuram, D.; Mohammed, Y.N.; Bajwa, A.T.; Irfan, A.; Jafar, A.; Rehman, Z.; Islam, Z.U.; Bajwa, T.; ul Islam, Z. Impact of hypoglycemia on hospitalized patients with hepatocellular carcinoma. Cureus 2024, 16, e64673. [Google Scholar] [CrossRef]
- Khunti, K.; Davies, M.; Majeed, A.; Thorsted, B.L.; Wolden, M.L.; Paul, S.K. Hypoglycemia and Risk of Cardiovascular Disease and All-Cause Mortality in Insulin-Treated People With Type 1 and Type 2 Diabetes: A Cohort Study. Diabetes Care 2015, 38, 316–322. [Google Scholar] [CrossRef]
- Barker, L.E.; Kirtland, K.A.; Gregg, E.W.; Geiss, L.S.; Thompson, T.J. Geographic distribution of diagnosed diabetes in the U.S.: A diabetes belt. Am. J. Prev. Med. 2011, 40, 434–439. [Google Scholar] [CrossRef]
- Chida, A.; Kawasaki, K.; Kuramoto, J.; Hayashi, H.; Kawahara, T.; Makiuchi, S.; So, E.; Shimizu, S.; Kishimoto, S.; Horie, S.; et al. Clinical characteristics of gastrointestinal stromal tumors with hypoglycemia. Oncol. Lett. 2024, 28, 568. [Google Scholar] [CrossRef]
- Baxter, R.C. The role of insulin-like growth factors and their binding proteins in tumor hypoglycemia. Horm. Res. Paediatr. 1996, 46, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.T.; Polonsky, K.S.; Rubenstein, A.H.; Kew, M.C.; Tager, H.S. Tumor hypoglycemia: Relationship to high molecular weight insulin-like growth factor-II. J. Clin. Investig. 1990, 85, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.W.; Rikhof, B.; van Doorn, J.; Bilo, H.J.; Alleman, M.A.; Honkoop, A.H.; van der Graaf, W.T. Non-islet cell tumour-induced hypoglycaemia: A review of the literature including two new cases. Endocr. Relat. Cancer 2007, 14, 979–993. [Google Scholar] [CrossRef]
- Xu, R.; Chen, X.-D.; Ding, Z. Perioperative nutrition management for gastric cancer. Nutrition 2022, 93, 111492. [Google Scholar] [CrossRef]
- Crudele, L.; De Matteis, C.; Novielli, F.; Petruzzelli, S.; Di Buduo, E.; Graziano, G.; Cariello, M.; Piccinin, E.; Gadaleta, R.M.; Moschetta, A. Fasting hyperglycaemia and fatty liver drive colorectal cancer: A retrospective analysis in 1145 patients. Intern. Emerg. Med. 2024, 19, 1267–1277. [Google Scholar] [CrossRef]
- De Matteis, C.; Crudele, L.; Gadaleta, R.M.; Di Buduo, E.; Novielli, F.; Petruzzelli, S.; Cariello, M.; Moschetta, A. Low adherence to Mediterranean diet characterizes metabolic patients with gastrointestinal cancer. Nutrients 2024, 16, 630. [Google Scholar] [CrossRef]
- Miller, S.I.; Wallace, R.J., Jr.; Musher, D.M.; Septimus, E.J.; Kohl, S.; Baughn, R.E. Hypoglycemia as a manifestation of sepsis. Am. J. Med. 1980, 69, 905–910. [Google Scholar] [CrossRef]
- Fischer, K.F.; Lees, J.A.; Newman, J.H. Hypoglycemia in hospitalized patients. N. Engl. J. Med. 1986, 315, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, N.; Bhattacharya, A.; Vajifdar, H. Preoperative predictors of mortality in adult patients with perforation peritonitis. Indian. J. Crit. Care Med. 2011, 15, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Razavi Nematollahi, L.; Kitabchi, A.E.; Stentz, F.B. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism 2009, 58, 443–448. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | GIST Hospitalizations with Hypoglycemia (%) | GIST Hospitalizations Without Hypoglycemia (%) | p Value | |
|---|---|---|---|---|
| Age (in years) | 63 | 66.63 | -- | |
| Sex | Male | 49.18 | 50.82 | 0.85 |
| Female | 50.82 | 49.59 | ||
| Race | White | 57.38 | 59.9 | 0.71 |
| Black | 26.23 | 21.7 | ||
| Hispanic | 9.84 | 6.39 | ||
| Others | 6.56 | 9.87 | ||
| Quartile of median household income for zip code | 0−25th | 19.67 | 26.23 | 0.59 |
| 26th−50th | 27.87 | 23.62 | ||
| 51st−75th | 27.87 | 24.01 | ||
| 76th−100th | 24.59 | 26.14 | ||
| Primary payer | Medicare | 69.93 | 57.44 | 0.32 |
| Medicaid | 14.75 | 9.02 | ||
| Private | 19.67 | 29.03 | ||
| Others | 1.64 | 4.5 | ||
| Hospital teaching status and location | Rural | 0.0 | 38.9 | 0.22 |
| Urban non-teaching | 9.84 | 12.47 | ||
| Urban teaching | 90.16 | 83.64 | ||
| Hospital bed-size | Small | 18.03 | 14.71 | 0.52 |
| Medium | 29.51 | 25.81 | ||
| Large | 52.46 | 59.48 | ||
| Hospital region | North-east | 14.75 | 20.49 | 0.13 |
| Mid-west | 14.75 | 21.0 | ||
| South | 50.82 | 36.72 | ||
| West | 19.67 | 21.79 | ||
| Complication | GIST with Hypoglycemia (%) | GIST Without Hypoglycemia (%) | Adjusted Odds Ratio (OR) * | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|
| Mortality | 12.6 | 3.1 | 4.16 | 2.06–8.37 | 0.001 |
| Complications | GIST with Hypoglycemia (%) | GIST Without Hypoglycemia (%) | Adjusted Odds Ratio (OR *) | 95% Confidence Interval |
|---|---|---|---|---|
| Malnutrition | 78 | 14.2 | 5.63 | 3.37–9.40 |
| Sepsis | 30.8 | 7.6 | 4.00 | 2.24–7.14 |
| Ascites | 15.5 | 4.2 | 3.43 | 1.63–7.19 |
| Bowel Perforation | 2.3 | 1.0 | 2.27 | 0.54–9.49 |
| Peritonitis | 8.5 | 2.6 | 2.91 | 1.17–7.22 |
| Intestinal Obstruction | 4.7 | 3.0 | 1.50 | 0.53–4.26 |
| Gastrointestinal Bleed | 20.2 | 12.1 | 1.50 | 0.76–2.96 |
| Iron deficiency anemia | 17.1 | 12.3 | 1.12 | 0.51–2.49 |
| Complications | Adjusted Odds Ratio of GIST Hospitalizations with and Without Hypoglycemia (aOR) * | 95% Confidence Interval | p Value |
|---|---|---|---|
| Mortality | 1.38 | 1.27–1.49 | <0.001 |
| Ascites | 1.49 | 1.34–1.65 | 0.721 |
| Peritonitis | 1.10 | 1.04–1.17 | <0.001 |
| Intestinal Obstruction | 4.91 | 3.44–7.05 | <0.001 |
| Gastrointestinal Bleed | 0.68 | 0.23–1.93 | 0.470 |
| Iron deficiency anemia | 3.54 | 1.62–7.74 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginjupalli, M.; Jayakumar, J.; Forlemu, A.; Sharma, A.R.; Bandaru, P.; Kumar, V.; Nalluri, K.S.D.; Reddy, M. Impact of Hypoglycemia on Morbidity, Mortality, and Resource Utilization in Gastrointestinal Stromal Tumor: A Nationwide Analysis. Gastroenterol. Insights 2025, 16, 36. https://doi.org/10.3390/gastroent16040036
Ginjupalli M, Jayakumar J, Forlemu A, Sharma AR, Bandaru P, Kumar V, Nalluri KSD, Reddy M. Impact of Hypoglycemia on Morbidity, Mortality, and Resource Utilization in Gastrointestinal Stromal Tumor: A Nationwide Analysis. Gastroenterology Insights. 2025; 16(4):36. https://doi.org/10.3390/gastroent16040036
Chicago/Turabian StyleGinjupalli, Manasa, Jayalekshmi Jayakumar, Arnold Forlemu, Anuj Raj Sharma, Praneeth Bandaru, Vikash Kumar, Kameswara Santosh Dheeraj Nalluri, and Madhavi Reddy. 2025. "Impact of Hypoglycemia on Morbidity, Mortality, and Resource Utilization in Gastrointestinal Stromal Tumor: A Nationwide Analysis" Gastroenterology Insights 16, no. 4: 36. https://doi.org/10.3390/gastroent16040036
APA StyleGinjupalli, M., Jayakumar, J., Forlemu, A., Sharma, A. R., Bandaru, P., Kumar, V., Nalluri, K. S. D., & Reddy, M. (2025). Impact of Hypoglycemia on Morbidity, Mortality, and Resource Utilization in Gastrointestinal Stromal Tumor: A Nationwide Analysis. Gastroenterology Insights, 16(4), 36. https://doi.org/10.3390/gastroent16040036






