Abstract
Background: Esophageal adenocarcinoma (EAC) and squamous cell carcinoma (ESCC) follow divergent incidence trajectories in the United States. Rising use of electronic nicotine delivery systems (ENDS) and evolving demographic risk profiles may be reshaping these trends. We aimed to characterize national incidence patterns of EAC and ESCC from 2000 through 2022—stratified by age, sex, and race/ethnicity—and to place these in the context of changing behavioral exposures. Methods: We performed a retrospective cohort study using Surveillance, Epidemiology, and End Results SEER 21 registry data (covering 48% of the U.S. population). We included first-primary, histologically confirmed EAC (ICD-O-3 codes 8140–8576) and ESCC (8050–8084) in individuals aged ≥ 15 years diagnosed between 2000 and 2022. Age-adjusted incidence rates (per 100,000 person-years; 2000 U.S. standard) and annual percent changes (APCs) were estimated via Joinpoint regression models. Results: A total of 90,290 EAC and 47,916 ESCC cases were identified. EAC incidence increased from 2.3 to 2.8 per 100,000 (APC +0.90%; 95% CI, 0.45–1.35), with the largest relative rises in ages 15–39 years (APC +1.50%) and among women (APC +2.65%). Non-Hispanic Black and American Indian/Alaska Native populations experienced the most pronounced EAC increases. Overall ESCC incidence declined (APC −0.78%; 95% CI, −1.10 to −0.46), though Asian/Pacific Islander (+3.59%) and American Indian/Alaska Native (+1.58%) groups saw rising rates. Conclusions: EAC incidence continues to climb—especially in younger adults, women, and select racial/ethnic minorities—while ESCC declines are uneven. These histology-specific patterns highlight the urgency of tailored prevention, targeted early-detection efforts, and mechanistic studies on emerging exposures such as vaping.
1. Introduction
Esophageal cancer remains a significant global health challenge, with two primary histologic subtypes—adenocarcinoma (EAC) and squamous cell carcinoma (ESCC)—exhibiting divergent etiologies, risk factors, and epidemiologic trajectories. In the United States, EAC incidence has risen dramatically over the past five decades, whereas ESCC has declined, particularly in high-income regions. Notably, EAC is now the predominant subtype among men in Western countries, accounting for the bulk of new cases [1].
1.1. Risk Factors for EAC
Established risk factors for EAC include chronic gastroesophageal reflux disease (GERD), central adiposity and overall obesity, male sex, and progression through Barrett’s esophagus [2]. Over the last decade, electronic nicotine delivery systems (ENDS)—commonly known as vaping—have surged in popularity among adolescents and young adults [3,4,5]. Although combustible tobacco remains a potent carcinogen for both EAC and ESCC, this shift toward ENDS raises concern about their potential oncogenicity. Early mechanistic studies demonstrate that ENDS aerosols—comprising nicotine, propylene glycol, glycerol, and flavoring agents—generate reactive aldehydes (formaldehyde, acrolein), reactive oxygen species, and metal nanoparticles, which can induce mucosal inflammation, oxidative DNA damage, and epithelial barrier disruption in the upper aerodigestive tract [4,5,6,7,8,9,10]. Moreover, observational and experimental data link ENDS exposure to epithelial barrier dysfunction and inflammation that could exacerbate reflux symptoms and esophageal irritation, known precursors to Barrett’s esophagus and EAC [7,11,12].
1.2. Risk Factors for ESCC
By contrast, ESCC remains strongly associated with combustible tobacco smoking, heavy alcohol consumption, low socioeconomic status, nutritional deficiencies (micronutrient insufficiency), and chronic mucosal injury (caustic ingestion, microbial dysbiosis) [8,13,13]. Although historical declines in smoking and occupational inhalants have driven ESCC incidence downward, emerging evidence suggests that these gains may be stagnating—or even reversing—in certain racial and ethnic subgroups [14].
Given these divergent and evolving risk landscapes, and the paucity of studies that compare EAC and ESCC trends in parallel—particularly among younger adults with rising ENDS exposure—there is an urgent need to re-examine national incidence patterns in a stratified, granular manner [9]. Leveraging the SEER 21 database from 2000 to 2022, this study aims to characterize age-, sex-, and race/ethnicity-specific incidence trends in EAC and ESCC, thereby informing targeted prevention and early detection strategies in high-risk populations.
Adult cigarette smoking has fallen steadily over the study window, while ENDS use has increased among adults and especially adolescents, underscoring shifting inhalational exposures that warrant etiologic study in relation to esophageal carcinogenesis [3,10,15]. Population-level declines in Helicobacter pylori (Hp) may also modulate GERD/Barrett’s pathways toward EAC [16,17,18,19]
2. Materials and Methods
This retrospective, population-based cohort study used incidence data from the Surveillance, Epidemiology, and End Results SEER 21 cancer registries, covering approximately 48% of the U.S. population. We identified all first primary esophageal cancers diagnosed between 1 January 2000, and 31 December 2022, via SEER*Stat v8.4.2, using International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histology codes: adenocarcinoma (codes 8140–8576) and squamous cell carcinoma (codes 8050–8084). Cases with other histologies (small cell, adenosquamous, or unspecified) were excluded.
For each subtype, we obtained annual age-adjusted incidence rates per 100,000 person-years, standardized to the 2000 U.S. standard population. Rates were stratified by age group (15–39, 40–64, 65–74, ≥75 years), sex (male, female), and race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, American Indian/Alaska Native (AI/AN), Asian/Pacific Islander).
We assessed temporal trends using Joinpoint Regression Program v4.9.1.0, calculating annual percent change (APC) with 95% confidence intervals. The software identifies statistically significant inflection points (joinpoints) in log-linear models of incidence over time, allowing quantification of periods of acceleration or deceleration [19,20,21].
All analyses adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Because SEER data are de-identified and publicly available, the Albany Medical College Institutional Review Board determined this study exempt from human subjects review.
3. Results
Table 1 shows the distribution of cases of EAC and ESCC by overall trends, age group, sex, and race.

Table 1.
The distribution of cases of EAC and ESCC by overall trends, age group, sex, and race.
3.1. Overall Trends
From 2000 to 2022, a total of 90,290 cases of EAC and 47,916 cases of ESCC were identified in the SEER 21 registries (Table 1). EAC incidence increased modestly but consistently over the study period, with an average annual percent change (APC) of 0.90%. In contrast, ESCC incidence declined from 1.9 to 1.2 per 100,000, reflecting a long-term downward trend. Figure 1 shows the cancer incidence rate per 100,000 people from 2000 to 2022 for EAC and ESCC.
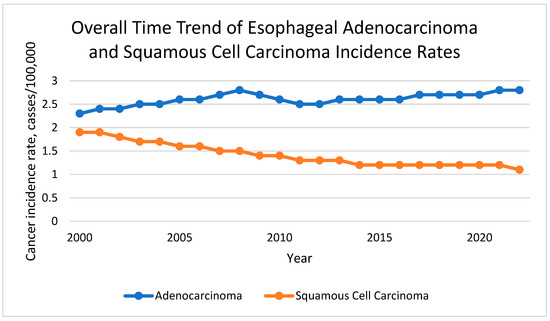
Figure 1.
Overall Time Trend of Esophageal Adenocarcinoma and Squamous Cell Carcinoma Incidence Rate.
3.2. Trends by Age
In EAC, the most pronounced increase was observed among adults aged 65–74 years (APC: 3.95%, 95% CI: 3.68–4.22), followed by individuals aged 75 and older (APC: 2.30%, 95% CI: 1.9–2.7). Middle-aged adults (40–64) also showed a significant rise (APC: 1.83%, 95% CI: 1.4–2.27). Although comprising only 1.22% of cases, young adults aged 15–39 experienced a significant relative increase (APC: 1.5%, 95% CI: 0.37–2.64; p < 0.001). Figure 2A shows the EAC incidence rates by age.
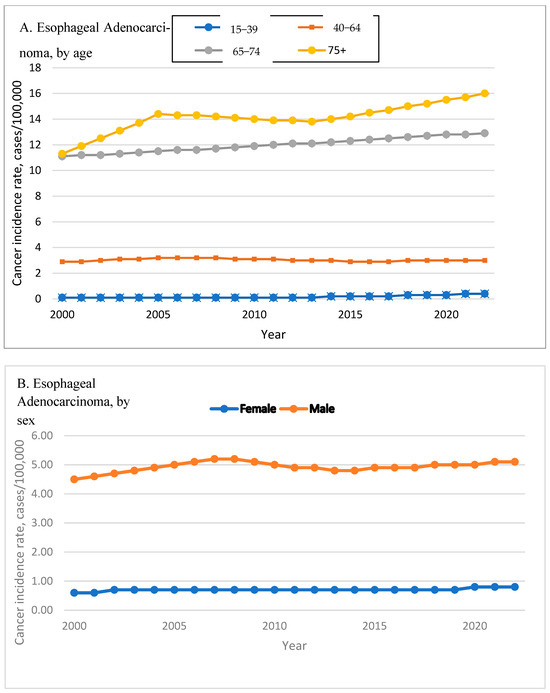
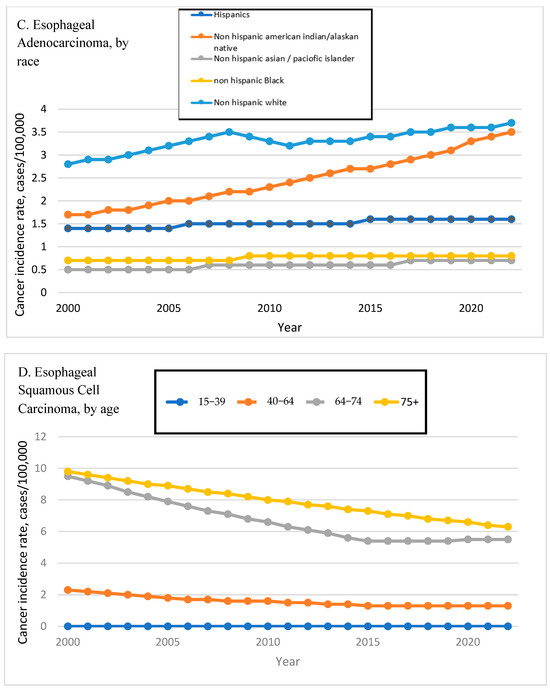
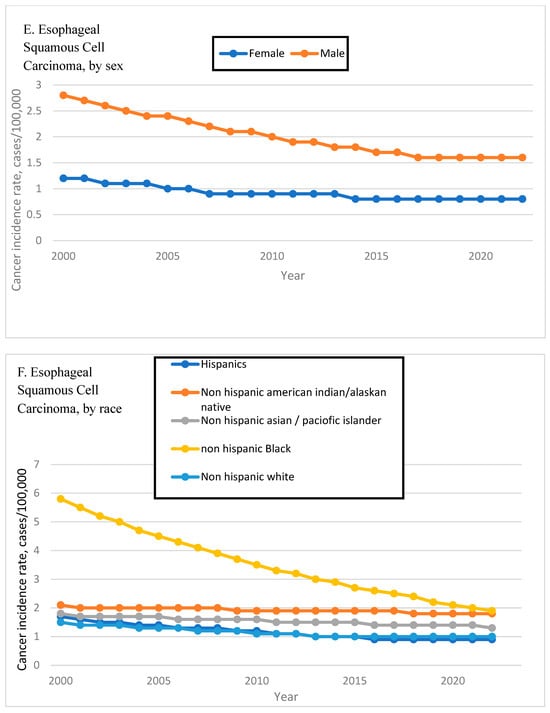
Figure 2.
Time Trend of Esophageal Adenocarcinoma and Squamous Cell Carcinoma Incidence Rates by Age, Sex, and Race. Esophageal adenocarcinoma incidence rates are shown by age (A), sex (B), and race (C). Esophageal squamous cell carcinoma incidence rates are shown by age (D), sex (E), and race (F).
ESCC trends were generally stable or declining across age groups. Adults aged 75+ exhibited the steepest decline (APC: −0.65%, 95% CI: −0.90 to −0.40), while the 40–64 age group saw a more modest but significant reduction (APC: −0.53%, 95% CI: −0.71 to −0.35). Incidence among 15–39-year-olds remained low and non-significantly decreased (APC: −0.9%, 95% CI: −1.79 to 0.0). Figure 2D shows the ESCC incidence rates by age.
3.3. Trends by Sex
Among EAC cases, men accounted for 85.28% of the total, with an APC of 2.51% (95% CI: 2.14–2.87), while women—who comprised 14.72%—had a slightly higher APC of 2.65% (95% CI: 2.42–2.88; p < 0.001). Figure 2B shows the EAC incidence rates by sex.
In ESCC, male incidence declined significantly (APC: −0.43%, 95% CI: −0.61 to −0.24), while female incidence remained stable (APC: 0.0%, 95% CI: −0.40 to 0.39; p = 0.98), reflecting a persistent sex disparity in squamous cell burden. Figure 2E shows the EAC incidence rates by age.
3.4. Trends by Race/Ethnicity
EAC incidence increased across all major racial groups. Black individuals had the highest APC at 6.54% (95% CI: 5.46–7.64), followed by AI/AN individuals (APC: 5.39%, 95% CI: 4.78–5.99). White individuals exhibited a moderate increase (APC: 3.11%, 95% CI: 2.33–3.89), and Hispanics, who represented the majority of EAC cases (87.21%), had an APC of 2.26% (95% CI: 2.02–2.51). The Asian/Pacific Islander group showed no significant change (APC: 0.39%, 95% CI: −5.46 to 6.6). Figure 2C shows the EAC incidence rates by race.
In ESCC, trends varied widely by race. Black individuals experienced a substantial decline (APC: −2.17%, 95% CI: −2.38 to −1.96), while White individuals showed a modest but significant reduction (APC: −0.40%, 95% CI: −0.68 to −0.11; p = 0.013). In contrast, both Asian/Pacific Islander (APC: 3.59%, 95% CI: 3.02–4.15) and AI/AN populations (APC: 1.58%, 95% CI: 1.12–2.05) experienced significant increases. Figure 2F shows the ESCC incidence rates by race.
4. Discussion
This comprehensive SEER analysis delineates divergent epidemiologic trajectories for EAC and ESCC in the United States and situates these trends amid plausible population-level exposures that have shifted over the last two decades. Combustible cigarette use has declined nationally, while electronic nicotine delivery systems (ENDS, “vaping”) have expanded—especially among youth and young adults—raising concern about their potential to contribute to esophageal injury via aldehyde/reactive oxygen species (ROS) generation and reflux-promoting physiology; however, our registry does not contain individual-level ENDS data, so ENDS should be viewed here as a potential (hypothesis-generating) factor rather than a demonstrated driver of incidence [10,22]. Mechanistic data show that ENDS aerosols can generate reactive aldehydes and induce deoxyribonucleic acid (DNA) damage and oxidative stress, supporting biologic plausibility for tissue injury along the aerodigestive tract [4,5].
These national trends—declining combustible smoking and rising ENDS use—may differentially affect EAC vs. ESCC risk and could contribute, among other factors, to the divergence we observed [10,23].
4.1. Persistent Rise of Esophageal Adenocarcinoma
4.1.1. Steady Increase Across Demographics
From 2000 to 2022, EAC incidence rose from 2.3 to 2.8 per 100,000 (APC +0.90%; 95% CI, 0.45–1.35; p < 0.001), consistent with prior reports of rising EAC incidence in Western populations [1]. This rise was ubiquitous across age, sex, and race strata, reflecting both legacy exposures (obesity, GERD, Barrett’s esophagus) and emerging factors.
4.1.2. Acceleration in Older Adults
The steepest APCs occurred in older cohorts: 65–74 years (3.95%; 3.68–4.22) and ≥75 years (2.30%; 1.90–2.70), which likely reflects the cohort effect of long-term GERD and obesity trends that gained momentum in the 1980s–1990s [13].
4.1.3. Early-Onset EAC
Although numerically small (1.22% of cases), EAC in adults aged 15–39 rose significantly (APC +1.50%; 0.37–2.64; p < 0.001). This mirrors patterns seen for early-onset gastrointestinal cancers and underscores the need to interrogate environmental and metabolic exposures that may accelerate carcinogenesis in younger cohorts [9].
One persisting risk factor that may be contributing to rising early-onset EAC is the shifting burden of excess adiposity in younger adults, as obesity rates among adults aged 18–35 have increased steadily in recent years [13]. Although electronic health record (EHR) sampling percentages do not equate directly to obesity prevalence, they highlight continued—and still substantial—representation of young adults in obesity surveillance. Excess central adiposity increases intra-abdominal pressure and promotes acid reflux, thereby exacerbating GERD and accelerating progression to Barrett’s esophagus [14]. Moreover, obesity-related hormonal dysregulation (elevated leptin, reduced adiponectin) and metabolic derangements create a pro-carcinogenic milieu that fosters the transformation of metaplastic Barrett’s epithelium into EAC [15]. Together, these data underscore how the obesity epidemic—whether measured by body mass index (BMI) prevalence or by continued high representation in BMI datasets—may help drive the ongoing rise in early-onset EAC among vulnerable young adults.
The rising popularity of glucagon-like peptide-1 (GLP-1) receptor agonists for obesity, diabetes, and heart failure management offers a compelling opportunity to study longitudinal obesity trends, as well as the broader impact on associated cancers like EAC. In the phase 3 STEP 1 trial, once-weekly semaglutide 2.4 mg produced a mean weight loss of 14.9% at 68 weeks and was associated with clinically meaningful improvements in obesity-related symptoms [16]. Likewise, daily liraglutide 3.0 mg yielded an average 8.0% weight reduction in adults with type 2 diabetes [17]. By decreasing central adiposity and reflux, GLP-1 receptor agonists may disrupt the obesity–GERD–Barrett’s esophagus cascade that predisposes to EAC. Future longitudinal studies should evaluate whether widespread adoption of GLP-1 receptor agonists translates into measurable reductions in both Barrett’s progression and EAC incidence.
4.1.4. Sex Convergence
Historically a male-predominant disease, EAC’s relative increase in women (APC +2.65%; 2.42–2.88) now slightly outpaces that in men (2.51%; 2.14–2.87; p < 0.001). While male sex remains an absolute risk factor—accounting for 85.3% of cases—this shift may reflect narrowing sex differences in obesity prevalence and related metabolic risk [13] and escalating ENDS use among young females, as female adolescents now consume flavored e-cigarettes at rates comparable to males [24,25].
4.1.5. Racial/Ethnic Disparities
EAC APCs were highest among non-Hispanic Black and AI/AN persons in our analysis, paralleling social-group disparities described in other settings and suggesting a need for stratified prevention and detection strategies [23].
Beyond social determinants and exposure patterns, inherited variation in alcohol-metabolizing enzymes may modulate ESCC risk. The aldehyde dehydrogenase 2 (ALDH2)*2 (rs671) inactive variant—prevalent in East Asian populations—impairs acetaldehyde clearance; in the presence of heavy drinking, carriers have markedly elevated ESCC risk [26,27,28]. A fast-activity alcohol dehydrogenase 1B (ADH1B) variant can further enhance acetaldehyde production. Beverage characteristics also matter: high-ethanol spirits and very hot beverages increase mucosal exposure to carcinogenic acetaldehyde and thermal injury [29,30]. These gene-environment interactions help contextualize rising ESCC rates in some Asian/Pacific Islander subgroups despite broader national declines.
4.2. Role of Tobacco and Vaping in EAC
4.2.1. Declining Cigarettes but Rising ENDS
Despite substantial declines in combustible smoking prevalence in the U.S. over the past two decades, EAC continued to climb, indicating that non-combustible exposures and other co-factors (notably obesity, GERD, and ENDS) are increasingly relevant [1,10]. National surveys document a rise in adult e-cigarette use to ~6.5% by 2023 and ongoing youth use driven by flavored products [10,31,32].
4.2.2. Plausible Mechanisms
ENDS aerosols contain nicotine, propylene glycol, vegetable glycerin, and flavoring agents that generate reactive aldehydes upon heating and can induce DNA damage, oxidative stress, and barrier dysfunction—processes implicated in carcinogenesis and tissue metaplasia [4,5,6,11,12,18,19,21]. Nicotine exposure can also influence esophageal physiology, potentially worsening reflux and hindering mucosal healing [5,12]. These overlapping mechanisms—when superimposed on obesity-related GERD—suggest that vaping is not an isolated cause but a compounding factor in the multifaceted pathogenesis of EAC [10,33].
4.3. Helicobacter pylori, Gastric Atrophy, and EAC Risk
Declines in Hp—through both secular trends and eradication campaigns—may interact with EAC risk through effects on acid output, reflux, and Barrett’s metaplasia. Certain strains of Hp, namely those eliciting corpus-predominant gastritis, can lead to atrophy of gastric parietal cells and subsequently, hypochlorhydria [34,35,36,37,38]. To that end, the secondary reduction in gastric acid secretion may protect against the development of GERD and Barrett’s esophagus [36,37]. Hp infection is also implicated in lowered ghrelin levels, contributing to reduced food intake and an indirect reduction in GERD risk, as obesity and high-intraabdominal pressure are strong risk factors for EAC [36]. Lastly, studies have demonstrated that Hp induces apoptosis of Barrett’s derived EAC cells at higher rates than normal esophageal cells through preferential fragmentation of cancerous cellular DNA [39]. The falling prevalence of Hp, whether it be through public health improvements or widespread antibiotic usage, may be linked to the observed trends in EAC incidence. Importantly, despite this well-studied relationship between Hp and EAC, there is currently no evidence of the role of Hp in the pathogenesis of ESCC [36,40].
4.4. Esophageal Squamous Cell Carcinoma: Uneven Decline
4.4.1. Overall Decrease
ESCC incidence declined from 1.9 to 1.2 per 100,000 (APC −0.78%; −1.02 to −0.54; p < 0.001), paralleling tobacco control, reduced heavy alcohol consumption, and improved nutritional status in many regions [8,15,23].
4.4.2. Subgroup Reversals
Declines in ESCC were uneven across racial demographics: non-Hispanic Black and non-Hispanic White persons saw the largest drops, while Asian/Pacific Islander and AI/AN groups experienced rising ESCC rates. These anomalies may reflect persistent or emerging risk factors—continued use of traditional tobacco, alcohol, nutritional deficiencies, and occupational/combustion exposures—and underscore the need for tailored prevention [20,23].
4.4.3. Young Adult ESCC
The 15–39 age group showed a non-significant decline (−0.90%; −1.79 to 0.00), but the persistence of ESCC in younger cohorts underscores the necessity of targeted surveillance for alarm features and sustained risk-reduction programs in subgroups with high exposure burdens [23].
4.5. Clinical and Public Health Implications
4.5.1. Integrated Cancer Prevention: Distinct Levers for EAC and ESCC
For EAC, pairing obesity management with GERD control is foundational. Central adiposity elevates intra-abdominal pressure and acid exposure, accelerating the GERD–Barrett’s–EAC continuum; clinically, this supports comprehensive weight-management programs (behavioral, pharmacologic) integrated with reflux education and judicious acid suppression [2,13,14,15,16,17,24,25]. Given the rising prevalence of ENDS, prevention and cessation strategies tailored to young adults should be embedded alongside obesity/GERD care, because ENDS aerosols deliver aldehydes and oxidants that injure mucosa and may exacerbate reflux physiology [4,5,6,7,10,11,12,18,19,21].
For ESCC, the essentials remain tobacco and alcohol control, micronutrient sufficiency, and reduction in combustion exposures. Evidence from International Agency for Research on Cancer (IARC) affirms alcohol’s causal role in ESCC, supporting policies that reduce harmful drinking while maintaining robust tobacco control [15,35].
4.5.2. Tobacco, Vaping, and Alcohol Policy: Align Controls with Current Use
While cigarette smoking has declined, adult e-cigarette use has increased and remains highest among 21–24-year-olds; youth use is concentrated in flavored products [10,27,28]. Federal measures—Tobacco 21 and the Food and Drug Administration’s (FDA) 2020 ENDS enforcement guidance—have created important guardrails, but loopholes (e.g., disposables, some tank systems) and variable state/local rules limit impact [26,33,34]. Policymakers should harmonize flavor restrictions across devices, strengthen retail compliance, and fund cessation tailored to ENDS users, particularly young adults [26,33,34]. For ESCC, renewed attention to alcohol-policy levers (pricing/taxation, availability, marketing limits) is warranted where incidence declines have stalled [15].
4.5.3. Early Detection and Clinical Pathways: Target High-Risk Groups
Because EAC often arises from Barrett’s esophagus, early detection should be risk-based rather than universal. The American College of Gastroenterology (ACG) 2022 guideline recommends targeted Barrett’s case-finding—especially men with chronic GERD plus additional risk factors—and structured surveillance using validated grading systems (e.g., Prague C & M) and dysplasia pathways [24,32]. Our observed increases among women and specific racial/ethnic groups argue for locally calibrated algorithms that reflect regional obesity/GERD patterns and tobacco/ENDS profiles, coupled with primary-care prompts and streamlined endoscopy access [24,25,32]. For ESCC, maintaining a low threshold for evaluation of alarm features in high-exposure subgroups, and integrating cessation and nutritional supports into primary care, remain pragmatic approaches [8,15,23].
4.5.4. Health Equity and Community Tailoring
Heterogeneous trajectories—steeper EAC increases in some Black and AI/AN populations and uneven ESCC declines among Asian/Pacific Islander and AI/AN groups—underscore the need for culturally and linguistically appropriate programs. Community-engaged vaping prevention, weight-management, and navigation supports to reduce endoscopy barriers (transportation, time off work, childcare) can improve reach. Equity metrics—screening completion, time-to-diagnosis, stage at detection—should be incorporated into quality dashboards to ensure that gains are shared across groups [23,24,25].
4.6. Health-System Implementation Priorities
4.6.1. Primary-Care Enablement and Risk Capture
Most at-risk patients present in primary care. EHRs should include structured fields for GERD duration, tobacco/ENDS use, and central adiposity, linked to decision support that flags candidates for Barrett’s case-finding and auto-populates referral order sets with biopsy/surveillance standards [24,25,32]. Embedding tobacco and ENDS cessation orders (including pharmacotherapy) during GERD care exploits teachable moments [10,27,28].
4.6.2. Endoscopy Quality and Capacity
If risk-based case-finding expands, endoscopy units should plan for capacity and quality safeguards: standardized indications, adherence to Prague C & M and ACG/American Society for Gastrointestinal Endoscopy (ASGE) protocols, and tracking of key indicators (biopsy adequacy, dysplasia adjudication, interval adherence) [24,25,32]. In low-access regions, partnerships with regional centers and, where feasible, validated non-endoscopic cell-collection tools for triage may smooth demand (pending local validation).
4.6.3. Clinical Integration of Prevention
Systems can align incentives by combining reflux, weight, and tobacco/ENDS services into integrated pathways with shared outcomes (symptom control, quit rates, weight loss). Coverage for evidence-based weight management and cessation pharmacotherapy should be standardized, particularly for young adults, who anchor current ENDS use [10,27,28].
4.7. Policy and Regulatory Considerations
4.7.1. Tobacco and ENDS
Given rising adult ENDS use and persistent youth flavor-driven demand, comprehensive approaches that harmonize flavor restrictions across ENDS device categories, strengthen age-verification and retail compliance, and fund cessation tailored to e-cigarette users are warranted [26,33,34]. Extending Tobacco 21 enforcement with routine compliance checks can slow initiation among late adolescents and young adults [26,34]. Tobacco taxes and mass-media campaigns remain cost-effective levers for combustible tobacco—relevant to both EAC and ESCC risk [1,15,39].
4.7.2. Alcohol and ESCC
In jurisdictions with stagnant ESCC declines or rising incidence, policymakers should revisit alcohol pricing (taxation), availability, and marketing restrictions—the highest-yield levers in international evidence—to reduce harmful drinking, paired with clinical screening and brief interventions [15].
4.7.3. Occupational and Environmental Exposures
For communities with combustion exposures (e.g., biomass/wood smoke) or polycyclic aromatic hydrocarbons, targeted workplace controls, clean-cookstove initiatives, and exposure monitoring can reduce ESCC-relevant carcinogens, requiring cross-sector coordination.
4.8. Research Directions
4.8.1. Linkage Studies Tying Incidence to Individual Exposure
Next steps include linking cancer registry data with individual-level exposure datasets (e.g., NHIS, NHANES) to quantify risks attributable to ENDS, combustible tobacco, alcohol, obesity, and GERD, moving beyond ecological inference [1,8,9,10,27,28,40]. These linkages can test whether ENDS intensity, flavor category, and device type track with Barrett’s and EAC after accounting for obesity and reflux.
4.8.2. Natural Experiments in Policy
The United States offers “natural experiments”—Tobacco 21, the 2020 ENDS enforcement guidance, and evolving state/local flavor bans. Rigorous quasi-experimental designs (interrupted time-series, difference-in-differences) can evaluate whether these rules alter ENDS initiation/persistence and, over longer windows, bend esophageal-cancer incidence curves (especially in younger cohorts) [26,33,34].
4.8.3. Mechanistic and Molecular Epidemiology
Mutational signature studies can test whether aldehyde/oxidant exposures from ENDS aerosols leave distinct patterns in EAC compared with tobacco-associated or reflux-dominant tumors. Paired tissue and exhaled-breath condensate studies could identify exposure biomarkers to enrich early-detection cohorts [4,5,6,11,12,18,19,21].
4.8.4. Immigration and Acculturation
Migrant studies show that cancer risks often shift toward host country profiles over time, with persistence of some origin-country risks across generations. For esophageal cancer, immigration can alter exposure mixtures (diet, alcohol, alcohol type/temperature, tobacco/ENDS adoption) and healthcare access, potentially reshaping EAC/ESCC incidence in these U.S. ethnic subgroups [40]. Accounting for nativity, duration in the U.S., and acculturation markers in future SEER-linked analyses would help disentangle environmental from genetic contributors.
4.8.5. Early Detection Innovation and Care-Path Trials
Prospective trials should evaluate risk-stratified Barrett’s case-finding in primary care—comparing standard referral with EHR-embedded prompts and nurse-led navigation—and track yield, dysplasia detection, and time-to-treatment [24,25,32]. Pragmatic studies can compare surveillance intervals and biopsy protocols within guideline ranges to optimize value.
4.8.6. Obesity and Reflux Interventions
Given the plausibility that central adiposity drives reflux and Barrett’s, weight-loss interventions—including GLP-1 receptor agonists used at scale—should be studied for intermediate endpoints (acid exposure, Barrett’s regression) and hard outcomes (EAC incidence) using pragmatic registries and claims cohorts [13,14,15,16,17].
4.8.7. Equity-Focused Implementation Science
Because our data reveal subpopulation heterogeneity, implementation trials should test culturally tailored vaping prevention, community health worker navigation for Barrett’s case-finding, and alcohol brief-intervention programs where trends are unfavorable, with outcomes including reach, uptake, and time-to-diagnosis [23,24,25,27,28].
4.9. Health-System Quality Metrics
To translate prevention and early detection into measurable gains, systems should adopt a concise set of indicators: (1) documentation rates of GERD duration, BMI/waist, and tobacco/ENDS status in primary care; (2) appropriate Barrett’s referrals per ACG criteria; (3) endoscopy quality (Prague C & M use, adherence to biopsy/surveillance protocols, dysplasia adjudication); (4) time from referral to endoscopy; (5) quit-rate tracking for tobacco/ENDS programs; and (6) equity metrics (completion rates by race/ethnicity and rurality) [24,25,32].
4.10. Strengths and Limitations
A principal strength of this analysis is the use of SEER-21 coverage (~48% of the U.S. population) over a 23-year window, enabling stable APC estimates across age, sex, and race/ethnicity. Findings are consistent with national exposure trends—declining cigarettes, rising adult ENDS use—and align with current guideline frameworks for early detection [1,10,24,25,27,28,32]. Limitations include the ecologic design (precluding individual-level exposure inference), potential histology misclassification over time, and lack of individual risk-factor data. SEER lacks individual data on smoking intensity, ENDS, alcohol type/temperature, Hp status, and GERD/BE, limiting causal inference [10,35,36,37,39,40]. Our SEER extract focused on incidence and did not analyze Summary Stage or overall survival; nor did it incorporate molecular subclassification. Future linkage studies are needed to address these gaps (see Research Directions) [29,30,31].
4.11. Policy Summary
These SEER-based trends argue for dual-track prevention: (1) EAC—integrate weight management and GERD care with ENDS prevention and cessation tailored to young adults; (2) ESCC—sustain and strengthen tobacco and alcohol control, with targeted efforts in subgroups where declines have stalled. Implement risk-based Barrett’s case-finding per ACG, invest in endoscopy quality capacity, and adopt equity metrics to ensure reach. At the regulatory level, harmonize flavor restrictions across ENDS device types, enforce Tobacco 21 compliance, and support alcohol-policy levers where ESCC risk remains high [1,10,15,24,25,26,33,34,35].
Author Contributions
V.H.M.: Conceptualization, Data Curation, Formal Analysis, Visualization, Writing—Original Draft, Writing—Review and Editing. K.N.-N.: Methodology, Validation, Writing—Review and Editing. M.T.: Supervision, Resources, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the lack of human subjects and publicly available data.
Informed Consent Statement
Patient consent was waived due to public nature of data available to general public through the SEER 21 database website.
Data Availability Statement
All data used in this analysis are publicly available through the SEER 21 database. Analytic methods and study materials will be made available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no financial, professional, or personal conflicts of interest relevant to this manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| ACG | American College of Gastroenterology |
| ADH1B | Alcohol dehydrogenase 1B |
| AI/AN | American Indian/Alaska Native |
| APC | Annual Percent Change |
| ASGE | American Society for Gastrointestinal Endoscopy |
| BE | Barrett’s esophagus |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| EAC | Esophageal Adenocarcinoma |
| EHR | Electronic Health Record |
| ENDS | Electronic Nicotine Delivery Systems (e-cigarettes/vaping) |
| ESCC | Esophageal Squamous Cell Carcinoma |
| FDA | U.S. Food and Drug Administration |
| GERD | Gastroesophageal Reflux Disease |
| GLP-1 | Glucagon-Like Peptide-1 |
| IARC | International Agency for Research on Cancer |
| ICD-O-3 | International Classification of Diseases for Oncology, 3rd Edition |
| NHANES | National Health and Nutrition Examination Survey |
| NHIS | National Health Interview Survey |
| NYTS | National Youth Tobacco Survey |
| Prague C & M | Circumferential and Maximal Extent Criteria (for Barrett’s) |
| SEER | Surveillance, Epidemiology, and End Results |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Fan, J.; Liu, Z.; Mao, X.; Tong, X.; Zhang, T.; Suo, C.; Chen, X. Global trends in the incidence and mortality of esophageal cancer from 1990 to 2017. Cancer Med. 2020, 9, 6875–6887. [Google Scholar] [CrossRef]
- Halland, M.; Katzka, D.; Iyer, P.G. Recent developments in pathogenesis, diagnosis and therapy of Barrett’s esophagus. World J. Gastroenterol. 2015, 21, 6479–6490. [Google Scholar] [CrossRef]
- Gentzke, A.S.; Wang, T.W.; Cornelius, M.; Park-Lee, E.; Ren, C.; Sawdey, M.D.; Cullen, K.A.; Loretan, C.; Jamal, A.; Homa, D.M. Tobacco Product Use and Associated Factors Among Middle and High School Students—NYTS, United States, 2021. MMWR Surveill. Summ. 2022, 71, 1–29. [Google Scholar] [CrossRef]
- ASGE STANDARDS OF PRACTICE COMMITTEE; Qumseya, B.J.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359.e2. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Tobacco 21 (Minimum Legal Sales Age 21; Compliance Guidance); FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- Jamal, A.; Park-Lee, E.; Birdsey, J.; West, A.; Cornelius, M.; Cooper, M.R.; Cowan, H.; Wang, J.; Sawdey, M.D.; Cullen, K.A.; et al. Tobacco product use among U.S. students—2024 NYTS. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 917–924. [Google Scholar] [CrossRef]
- Park-Lee, E.; Jamal, A.; Cowan, H.; Sawdey, M.D.; Cooper, M.R.; Birdsey, J.; West, A.; Cullen, K.A. Notes from the Field: E-cigarette and nicotine pouch use among middle and high school students—United States, 2024. MMWR. Morb. Mortal. Wkly. Rep. 2024, 73, 774–778. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Kistler, K.A.; Pennington, A.; Spyrou, A.; Kouretas, D.; Gillman, G. Aldehyde levels in e-cigarette aerosol. Food Chem. Toxicol. 2018, 111, 64–70. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, S.H.; Weng, M.W.; Wang, H.-T.; Huang, W.C.; Lepor, H.; Wu, X.-R.; Chen, L.-C.; Tang, M.-S. E-cigarette smoke damages DNA and reduces repair activity. Proc. Natl. Acad. Sci. USA 2018, 115, E1560–E1569. [Google Scholar]
- Niu, C.; Liu, Y.; Wang, J.; Liu, Y.; Zhang, S.; Zhang, Y.; Zhang, L.; Zhao, D.; Liu, F.; Chao, L.; et al. Risk factors for ESCC and its histological precursor lesions in China: A multicenter cross-sectional study. BMC Cancer 2021, 21, 1034. [Google Scholar] [CrossRef]
- Crotty Alexander, L.E.; Drummond, C.A.; Hepokoski, M.; Mathew, D.; Moshensky, A.; Willeford, A.; Das, S.; Singh, P.; Yong, Z.; Lee, J.H.; et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R834–R847. [Google Scholar] [CrossRef]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; NN8022-1922 Study Group. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes. JAMA 2015, 314, 687–699. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Reidel, B.; Radicioni, G.; Clapp, P.W.; Ford, A.A.; Abdelwahab, S.; Rebuli, M.E.; Haridass, P.; Alexis, N.E.; Jaspers, I.; Kesimer, M. E-cigarette use causes a unique innate immune response in the lung. Am. J. Respir. Crit. Care. Med. 2018, 197, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhuang, H.; Liu, Y. The association between obesity factor and esophageal cancer. J. Gastrointest. Oncol. 2012, 3, 226–231. [Google Scholar]
- Tarazi, M.; Chidambaram, S.; Markar, S.R. Risk Factors of Esophageal Squamous Cell Carcinoma beyond Alcohol and Smoking. Cancers 2021, 13, 1009. [Google Scholar] [CrossRef]
- Xie, S.H.; Lagergren, J. Social group disparities in the incidence and prognosis of oesophageal cancer. United Eur. Gastroenterol. J. 2018, 6, 343–348. [Google Scholar] [CrossRef]
- Bonner, E.; Chang, Y.; Christie, E.; Colvin, V.; Cunningham, B.; Elson, D.; Ghetu, C.; Huizenga, J.; Hutton, S.J.; Kolluri, S.K.; et al. The chemistry and toxicology of vaping. Pharmacol. Ther. 2021, 225, 107837. [Google Scholar] [CrossRef]
- Rader, B.; Hazan, R.; Brownstein, J.S. Changes in Adult Obesity Trends in the US. JAMA Health Forum. 2024, 5, e243685. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC) Working Group. Personal Habits and Indoor Combustions (Alcoholic Beverage Consumption); IARC Monographs; IRAC: Lyon, France, 2012; Volume 100E. [Google Scholar]
- Maity, R.; Dhali, A.; Biswas, J. Is Helicobacter pylori infection protective against esophageal cancer? World J. Gastroenterol. 2024, 30, 4168–4174. [Google Scholar] [CrossRef]
- Jones, A.D.; Bacon, K.D.; Jobe, B.A.; Sheppard, B.C.; Deveney, C.W.; Rutten, M.J. Helicobacter pylori induces apoptosis in Barrett’s-derived esophageal adenocarcinoma cells. J. Gastrointest. Surg. 2003, 7, 68–76. [Google Scholar] [CrossRef]
- Erőss, B.; Farkas, N.; Vincze, Á.; Tinusz, B.; Szapáry, L.; Garami, A.; Balaskó, M.; Sarlós, P.; Czopf, L.; Alizadeh, H. Helicobacter pylori infection reduces the risk of Barrett’s esophagus: A meta-analysis and systematic review. Helicobacter 2018, 23, e12504. [Google Scholar] [CrossRef]
- Lindkvist, B.; Johansen, D.; Stocks, T.; Concin, H.; Bjørge, T.; Almquist, M.; Häggström, C.; Engeland, A.; Hallmans, G.; Nagel, G.; et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: A prospective study of 580,000 subjects within the Me-Can project. BMC Cancer 2014, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Chen, H.-S.; Byrne, J.; Wheeler, B.; Feuer, E.J. Twenty years since Joinpoint 1.0: Continuing improvements for joinpoint regression analysis. Stat. Med. 2022, 41, 3102–3130. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dent, J.; Armstrong, D.; Bergman, J.J.G.H.M.; Gossner, L.; Hoshihara, Y.; Jankowski, J.A.; Junghard, O.; Lundell, L.; Tytgat, G.N.J.; et al. The Prague C & M Criteria for the endoscopic grading of Barrett’s esophagus. Gastroenterology 2006, 131, 1392–1399. [Google Scholar] [PubMed]
- International Agency for Research on Cancer (IARC). Drinking Coffee, Maté and very Hot Beverages; IARC Monographs; IRAC: Lyon, France, 2018; Volume 116. [Google Scholar]
- Vahratian, A.; Briones, E.M.; Jamal, A.; Marynak, K.L. Electronic Cigarette Use Among Adults in the United States, 2019–2023. NCHS Data Brief. 2025, CS356607. [Google Scholar]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Sassano, M.F.; Davis, E.S.; Keating, J.E.; Zorn, B.T.; Kochar, T.K.; Wolfgang, M.C.; Glish, G.L.; Tarran, R. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018, 16, e2003904. [Google Scholar] [CrossRef]
- Mizukami, K.; Sugano, K.; Takeshima, T.; Murakami, K. Disease trends after Helicobacter pylori eradication based on Japanese nationwide claims and the health check-up database. World J. Gastroenterol. 2023, 29, 692–705. [Google Scholar] [CrossRef]
- Yang, S.J.; Yokoyama, A.; Yokoyama, T.; Huang, Y.C.; Wu, S.Y.; Shao, Y.; Niu, J.; Wang, J.; Liu, Y.; Zhou, X.Q.; et al. Relationship between genetic polymorphisms of ALDH2 and ADH1B and esophageal cancer risk in males: A meta-analysis. World J. Gastroenterol. 2010, 16, 4210–4220. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Alcohol Consumption and Ethyl Carbamate; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 96. [Google Scholar]
- U.S. Food and Drug Administration. Justice Department and FDA Announce Federal Multi-Agency Task Force to Curb the Distribution and Sale of Illegal E-Cigarettes; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2024. [Google Scholar]
- Birdsey, J.; Cornelius, M.; Jamal, A.; Park-Lee, E.; Cooper, M.R.; Wang, J.; Sawdey, M.D.; Cullen, K.A.; Neff, L. Tobacco Product Use Among U.S. Middle and High School Students—United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1173–1182. [Google Scholar] [CrossRef]
- Conklin, D.J.; Ogunwale, M.A.; Chen, Y.; Theis, W.S.; Nantz, M.H.; Fu, X.-A.; Chen, L.-C.; Riggs, D.W.; Lorkiewicz, P.; Bhatnagar, A.; et al. Electronic cigarette-generated aldehydes. Aerosol. Sci. Technol. 2018, 52, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (NCI). Joinpoint Regression Program, Version 4.9.1.0; NCI: Bethesda, MD, USA, 2022. [Google Scholar]
- U.S. Food and Drug Administration. Enforcement Priorities for Electronic Nicotine Delivery Systems (Revised Guidance); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020. [Google Scholar]
- Canistro, D.; Vivarelli, F.; Cirillo, S.; Marquillas, C.B.; Buschini, A.; Lazzaretti, M.; Marchi, L.; Cardenia, V.; Rodriguez-Estrada, M.T.; Lodovici, M.; et al. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep. 2017, 7, 2028. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Cigarette Smoking Among Adults—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 633–641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).