Abstract
Helicobacter pylori is a primary cause of several gastrointestinal diseases, including chronic gastritis and gastric cancer. Unfortunately, standard treatments are increasingly failing due to rising antimicrobial resistance, particularly to clarithromycin. This necessitates the development of optimized H. pylori management strategies that minimize antibiotic use, reduce adverse effects, lower costs, and improve compliance. Vonoprazan-based regimens seem to be a viable option, as vonoprazan (VPZ) offers more consistent and potent acid suppression because it is not significantly affected by CYP2C19 genetic variations. This review comprehensively analyzed 43 clinical studies from the past five years in PubMed, evaluating the efficacy and safety of vonoprazan-based regimens for H. pylori eradication, including comparisons with established and novel therapies of varying doses and durations. The findings consistently demonstrate that VPZ-based therapies achieve comparable or superior eradication rates, alongside a more favorable safety profile and enhanced cost-effectiveness. While high-dose vonoprazan-amoxicillin (VA) therapy temporarily impacts gut microbiota and can persistently affect the antibiotic resistome, low-dose VA regimens show negligible effects. This highlights VA therapy as a promising candidate for an optimal H. pylori eradication strategy, though further long-term research, particularly in diverse global populations, is essential to definitively establish the best possible regimens.
1. Introduction
Helicobacter pylori is a spiral-shaped, Gram-negative, flagellated bacterium that predominantly colonizes the human gastric mucosa [1,2]. It is widely recognized as a major etiological agent of various gastrointestinal diseases, including chronic gastritis, peptic ulcer disease, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [1,2,3]. It has also been associated with other conditions such as anemia resulting from iron or B12 deficiency, idiopathic thrombocytopenic purpura, atopic dermatitis, neurological degenerative diseases and colorectal cancer [4,5]. Globally, H. pylori infects approximately 50% of the population, with prevalence varying significantly by region, socioeconomic status, and sanitation conditions [3,4,6,7,8,9]. Infection typically occurs in early childhood and persists lifelong if not treated, contributing to high recurrence rates in low-resource settings. Due to its strong association with gastric malignancies, the International Agency for Research on Cancer classifies H. pylori as a Group I carcinogen [3,4,10]. Eradication of H. pylori should be offered to all individuals with a confirmed active infection to reduce gastric inflammation, prevent disease progression, and lower gastric cancer risk [2,10].
Through the decades, the management of H. pylori infection has contended with setbacks such as increasing rates of antimicrobial resistance and concerns surrounding treatment efficacy, safety, and patient adherence [6,10,11,12,13,14,15]. Recent guidelines provided by American College of Gastroenterology (ACG) recommend a 14-day bismuth quadruple therapy (BQT) as the first-choice regimen, especially in regions with high clarithromycin resistance. It is also considered the preferred option for patients with a documented penicillin allergy. For treatment-naive patients, alternative first-line strategies include rifabutin-based triple therapy and dual therapy with amoxicillin and VPZ, a potassium-competitive acid blocker (PCAB) [10]. Each regimen presents distinct limitations. Bismuth quadruple therapy, while broadly acknowledged to be effective (eradication rate > 90%), is associated with a complex medication regimen, frequent gastrointestinal adverse effects and high cost. Issues mentioned above are reflected in reduced patient adherence [13,14,15,16,17].
The widespread emergence of antibiotic resistance, particularly to medications such as clarithromycin, metronidazole, and levofloxacin, is a significant barrier to successful treatment [6,15,16]. This appears to be a reason for declining efficacy of clarithromycin-based triple therapy as its eradication rate scarcely attains 80% [4,14,17]. Notably, both clarithromycin and levofloxacin are classified by the World Health Organization as ‘Watch’ list antibiotics due to their high resistance potential, requiring more stringent monitoring of their distribution [10,11]. As a result, the 2024 ACG guidelines specify that clarithromycin-based regimens should be restricted only to cases involving a history of failed first-line treatment and only if the persistent H. pylori strain has documented clarithromycin susceptibility. On the other hand, when clarithromycin resistance status remains unknown due to no access to susceptibility testing and better regimens are unavailable, VPZ-based triple therapy is favored over PPI-based triple therapy [10]. According to the latest guidelines, physicians are strongly recommended to choose empiric treatment based on local antimicrobial resistance to ensure a high probability of success [10,18].
An ideal first-line H. pylori eradication regimen would achieve consistently high cure rates (>90%) across diverse populations [11]. It would have a favorable safety profile, minimal disruption to the gut microbiota, low cost, simplified administration, global accessibility and strong patient adherence. While no regimen yet fulfills all these criteria, dual and triple therapies incorporating PCABs, particularly vonoprazan, represent a substantial step toward this therapeutic goal and are reshaping the landscape of H. pylori management [11,18].
VPZ is a PCAB that inhibits gastric acid secretion by reversibly blocking the H+/K+-ATPase enzyme in parietal cells through potassium ion competition [19]. Unlike proton pump inhibitors (PPIs), VPZ does not require activation in an acidic environment and is not significantly affected by CYP2C19 genetic polymorphisms, resulting in more rapid, consistent, and potent acid suppression across diverse patient populations [4,6,11,19,20]. In contrast, PPIs are prodrugs that must first be activated in the acidic canaliculi of parietal cells before irreversibly binding to the H+/K+-ATPase, leading to a slower onset of action. Their efficacy is also influenced by CYP2C19 metabolism, which contributes to interpatient variability in acid suppression and eradication rates [4,6,11]. Vonoprazan’s ability to raise and sustain intragastric pH above 5–6 shortly after administration creates an optimal environment for antibiotic efficacy, contributing to improved H. pylori eradication rate [21,22]. VPZ is rapidly absorbed following oral administration, reaching peak plasma concentration within two hours, and has a prolonged plasma half-life of approximately nine hours. Its absorption is not affected by food intake, offering dosing flexibility [6]. In addition to its role in H. pylori eradication, it has also shown superior therapeutic outcomes in acid-related disorders such as gastroesophageal reflux disease and peptic ulcer disease [6,23,24,25,26]. A comprehensive comparison of VPZ, a representative PCAB, versus PPI is shown in Table 1.

Table 1.
Comparison of vonoprazan vs. PPI.
This review paper presents the available data on the efficacy and safety of vonoprazan-based regimens in the eradication of H. pylori, with a focus on the results of clinical trials and meta-analyses. The results derived from these studies may contribute to a better understanding of clinical applicability of vonoprazan, leading to optimized management of H. pylori infection and the subsequent avoidance or mitigation of numerous gastrointestinal disorders.
2. Materials and Methods
A comprehensive literature search was conducted in the PubMed database to identify studies evaluating the clinical efficacy and safety of vonoprazan-based therapies in the eradication of Helicobacter pylori. The search was performed on 24 July 2025 to include the most recent and relevant publications. The following search terms were used: “Vonoprazan” OR “Helicobacter pylori infection”. Only freely accessible full-text articles were considered, with preference given to clinical trials, randomized controlled trials, and relevant monographs or clinical documents. The inclusion criteria focused on studies assessing vonoprazan-based therapies in comparison with standard or alternative treatment regimens, including variations in dosage and treatment duration. Publications that did not address clinical outcomes related to efficacy or safety were excluded. The selection and analysis of literature were carried out by the authors through independent database exploration and critical reading of abstracts and full texts. Duplicates were excluded by comparing study titles, authors, publication dates, and DOI numbers. Figure 1 presents a flowchart of the study selection process.
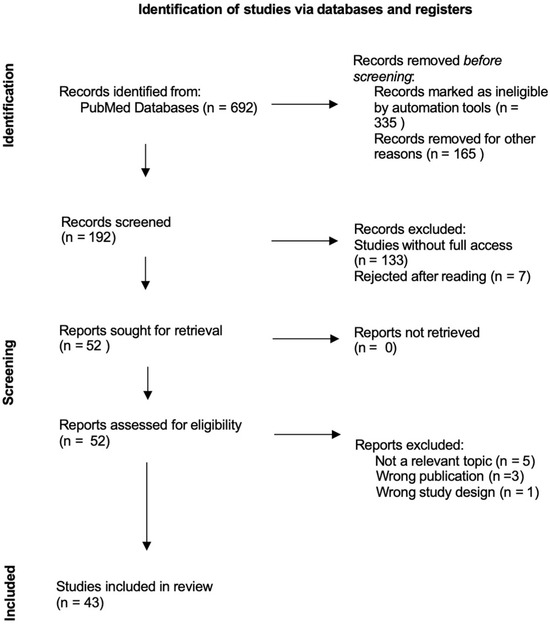
Figure 1.
Reporting Items of Narrative Review flow diagram.
3. Discussion
Table 2 presents 25 studies included in this review: meta-analyses, randomized controlled trials (RCTs), multicenter RCTs (MRCTs), prospective RCTs (PRCTs), and observational studies conducted primarily in Asia, Europe, and the United States evaluating H. pylori eradication rates with vonoprazan-based therapies versus other established and innovative regimens. Overall, VPZ-based therapies, particularly dual or triple regimens, demonstrated non-inferior or superior eradication rates across most analyses, with favorable safety, compliance, and outcomes even in antibiotic-resistant populations.

Table 2.
Characteristics of the principal studies incorporated into the qualitative examination.
Table 3 summarizes how vonoprazan-based therapies consistently demonstrate superior or non-inferior efficacy when compared to dual, triple, and quadruple regimens of varying dosages and durations. This holds true across diverse clinical scenarios, including first-line treatment, cases of clarithromycin resistance, and in penicillin-allergic populations. Beyond their effectiveness, these therapies offer enhanced tolerability, fewer adverse events, and improved patient compliance. Furthermore, VPZ-based dual therapies exhibit minimal impact on the gut microbiota and reduced susceptibility to CYP2C19 genetic variability, positioning them as a clinically effective and cost-efficient option for H. pylori eradication. Taken together, the reviewed evidence indicates that vonoprazan-based dual therapy may represent one of the most promising approaches, combining high efficacy with simplified administration and favorable safety.

Table 3.
Summary of study principal findings of Vonoprazan-Based Therapies for H. pylori eradication.
Vonoprazan-based therapies are used differently worldwide, reflecting regional resistance patterns and guidelines. In East Asia, dual and quadruple regimens are common, while in the US and Europe their use is limited but shows comparable or improved efficacy compared to traditional PPI-based therapies.
3.1. Efficacy of Vonoprazan-Based Therapies Compared to PPI-Based Therapies
A meta-analysis of nine RCTs published between 2016 and 2022 (n = 1715) evaluated the efficacy of PCAB-based therapy, primarily vonoprazan, versus PPI-based regimens for H. pylori eradication. In first-line treatment (seven studies), vonoprazan-based therapy achieved a significantly higher eradication rate than PPI-based therapy (91.89% vs. 79.95%), with a pooled relative risk (RR) of 1.13 (95% CI: 1.04–1.22). Subgroup and sensitivity analyses confirmed the robustness of these findings despite moderate heterogeneity (I2 = 69%, p < 0.01). Japanese studies demonstrated a significantly greater treatment effect (RR = 1.24; 95% CI: 1.14–1.34) compared with non-Japanese studies (RR = 1.06; 95% CI: 1.01–1.12; interaction p < 0.01). For second- and third-line therapies, PCAB and PPI regimens showed comparable efficacy, with no statistically significant differences (RR = 0.89 and 1.42, respectively). These results may reflect limited data and variation in antibiotic selection. Across the included studies, the most frequent comparison was vonoprazan 20 mg versus lansoprazole 30 mg. Treatment duration varied from 7 or 14 days to as long as 6 weeks [25].
A meta-analysis of four randomized controlled trials, encompassing 1560 treatment-naive patients with Helicobacter pylori infection, evaluated the efficacy of a 10-day vonoprazan-based regimen compared to a standard 14-day PPI-based therapy. In the intention-to-treat (ITT) analysis, eradication rates were higher in the vonoprazan group (88.7%) than in the PPI group (82.9%), with an odds ratio (OR) of 1.53 (95% CI: 0.85–2.75; p = 0.16), though this difference did not reach statistical significance. However, the per-protocol (PP) analysis revealed a significant advantage for vonoprazan, with eradication rates of 91.8% versus 86.8%, respectively (OR = 1.72; 95% CI: 1.23–2.42; p = 0.002) [29].
In a randomized MRCT conducted across the United States and Europe, 1046 treatment-naive patients with confirmed Helicobacter pylori infection were randomized to receive vonoprazan-based triple therapy (VAC), vonoprazan-based dual therapy (VA), or lansoprazole-based triple therapy (PAC). The primary endpoint assessed noninferiority of VPZ regimens compared to lansoprazole triple therapy in patients infected with strains susceptible to both clarithromycin and amoxicillin. In this nonresistant population, eradication was achieved in 84.7% of patients receiving VAC, 78.5% receiving VA, and 78.8% receiving PAC. VAC met the noninferiority threshold with a treatment difference of 5.9% (95% CI: 0.8–12.6; p < 0.001). VA also demonstrated noninferiority, with a treatment difference of –0.3% (95% CI: −7.4 to 6.8; p = 0.007). In the per-protocol population (PPp), eradication rates were 90.4% for VAC, 81.2% for VA, and 82.1% for PAC. These findings suggest that vonoprazan regimens maintain at least equivalent efficacy to PPI-based therapy in this key treatment-naive population [23].
A randomized controlled trial involving 192 patients aged 18–75 years with confirmed H. pylori infection was conducted at the Department of Medicine/Gastroenterology in Karachi, Pakistan. Participants were randomized into two treatment groups: Group A received standard triple therapy consisting of amoxicillin, clarithromycin, and omeprazole for 14 days, while Group B received dual therapy comprising amoxicillin and vonoprazan for the same duration. The primary outcome was H. pylori eradication, assessed four weeks post-treatment using a stool antigen test. Among participants who completed treatment, 73/87 in Group A achieved eradication compared with 86/92 in Group B. This difference was statistically significant, favoring vonoprazan-based dual therapy. Moreover, patients in the vonoprazan group experienced fewer adverse events, with significantly lower rates of nausea/vomiting (p = 0.035) and bloating (p = 0.045). These findings suggest that dual therapy with vonoprazan and amoxicillin provides a significantly higher H. pylori eradication rate than conventional PPI-based triple therapy, while also being better tolerated [30]. The improved first-line efficacy of vonoprazan likely stems from its rapid and sustained acid suppression, which enhances the activity of time-dependent antibiotics such as amoxicillin.
This randomized clinical trial carried by Waqar et al., compared the efficacy, compliance, and cost-effectiveness of a 7-day vonoprazan-based triple therapy versus a 14-day esomeprazole-based triple therapy in 122 patients with a confirmed H. pylori infection via stool antigen test. Participants were randomly assigned to receive either vonoprazan, amoxicillin, and levofloxacin for 7 days (n = 61), or esomeprazole, amoxicillin, and levofloxacin for 14 days (n = 58). Eradication rates were 95.1% in the vonoprazan group and 93.1% in the esomeprazole group, with no statistically significant difference (p = 0.64). Patient compliance reached 100% in the vonoprazan group and 95.0% in the esomeprazole group (p = 0.07). Despite a shorter treatment duration, vonoprazan therapy demonstrated comparable efficacy, higher compliance, and better affordability. The study suggests that short-course vonoprazan therapy may offer clinical and economic advantages, particularly in low-resource settings like Pakistan. However, findings are limited by the single-center design and small sample size, warranting further multicenter research [7].
3.2. Efficacy of Vonoprazan-Based Dual or Triple Therapies Compared to Bismuth Quadruple Therapies Recommended in China
It is worth noticing that studies mentioned in this section were conducted in China. Subsequently, the term ‘bismuth quadruple therapy’ refers to a 14-day regimen comprising PPI, bismuth and two antibiotics—typically amoxicillin and clarithromycin. This therapy is consistent with the recent Chinese national clinical practice guideline on Helicobacter pylori [43]. However, this variant differs from a standard bismuth quadruple therapy recommended in Western countries, which consists of PPI, bismuth, metronidazole and tetracycline [2,10]. This discrepancy reflects regional differences in antibiotic resistance patterns.
A multicenter, open-label, RCT was conducted across three institutions in eastern China to compare the efficacy and safety of 10-day vonoprazan amoxicillin therapy with 14-day bismuth-based quadruple therapy for first-line Helicobacter pylori eradication. BQT used in this study was composed of rabeprazole, amoxicillin, clarithromycin, and colloidal bismuth. A total of 314 treatment-naive patients were randomly assigned in a 1:1 ratio to receive either the VA or BQT regimen. Eradication was assessed using the 13C-urea breath test at least four weeks post-treatment. In the ITT analysis, eradication rates were 86.0% for VA and 89.2% for BQT (p = 0.389). PP analysis showed eradication success rates of 90.8% for VA and 91.3% for BQT (p = 0.884). Noninferiority was demonstrated across all three analyses. Overall, the 10-day VA regimen achieved eradication rates exceeding 90% in PP analysis and demonstrated comparable efficacy to the 14-day BQT regimen in all analytical models, supporting its role as an effective and better-tolerated alternative for initial H. pylori therapy [16].
Another study conducted by Yang et al. came to similar, yet even more optimistic conclusions, that shorter-duration vonoprazan-based dual therapy may be a highly efficient and patient-friendly first-line treatment option for H. pylori infection, considering it showed added benefits of fewer adverse effects and superior cost-effectiveness. The trial involved 600 H. pylori infected patients. Participants were randomly assigned in equal proportions to one of three groups: group A received vonoprazan and amoxicillin for 14 days; group B received the same medications for 10 days; and group C was treated with rabeprazole, bismuth potassium citrate, tinidazole, and clarithromycin for 14 days. PP analysis demonstrated that the H. pylori eradication rates were 92.5% in group A, 91.6% in group B, and 80.1% in group C. The difference in efficacy between groups A and B was not statistically significant (p > 0.05), whereas both groups achieved significantly higher eradication rates compared to Group C (p < 0.05). In conclusion, the 10-day vonoprazan-amoxicillin regimen demonstrated comparable efficacy to the 14-day regimen [31].
A prospective MRCT involving 600 patients with Helicobacter pylori infection assessed the efficacy, safety, and cost-effectiveness of a 14-day VAB (vonoprazan, amoxicillin, and bismuth) in comparison to the BQT (esomeprazole, clarithromycin, amoxicillin, and bismuth). Eradication rates were similar between two groups, with no statistically significant differences observed in either the ITT analysis (83.7% VAB vs. 83.2% BQT, p = 0.869) or the PP population (90.9% vs. 89.7%, p = 0.614), respectively. Patients receiving vonoprazan-based regimen experienced significantly fewer adverse events compared to those randomly assigned for BQT (13.7% vs. 28.6%, p < 0.001). From an economic standpoint, the vonoprazan-based therapy was also more cost-effective. Overall, this study supports the use of vonoprazan-based triple therapy as a clinically effective, better-tolerated, and more affordable first-line option for H. pylori eradication [33].
Huang XP et al.’s multicenter, randomized study evaluated the efficacy and safety of vonoprazan-amoxicillin therapy compared to bismuth quadruple therapy in Chinese patients with H. pylori infection. Among five treatment groups, VA-7 and VBQT were terminated early due to insufficient eradication. In stage two, both VA-10 and VA-14 achieved significantly higher eradication rates than BQT: 93.2% and 92.2% vs. 80.2% in the ITT analysis (p = 0.022 and 0.046), and 94.0% and 93.9% vs. 80.9% in the PP analysis. Adverse events occurred less frequently with VA-10 and VA-14 than with BQT (25.27% and 13.73% vs. 37.62%, p < 0.001) and were milder in severity. These findings demonstrate that 10- or 14-day VA regimens provide superior eradication with improved tolerability over BQT in the Chinese population [17].
A meta-analysis, encompassing 13 randomized controlled trials and 4023 patients, systematically evaluated the effectiveness and safety of high-dose vonoprazan dual therapy for H. pylori eradication. The investigation compared VA against high-dose PPI-based dual therapy (PA) and PPI-bismuth quadruple therapy. Overall, VA therapy demonstrated favorable eradication rates of 88.81% (ITT) and 93.56% (PP). Specifically, VA proved statistically superior to PA, achieving higher eradication rates (ITT: 86.95% vs. 82.43%, p = 0.04; PP: 92.38% vs. 88.25%, p = 0.02). Furthermore, VA also surpassed BQT in efficacy (ITT: 90.85% vs. 85.90%, p = 0.03; PP: 94.85% vs. 90.24%, p = 0.03). A significantly lower incidence of adverse reactions was observed with VA therapy (14.56% vs. 26.00%, RR = 0.57, p < 0.0001), coupled with improved patient compliance (96.29% vs. 93.56%, RR = 1.03, p = 0.003). These findings suggest that VA therapy presents a highly promising and potentially superior alternative for H. pylori eradication, offering robust efficacy, a more favorable safety profile, and enhanced adherence compared to existing regimens [32].
Overall, dual or triple vonoprazan-based therapies appear to have non-inferior or superior efficacy compared to bismuth-based quadruple therapy recommended in China, while also proving better tolerated. Simplified regimens such as vonoprazan–amoxicillin dual therapy may play a central role in the future management of H. pylori. By reducing antibiotic exposure and treatment complexity, these strategies have the potential to improve adherence and limit the development of resistance.
3.3. Efficacy Assessment of Vonoprazan-Based and PPI-Based Bismuth Quadruple Regimens Recommended in China
A prospective, randomized clinical trial from China enrolled 234 treatment-naive H. pylori-positive patients, who were randomly assigned equally to three regimens: VBQT-10 (vonoprazan, amoxicillin, furazolidone, and colloidal bismuth) for 10 days, VBQT-14 for 14 days, and PPI-based bismuth quadruple therapy for 14 days (BQT-14). In the intention-to-treat analysis, eradication rates were 96.2% (VBQT-10), 94.9% (VBQT-14), and 93.6% (BQT-14), while the per-protocol analysis showed rates of 98.6%, 97.4%, and 94.8%, respectively. Adverse event rates were 12.8% for VBQT-10, 3.8% for VBQT-14, and 6.4% for BQT-14, with no statistically significant difference (p = 0.096). The cost-effectiveness ratio favored VBQT-10 (1.32) over VBQT-14 (1.88) and BQT-14 (3.06). These findings suggest that VBQT-10 is a cost-effective, safe, and noninferior alternative to longer or PPI-based regimens for H. pylori eradication [35].
This randomized, double-blind, parallel-group study investigated and compared the safety and pharmacokinetics (PK) of bismuth in H. pylori-positive Korean adults receiving either vonoprazan- or lansoprazole-based quadruple therapy. A total number of 30 participants were assigned to a 14-day regimen and 26 completed the study with negative tested for H. pylori via urea breath test, indicating effective eradication in both groups (12/15, 80% in the vonoprazan group, 14/15 93% in the lansoprazole group). Steady-state pharmacokinetic parameters of bismuth-including Cmax, AUCτ, tmax, CL/F, and Vz/F—were calculated using a noncompartmental model. The systemic exposure to bismuth was nearly identical between groups, with only a 5% difference observed. Steady-state plasma concentrations confirmed appropriate drug accumulation between days 12 and 14. Overall, vonoprazan-based therapy demonstrated equivalent bismuth exposure and tolerability to the lansoprazole-based regimen [36].
This prospective, randomized, single-center trial evaluated the efficacy and safety of VBQT for H. pylori eradication in a population with high antibiotic resistance. A total of 101 patients with confirmed H. pylori infection were randomly assigned to receive either a 7-day or 14-day VBQT regimen. The eradication rates were 84.4% (95% CI: 74.3–94.2) for the 7-day group and 94% (95% CI: 87.4–100) for the 14-day group, although the difference did not reach statistical significance (risk difference 0.25, 95% CI: 0.03–0.53, p = 0.11). Notably, the 14-day regimen achieved a 100% eradication rate in patients with clarithromycin-resistant H. pylori, whereas the two resistant cases in the 7-day group failed treatment. Antibiotic resistance rate for clarithromycin achieved 33.3%. Genetic analysis revealed that all participants were CYP3A4 rapid metabolizers, and 72.3% of patients were intermediate metabolizers based on CYP3A5 polymorphisms. However, H. pylori eradication rates were only marginally diminished in CYP3A5 rapid metabolizers compared to poor or intermediate metabolizers. This study supports the use of 14-day VBQT as a highly effective regimen, particularly in regions with high clarithromycin resistance [37].
3.4. Evaluation of Vonoprazan-Based Therapy Incorporating Berberine
The guideline development group conditionally recommends incorporating Chinese herbal medicine into bismuth quadruple therapy for H. pylori eradication, based on evidence of low certainty. Berberine, derived from Rhizoma coptidis, may serve as an alternative in empirical treatment settings where BQT demonstrates suboptimal eradication rates or in regions where bismuth is inaccessible. It may also be considered in patients with bismuth allergy. Specific herbal options have shown eradication effects comparable to conventional bismuth-based therapy [43].
Chen et al. conducted a randomized controlled trial, in which they compared three H. pylori treatment regimens: berberine triple therapy (Group A), vonoprazan quadruple therapy (Group B), and rabeprazole quadruple therapy (Group C). Among 300 enrolled patients, 263 completed the study. ITT analysis showed eradication rates of 70.0% (A), 77.0% (B), and 69.0% (C); PP analysis showed 81.4%, 86.5%, and 78.4%, respectively. No statistically significant differences were observed among the groups in either analysis (p > 0.05). Symptom improvement, adverse events, and compliance were also comparable (p > 0.05). Overall, berberine triple therapy demonstrated similar efficacy and safety to standard quadruple therapies, supporting its use as an alternative for initial H. pylori treatment [38].
3.5. Efficacy Assessment of Vonoprazan-Based Dual and Triple Therapies
Yang Xe et al. conducted a multicenter randomized trial so as to evaluate the efficacy, safety, and cost-effectiveness of high-dose dual therapy (HDDT) and standard regimens for H. pylori eradication. A total of 367 untreated patients were randomly assigned to two groups: 14-day high-dose vonoprazan-amoxicillin (H-VA-14), and 14-day vonoprazan-amoxicillin-clarithromycin. Individuals in both H-VA-14 and VAC groups were given 20 mg of VPZ b.i.d. Participants in H-VA-14 group received 1000 mg amoxicillin t.i.d, while those from VAC group 1000 mg b.i.d. The VA therapy demonstrated non-inferiority to VAC in both analyses: ITT (84.15% vs. 83.15%, p = 0.795) and PP (96.75% vs. 93.75%, p = 0.212). No significant differences in adverse events, compliance, or symptom improvement were noted between any of the two treatment groups. Compliance rates exceeded 95% across all regimens, and overall symptom relief was over 93%. The incidence of adverse events varied depending on regimen, at 9.84% (H-VA-14) and 11.41% (VAC), with no serious events reported. The H-VA-14 therapy was the most cost-effective option, given that it was 17.3% less expensive than VAC. The study supports vonoprazan-based HDDT as a viable first-line treatment, with eradication outcomes comparable to more complex regimens and favorable cost and safety profiles [15].
This prospective observational study evaluated the efficacy and safety of vonoprazan–amoxicillin dual therapy compared to vonoprazan-based triple therapy using vonoprazan, amoxicillin, clarithromycin in Helicobacter pylori treatment-naive junior high school students in Japan. A total of 60 students received VA therapy, while 161 received VAC therapy, both for a 7-day duration. In ITT analysis, eradication rates were 85.0% (95% CI: 75.8–94.2%) for the VA group and 82.0% (95% CI: 76.0–87.9%) for the VAC group. PP analysis showed similar results: 86.4% (95% CI: 77.4–95.5%) for VA and 84.1% (95% CI: 78.3–89.8%) for VAC. VA therapy demonstrated non-inferiority compared to VAC therapy; the pre-specified non-inferiority criterion was met, with p-values of 0.018 for the ITT analysis and 0.020 for the PP analysis. Adverse events occurred in 10.0% of the VA group and 19.8% of the VAC group (p = 0.108), with most symptoms being mild and self-limiting. These findings suggest that 7-day VA therapy offers comparable eradication efficacy to VAC therapy while reducing antibiotic exposure and adverse event incidence [27].
This randomized, multicenter clinical trial evaluated the efficacy of 7-day VA therapy compared to VAC regimen in 335 H. pylori-positive, treatment-naive patients across seven Japanese centers. Eradication rates in ITT analysis were 84.5% for VA and 89.2% for VAC (p = 0.203), and 87.1% vs. 90.2% (p = 0.372) in PP analysis, demonstrating non-inferiority of VA only in the PP population. In clarithromycin-resistant strains, VA showed superior efficacy (92.3% vs. 76.2%, p = 0.048), whereas in susceptible strains, VAC was significantly more effective (95.1% vs. 85.5%, p = 0.011). Eradication outcomes in the VA group were unaffected by clarithromycin resistance (p = 0.267), in contrast to the VAC group (p < 0.001). Adverse events occurred in 27.4% of VA and 30.5% of VAC patients (p = 0.524), with most classified as mild. Treatment compliance was high in both groups. These results support VA therapy as an effective and well-tolerated first-line option, particularly in regions with high clarithromycin resistance [28].
A prospective, open-label randomized controlled clinical trial was conducted by Shekeban et al. at Alexandria Main University outpatient clinics to compare the efficacy, safety, and adherence of VA and VAC therapies with standard PAC for H. pylori eradication. The regimens were administered for 14 days and involved 132 patients. Eradication rates by PP analysis were 70% for PAC, 76.2% for VA, and 79.2% for VAC (p = 0.777). ITT analysis yielded eradication rates of 84.1% for PAC, 88.6% for VA, and 86.4% for VAC in the best-case scenario, showing no statistically significant differences (p = 0.754–0.824). The most frequent adverse event in PAC and VAC groups was taste disturbance (73.5% and 76.3%), while the VA group reported the fewest adverse events overall. Compliance was high across all groups, with no significant differences in adherence. All three regimens demonstrated suboptimal eradication rates, indicating a need for improved dosing strategies and novel therapies. The assessment revealed that vonoprazan-based dual therapy achieved comparable efficacy, alongside a notably lower incidence of adverse events and improved cost-effectiveness [39].
Based on these findings, the future course of H. pylori eradication appears to favor simplified vonoprazan–amoxicillin dual therapy, which demonstrates comparable or superior efficacy to triple regimens while reducing antibiotic exposure and adverse events. This approach is particularly promising in regions with high clarithromycin resistance, where conventional triple therapies lose effectiveness. Moving forward, optimizing dosing strategies and validating dual therapy in broader, diverse populations will be key to establishing it as a global first-line standard.
3.6. Optimization of Vonoprazan Dual Therapy
The optimal duration and dosage for vonoprazan amoxicillin dual therapy continue to be areas of active research, with some studies demonstrating non-inferiority for shorter durations lasting 10 days compared to longer ones or traditional regimens [16,29,31]. A multicenter, prospective, randomized controlled trial carried by Peng et al. recruited 516 patients with treatment-naive H. pylori infection from five clinical facilities across China. Individuals were allocated equally to three regimens: a 10-day high-dose amoxicillin regimen (H-VA-10), a 10-day low-dose amoxicillin regimen (L-VA-10), and a 14-day high-dose amoxicillin regimen (H-VA-14). The H-VA-10 regimen demonstrated non-inferiority to H-VA-14, with similar eradication rates in both ITT (86.6% vs. 89.5%, p = 0.021) and PP analyses (90.9% vs. 94.5%, p = 0.013). In contrast, the L-VA-10 regimen was significantly less effective than H-VA-14 (ITT: 79.7% vs. 89.5%, p = 0.011; PP: 82.0% vs. 94.5%, p < 0.001). Regarding safety, the incidence of adverse events was similar across all three treatment groups, ranging from 17.4% for L-VA-10 to 22.7% H-VA-14, primarily comprising mild to moderate reactions such as oral malodor, nausea, and rash. Overall, the H-VA-10 and H-VA-14 regimens yielded favorable therapeutic outcomes for H. pylori eradication, while the L-VA-10 regimen proved less effective [13].
A single-center, randomized non-inferiority trial, carried out by Hu et al. from Nanchang University, enrolled 110 treatment-naive H. pylori patients in China to evaluate a 14-day vonoprazan-amoxicillin dual therapy using either a low-dose (1 g amoxicillin b.i.d.) or high-dose (1 g amoxicillin three t.i.d.) regimen. Eradication rates for L-VA-14 was 89.1%, while H-VA-14 achieved 87.3% in ITT analysis and 94.1% vs. 95.9% in PP analysis, respectively, with non-inferiority successfully demonstrated and no significant difference observed between the two amoxicillin dosages (difference: 1.8%). The incidence of adverse events was statistically similar across both groups (L-VA-14 29.1% vs. H-VA-14 20.0%, p = 0.268), with all events being mild or moderate and no treatment discontinuations. High patient compliance was also noted (98.2%). These findings indicate that both 14-day vonoprazan-amoxicillin dual therapy regimens provide satisfactory first-line efficacy for H. pylori infection [21].
Under the supervision of the same scientist, PhD Yi Hu, a similar trial, yet on a larger scale was conducted across 12 centers in China. It involved 504 H. pylori-positive, treatment-naive patients who were randomized to receive vonoprazan combined with either low-dose or high-dose dual therapy for 14 days. This time, despite evaluating efficacy of H. pylori management, they also assessed the influence of therapeutic interventions on the gut microbiota and its associated antibiotic resistome. Eradication rates were comparable between groups, with L-VA-14 achieving 85.3% and H-VA-14 achieving 86.5% in ITT analysis and 88.8% and 92.4% in PP analysis, respectively, meeting the predefined non-inferiority margin in both analyses (p = 0.0022 and p = 0.0085, respectively). Adverse events were fewer in the L-VA-14. Notably, beta-lactam resistance gene abundance rose significantly at week 2 in both groups but returned to baseline only in the L-VA-14 group. At week 2, the H-VA-14 group exhibited higher resistome diversity than the L-VA-14 group (p = 0.017), with distinct resistome clustering between regimens. Overall, L-VA-14 therapy demonstrated non-inferior efficacy to H-VA-14, with fewer side effects and a more favorable short-term impact on the gut resistome [40].
Results from studies mentioned above have their reflection in recent guidelines. A vonoprazan amoxicillin dual therapy is recommended as a frontline treatment option for H. pylori infection in treatment-naive patients, based on a conditional recommendation stemming from moderate quality evidence [10]. High-dose VA therapy lasting for either 10 or 14 days and L-VA-14 seem to be similarly effective options, but they differ slightly in terms of adverse effects and gut microbiota impact.
3.7. Special Populations and Considerations
3.7.1. Vonoprazan–Tetracycline Dual Therapy in Penicillin-Allergic Patients
Wen Gao et al. provided a study, which investigated vonoprazan–tetracycline (VT) dual therapy as an alternative to bismuth quadruple therapy for H. pylori eradication in penicillin-allergic, treatment-naive adults. In an equally randomised trial involving 300 participants, both groups received 14-day regimens and were assessed through ITT, mITT, and per-protocol analyses. VT therapy achieved eradication rates of 92.0% (ITT), 94.5% (mITT), and 95.1% (PP), closely matching BQT outcomes of 89.3%, 93.1%, and 97.7%, respectively, demonstrating non-inferiority across all measures (p ≤ 0.001). Treatment-emergent adverse events (TEAEs) were significantly less frequent in the VT group (14.0%) compared to BQT (48.0%), with fewer therapy discontinuations (2.0% vs. 8.7%, p = 0.010). Despite equal adherence, safety outcomes clearly favored VT therapy. These findings suggest that VT dual therapy offers a safer and equally effective first-line option for patients unable to receive penicillin [18].
3.7.2. Association of Higher Body Mass Index (BMI) with Enhanced VA Regimen Efficacy
Kasai et al. retrospectively investigated factors influencing the success of vonoprazan-based second-line H. pylori eradication therapy in 33 patients from seven Japanese facilities who had failed prior first-line therapy. The overall eradication success rate for this cohort was 81.8%. A key finding was a significantly higher eradication rate in patients with a Body Mass Index (BMI) ≥ 23.8 kg/m2 compared to those with lower BMI (p = 0.007), as indicated by ROC analysis (AUC = 0.796). No patients with severe obesity (≥30 kg/m2) were present in the cohort. Conversely, no significant correlations were observed between eradication success and other patient characteristics examined, including age, height or weight. These findings suggest that a higher BMI may be a positive predictor for the success of vonoprazan-based second-line H. pylori eradication therapy [41]. A possible explanation for this observation may relate to physiological differences in gastric environment and treatment pharmacokinetics across BMI categories. In patients with very low BMI, reduced body mass and possible malnutrition could alter drug absorption or metabolism, potentially reducing antibiotic efficacy.
3.7.3. Impact of CYP3A Genotype on VBQT Efficacy
Vonoprazan is primarily processed via CYP3A4/5 pathways, in contrast to proton pump inhibitors which are largely metabolized by CYP2C19. This distinct metabolic profile offers therapeutic advantages, as VPZ-based regimens are less susceptible to the efficacy variability influenced by CYP2C19 polymorphisms that significantly impact omeprazole or lansoprazole therapies for H. pylori [6,37]. Genotypic profiling showed that all individuals carried the CYP3A4 rapid metabolizer variant, while 72.3% were classified as intermediate metabolizers based on CYP3A5 status. Although eradication rates were slightly lower among CYP3A5 rapid metabolizers, the difference was minimal when compared to poor and intermediate metabolizers [37].
3.7.4. Vonoprazan-Based Therapy for Resistant H. pylori Strains
Chey et al. verified that vonoprazan dual therapy eradicated 69.6% of resistant infections, a substantial improvement over lansoprazole triple therapy (31.9%, p < 0.001). Across the overall patient cohort, both VA (80.8%) and VAC (77.2%) therapies demonstrated significantly higher eradication rates compared to lansoprazole triple therapy (68.5%). These results highlight the considerable therapeutic advantage of vonoprazan-based regimens, particularly in addressing resistant H. pylori infections [23].
Another PRCT carried out by Suzuki et al. in Japan, proved that in the context of clarithromycin-resistant strains, VA-dual therapy demonstrated a markedly superior eradication rate of 92.3%, significantly outperforming VAC-triple therapy, which achieved only 76.2% (p = 0.048). This emphasizes VA therapy’s robust performance, as its efficacy remained remarkably consistent irrespective of the strain’s clarithromycin susceptibility (85.5% for susceptible vs. 92.3% for resistant, p = 0.267). Conversely, VAC-triple’s effectiveness was severely compromised by clarithromycin resistance, dropping from 95.1% in susceptible strains to 76.2% in resistant ones (p < 0.001) [28]. According to latest ACG guidelines, in individuals naive to H. pylori treatment and with unknown clarithromycin susceptibility, PCAB-clarithromycin triple therapy is suggested as a preferred option over PPI-clarithromycin triple therapy. This guidance is a conditional recommendation, stemming from moderate quality evidence [10]. Tungtrongchitar et al. found that 14-day vonoprazan-based quadruple therapy eradicated all clarithromycin-resistant cases, whereas none were cured with the 7-day regimen. The treatment regimen combined vonoprazan, bismuth subsalicylate, metronidazole, and tetracycline at standard doses. Among 18 cultured isolates, resistance was detected in 33.3% for clarithromycin, 29.4% for metronidazole, and 27.7% for levofloxacin, with 11.1% showing dual resistance and no amoxicillin resistance observed. Despite these challenges, the extended vonoprazan-based regimen maintained high efficacy, underscoring its value against resistant H. pylori strains [37].
Vonoprazan-based regimens, especially dual and quadruple therapies, show strong potential as future first-line options due to their consistent efficacy against clarithromycin-resistant H. pylori strains. Optimizing treatment duration and regimen composition will be crucial to overcoming rising antimicrobial resistance and improving global eradication outcomes.
3.8. Impact of VA Therapy on the Gut Microbiome
Although several clinical trials confirmed the non-inferiority or superiority of vonoprazan-amoxicillin regimens compared to traditional and more complex ones, a question has arisen whether these therapies affect gut microbiota. Hu et al. decided to investigate the impact of VA therapy on gut microbiota and short-chain fatty acids (SCFAs) in 119 H. pylori-positive patients. They were randomized to receive either low-dose or high-dose regimens for 7 or 10 days. Fecal analysis revealed that H. pylori-positive individuals had significantly higher microbial richness and diversity than H. pylori-negative controls. High-dose VA therapy temporarily reduced microbial diversity and altered bacterial composition post-eradication, yet these changes were largely reversed by the confirmation time point. In contrast, low-dose VA therapy had negligible effects on microbiota structure across all time points. Taxonomic shifts following high-dose treatment included a transient decline in SCFA-producing genera such as Anaerostipes, Dialister, and Lachnospira. Interestingly, H. pylori eradication with high-dose VA therapy led to a temporary increase in Proteobacteria and decrease in Firmicutes and Bacteroidetes. Despite these fluctuations, both microbiota diversity and SCFA profiles recovered post-treatment. Overall, VA dual therapy demonstrated a minimal and reversible impact on gut microbial ecology, especially with low-dose regimens [42]. Yi Hu, who again supervised the next study, discovered that VA therapy induced transient alterations in gut microbiota diversity and composition, with most changes reverting to pretreatment levels by week 8–10. At finer taxonomic levels, Enterobacteriaceae and Klebsiella expanded transiently during therapy but returned to baseline thereafter. However, specific microbiota shifts, such as the reduction in Actinomycetota, were uniquely observed in the H-VA-14 group. While both L-VA-14 and H-VA-14 therapies initially increased beta-lactam-related resistance genes, the H-VA-14 group’s abundance remained elevated compared to baseline at week 8–10 (p = 0.017), unlike L-VA-14. Furthermore, H-VA-14 exhibited higher overall resistome alpha diversity and distinct resistome composition compared to L-VA-14 at week 2. In summary, although VA therapy generally allows for gut microbiota recovery, the higher amoxicillin dose can lead to more pronounced and persistent impacts on the antibiotic resistome [40]. These findings suggest that while VA therapy, particularly at low doses, exerts limited and reversible effects on the gut ecosystem, the use of high-dose amoxicillin may carry broader implications. The transient reduction in SCFA-producing genera could theoretically influence mucosal integrity and host metabolism, while the sustained enrichment of resistance genes raises concerns about long-term antimicrobial stewardship.
3.9. Adverse Effects and Tolerability
Table 4 summarises the safety profiles of vonoprazan-based regimens compared to bismuth quadruple therapy and other triple therapies. Across studies, VA therapies consistently demonstrated lower or comparable overall AE rates, particularly with significantly fewer cases of altered taste and diarrhea compared to BQT. In Yan et al., the AE incidence was 21.0% in the VA dual group versus 43.9% in the BQT group (p < 0.001), with altered taste (0.6% vs. 26.1%) and diarrhea (6.4% vs. 15.3%) notably reduced in the VA arm; most AEs were mild to moderate, and no severe events occurred [16]. Similar patterns were observed in trials of VA triple therapy, such as VA-10 and VA-14, which reported overall AE rates of 25.27% and 13.73%, respectively, compared to 37.62% for BQT (p < 0.001). Common events like bitter taste, dizziness, abdominal discomfort, and nausea were significantly less frequent with VA regimens, while compliance exceeded 90% in all groups without significant differences [17]. Comparative analyses with other triple therapies (PAC, VAC) found numerically lower AE rates with VA dual therapy—26.1% versus 34.5% for PAC (p = 0.22) and 25.8% versus 32.0% for VAC (p = 0.08)—although these differences were not statistically significant, and compliance rates remained similar (80–87%) [12]. In four studies analysed by Zhang et al., VA dual therapy showed a lower overall AE incidence (21.2%, 95% CI: 11.8–30.7%) versus triple therapy (26.5%, 95% CI: 17.8–35.2%), approaching statistical significance (p = 0.06), with diarrhea occurring significantly less often (RR = 0.64, p = 0.01). Serious AEs were rare (<2%), and discontinuations due to AEs were infrequent, most often due to amoxicillin allergy [14]. Additional evidence from Chen et al. showed the H-VA-14 regimen had the lowest AE incidence (2.3%) compared to VBQT (19.5%) and BQT (14.0%) (p < 0.05), with nausea most frequent in VBQT, though subgroup differences were not statistically significant [34]. Xu-Er Yang et al. reported AE rates of 9.84% in the VA-14 group versus 11.41% in the VAC-14 group (p = 0.624), with gastrointestinal discomfort and altered taste being the most common mild symptoms. Across all included studies, serious adverse events were rare, most side effects resolved spontaneously, and treatment adherence was high, typically above 90%, underscoring the good overall tolerability of vonoprazan-based therapies [15].

Table 4.
Summary of the adverse effects in studies in the narrative review.
4. Conclusions
Given the global prevalence of H. pylori and the escalating challenge of antibiotic resistance, current complex regimens heavily reliant on multiple antibiotics are proving increasingly suboptimal. Consequently, there is an urgent need for therapeutic optimization [11,12,23]. Vonoprazan-amoxicillin dual therapy emerges as a compelling alternative, primarily due to vonoprazan’s potent, sustained acid suppression. This prolonged gastric acid suppression is crucial as it both enhances H. pylori’s vulnerability by stimulating active replication and improves the stability and bioavailability of acid-sensitive antibiotics like amoxicillin [10]. Coupled with amoxicillin’s generally low global resistance rates, this regimen offers a streamlined yet highly effective approach to eradication. This has been proved by multiple studies. For an instance, Yan et al. concluded that the 10-day VA regimen is an effective and better-tolerated alternative for initial H. pylori treatment, achieving eradication rates over 90% and comparable efficacy to 14-day BQT [16]. Similarly, Yang et al. reported that 10-day and 14-day VA regimens were equally effective, both significantly surpassing BQT [31]. A meta-analysis by Zhang et al. further supports VA therapy as a highly promising option, with superior eradication rates compared to PA and BQT regimens [32]. Vonoprazan-based quadruple therapies also achieved favorable outcomes. Lu et al. found VBQT-10 to be more effective and cost-efficient than 14-day BQT, with similar safety [35], while other studies confirmed comparable tolerability to PPI-based quadruple therapy along with similar bismuth exposure [36] and efficacy in regions with high clarithromycin resistance [37]. The 2024 ACG guidelines recommend clarithromycin-based regimens only for patients with a history of failed first-line treatment and confirmed clarithromycin-susceptible H. pylori strains. If clarithromycin resistance is unknown and better options aren’t available, vonoprazan-based triple therapy is preferred over PPI-based triple therapy [10,12]. However, recent RCTs prove that even VA therapy performs equally or better than triple regimens containing PCAB or PPI, clarithromycin and amoxicillin [15,27,28]. Yang et al. demonstrated that H-VA-14 was non-inferior to VAC and more cost-effective, with favorable safety [15]. Gotoda et al. and Suzuki et al. confirmed that shorter or standard VA courses provide efficacy comparable to VAC while reducing antibiotic exposure and adverse events, especially in regions with high clarithromycin resistance [27,28]. For penicillin-allergic patients, BQT remains preferred, though VT has been highlighted as a promising alternative with non-inferior efficacy [10,18].
While vonoprazan-amoxicillin regimens are promising for H. pylori eradication, research by Hu et al. indicates their impact on gut microbiota varies. High-dose regimens temporarily reduce microbial diversity and alter bacterial composition, while low-dose VA has negligible effects. Importantly, higher amoxicillin exposure may increase resistance-related genes [40,42].
The VA therapy consistently demonstrates a more favorable safety profile than bismuth quadruple therapy, with significantly lower adverse event incidence (21.0% for VA vs. 43.9% for BQT [16]; 13.73–25.27% for VA vs. 37.62% for BQT [17]), particularly reducing occurrences of altered taste and diarrhea [16,17,30,39]. Although comparisons with PAC or VAC show less pronounced differences, tolerability and compliance remain high [12,14]. Specific vonoprazan-based approaches like H-VA-14 further exhibit exceptionally low AE incidence (2.3%), proving significantly safer than VBQT and BQT [34]. Overall, serious adverse events with vonoprazan-based regimens are rare, and most side effects are mild and self-resolving, leading to high treatment adherence [15,16,17].
Vonoprazan-amoxicillin therapy emerges as a strong candidate, significantly advancing us toward the ideal due to its proven efficacy and favorable characteristics demonstrated in various studies. It often demonstrated high cure rates (over 90% [13,16,32]), an advantageous safety profile, minimal gut microbiota disruption [39,40], lower cost [15,16,31,34,39], simpler administration, global accessibility, and strong patient adherence [7,15,32,39]. However, further research, particularly long-term trials in regions like the United States and Europe, is crucial to cement VA therapy’s role in H. pylori management. This continued investigation is essential to fully assess the best possible regimens for Helicobacter pylori eradication across different populations worldwide.
5. Limitations of the Review
Most studies were conducted in Asia, which limits the generalizability of the results to other populations. There is significant variation in treatment duration, drug dosages, and therapeutic regimens, making comparisons difficult. Data on second- and third-line therapies are limited and often come from small, single-center studies. Long-term follow-up on efficacy and safety is lacking, as are data for specific populations (e.g., children, the elderly, or patients with allergies). Reporting of adverse events and treatment efficacy was inconsistent, and some studies may be affected by publication bias.
Author Contributions
Conceptualization, K.K. and E.H.; methodology, S.K.; software, N.P.; validation, D.P., E.H. and M.T.; formal analysis, K.K.; investigation, S.K.; resources, N.P.; data curation, W.M.; writing—original draft preparation, K.M.; writing—review and editing, D.P.; visualization, E.H.; supervision, K.K.; project administration, E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACG | American College of Gastroenterology |
| BQT | bismuth quadruple therapy |
| PCAB | potassium-competitive acid blocker |
| PPI | proton pump inhibitor |
| VPZ | vonoprazan |
| ITT | intention-to-treat |
| mITT | modified in intention-to-treat |
| PP | per-protocol |
| RR | relative risk |
| OR | odds ratio |
| RCT | randomized clinical trial |
| RMCT | randomized multicenter clinical trial |
| VA | vonoprazan-based dual therapy/vonoprazan amoxicillin therapy |
| VAC | vonoprazan-based triple therapy/vonoprazan amoxicillin clarithromycin therapy |
| PAC PPI | based triple therapy/PPI amoxicillin clarithromycin therapy |
| VAB | vonoprazan amoxicillin bismuth therapy |
| BQT PPI | bismuth quadruple therapy |
| VBQT | vonoprazan-based bismuth therapy |
| PK | pharmacokinetics |
| HDDT | High-dose dual therapy |
| L-VA-10 | low-dose dual therapy/low-dose vonoprazan amoxicillin therapy for 10 days |
| L-VA-14 | low-dose dual therapy/low-dose vonoprazan amoxicillin therapy for 14 days |
| H-VA-14 | high-dose dual therapy/high-dose vonoprazan amoxicillin therapy for 14 days |
| PA PPI | based dual therapy |
| TEAEs | treatment-emergent adverse events |
| BMI | body mass index |
| ROC | receiver operating characteristic |
| SCFAs | short-chain fatty acids |
| VAT | vonoprazan-amoxicillin-tetracycline therapy |
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, B.; Pang, X.Y.; Gao, W.W. Efficacy and safety of Vonoprazan-based treatment of Helicobacter pylori infection: A systematic review and network meta-analysis. BMC Infect. Dis. 2024, 24, 953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Zhang, H.; Zhu, X.J.; Yao, N.; Yin, J.M.; Liu, J.; Dan, H.J.; Pang, Q.M.; Liu, Z.H.; Shi, Y.Q. Efficacy and safety of vonoprazan and high-dose amoxicillin dual therapy in eradicating Helicobacter pylori: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2024, 64, 107331. [Google Scholar] [CrossRef] [PubMed]
- Kanu, J.E.; Soldera, J. Treatment of Helicobacter pylori with potassium competitive acid blockers: A systematic review and meta-analysis. World J. Gastroenterol. 2024, 30, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waqar, F.; Noor, M.; Farhat, K.; Ali, S.; Haider, E.; Fatima Gillani, S.F. Efficacy and Cost-Effectiveness, Comparison Of 7-Days Vonoprazan Versus 14-Days Esomeprazole Based Triple Therapies For Treating Helicobacter Pylori Infection In Pakistani Population: A Randomized Clinical Trial. J. Ayub Med. Coll. 2023, 35 (Suppl. 1), S746–S751. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lv, Y.M.; Yang, P.; Jiang, Y.Z.; Qin, X.R.; Wang, X.Y. Safety and effectiveness of vonoprazan-based rescue therapy for Helicobacter pylori infection. World J. Gastroenterol. 2023, 29, 3133–3144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, X.; Wang, J.; Du, Q.; Tian, D.; Hu, N.; Liu, D.; Zhou, F.; Xie, L.; Gu, L.; Kudou, K.; et al. Efficacy and Safety of Vonoprazan-Based Quadruple Therapy for the Eradication of Helicobacter pylori in Patients with Peptic Ulcers: A Pooled Analysis of Two Randomized, Double-Blind, Double-Dummy, Phase 3 Trials. Biol. Pharm. Bull. 2024, 47, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Howden, C.W.; Moss, S.F.; Morgan, D.R.; Greer, K.B.; Grover, S.; Shah, S.C. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2024, 119, 1730–1753. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kusano, C.; Horii, T.; Ichijima, R.; Ikehara, H. The Ideal Helicobacter pylori Treatment for the Present and the Future. Digestion 2022, 103, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.G.; Mei, Y.Z.; Jiang, X.; Zheng, A.J.; Ding, Y.B. Vonoprazan-amoxicillin dual therapy for Helicobacter pylori eradication: A systematic review and meta-analysis of randomized controlled trials. Saudi J. Gastroenterol. 2023, 29, 347–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, X.; Yao, J.Y.; Ma, Y.Q.; Li, G.H.; Chen, H.W.; Wan, Y.; Liang, D.S.; Zhang, M.; Zhi, M. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen with Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin. Gastroenterol. Hepatol. 2024, 22, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Lin, B.S.; Li, Y.Y.; Ding, Y.M.; Han, Z.X.; Ji, R. Efficacy and Safety of Vonoprazan and Amoxicillin Dual Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis. Digestion 2023, 104, 249–261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.E.; Zhang, S.J.; Liu, Y.; Yao, S.Y.; Zhang, S.X.; Liu, X.M.; Liang, L.X.; Wang, F. Amoxicillin high-dose dual therapy for Helicobacter pylori primary eradication: Proton pump inhibitor and potassium-competitive acid blocker, which’s better? World J. Gastroenterol. 2025, 31, 100863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, T.L.; Wang, J.H.; He, X.J.; Zhu, Y.B.; Lu, L.J.; Wang, Y.J.; Wang, Z.W.; Gao, J.G.; Xu, C.F.; Ma, H.; et al. Ten-Day Vonoprazan-Amoxicillin Dual Therapy vs Standard 14-Day Bismuth-Based Quadruple Therapy for First-Line Helicobacter pylori Eradication: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2024, 119, 655–661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, X.P.; Liu, Y.J.; Lin, S.W.; Shao, Y.F.; Qiu, F.; Qiu, Q.W.; Xu, Z.K.; Chen, J.X.; Chen, L.H.; Lin, Z.Q.; et al. Vonoprazan-amoxicillin dual therapy for Helicobacter pylori eradication in Chinese population: A prospective, multicenter, randomized, two-stage study. World J. Gastroenterol. 2024, 30, 3304–3313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, W.; Liu, J.; Wang, X.; Li, J.; Zhang, X.; Ye, H.; Li, J.; Dong, X.; Liu, B.; Wang, C.; et al. Simplified Helicobacter pylori therapy for patients with penicillin allergy: A randomised controlled trial of vonoprazan-tetracycline dual therapy. Gut 2024, 73, 1414–1420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miftahussurur, M.; Pratama Putra, B.; Yamaoka, Y. The Potential Benefits of Vonoprazan as Helicobacter pylori Infection Therapy. Pharmaceuticals 2020, 13, 276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeuchi, T.; Furuta, T.; Fujiwara, Y.; Sugimoto, M.; Kasugai, K.; Kusano, M.; Okada, H.; Suzuki, T.; Higuchi, T.; Kagami, T.; et al. Randomised trial of acid inhibition by vonoprazan 10/20 mg once daily vs rabeprazole 10/20 mg twice daily in healthy Japanese volunteers (SAMURAI pH study). Aliment. Pharmacol. Ther. 2020, 51, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, X.; Liu, X.S.; He, C.; Ouyang, Y.B.; Li, N.S.; Xie, C.; Peng, C.; Zhu, Z.H.; Xie, Y.; et al. Fourteen-day vonoprazan and low- or high-dose amoxicillin dual therapy for eradicating Helicobacter pylori infection: A prospective, open-labeled, randomized non-inferiority clinical study. Front Immunol. 2023, 13, 1049908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Zhang, R.; Fang, Y.; Zhao, R.; Fu, Y.; Ren, P.; Zhan, Q.; Shao, M. P-CAB versus PPI in the eradication of Helicobacter pylori: A systematic review and network meta-analysis. Ther. Adv. Gastroenterol. 2024, 17, 17562848241241223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chey, W.D.; Mégraud, F.; Laine, L.; López, L.J.; Hunt, B.J.; Howden, C.W. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology 2022, 163, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Cheng, J.; Lao, Y.J.; Huang, K.; Mou, J.L.; Hu, F.; Lin, M.L.; Lin, J. The efficacy and safety of vonoprazan-amoxicillin dual therapy in eradicating Helicobacter pylori: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simadibrata, D.M.; Syam, A.F.; Lee, Y.Y. A comparison of efficacy and safety ofpotassium-competitive acid blocker and proton pump inhibitor in gastric acid- related diseases: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2022, 37, 2217–2228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, B.S.; Zhou, Y.; Meng, C.X.; Takagi, T.; Tian, Y.S. Vonoprazan vs proton pump inhibitors in treating post-endoscopic submucosal dissection ulcers and preventing bleeding: A meta-analysis of randomized controlled trials and observational studies. Medicine 2020, 99, e19357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gotoda, T.; Kusano, C.; Suzuki, S.; Horii, T.; Ichijima, R.; Ikehara, H. Clinical impact of vonoprazan-based dual therapy with amoxicillin for H. pylori infection in a treatment-naïve cohort of junior high school students in Japan. J. Gastroenterol. 2020, 55, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Ichijima, R.; Ohyauchi, M.; Ito, H.; Kawamura, M.; Ogata, Y.; Ohtaka, M.; et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: A multicentre randomised trial in Japan. Gut 2020, 69, 1019–1026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, W.; Wang, Q.; Zhang, X.; Wang, L. Ten-day vonoprazan-based versus fourteen-day proton pump inhibitor-based therapy for first-line Helicobacter pylori eradication in China: A meta-analysis of randomized controlled trials. Int. J. Immunopathol. Pharmacol. 2024, 38, 3946320241286866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zuberi, B.F.; Ali, F.S.; Rasheed, T.; Bader, N.; Hussain, S.M.; Saleem, A. Comparison of Vonoprazan and Amoxicillin Dual Therapy with Standard Triple Therapy with Proton Pump Inhibitor for Helicobacter Pylori eradication: A Randomized Control Trial. Pak. J. Med Sci. 2022, 38(Pt. II), 965–969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, F.; Yu, B.; Qin, L.; Dai, X. A randomized clinical study on the efficacy of vonoprazan combined with amoxicillin duo regimen for the eradication of Helicobacter pylori. Medicine 2023, 102, e35610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.M.; Wang, M.D.; Yang, S.Y.; Hu, J.Q.; Zhu, B.Q.; Wei, Y.K.; Zhang, C.L.; Long, E.W. The efficacy and safety of vonoprazan-based high-dose dual therapy for eradication of Helicobacter pylori: A systematic review and meta-analysis. J. Infect. Public Health 2025, 18, 102768. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.W.; Xiong, S.; Jia, Y.G.; Xiao, D.; Tan, S.Y.; Cao, J.W.; Sun, J.; Tian, X.; Li, S.Y.; Chen, R.H.; et al. Comparison of vonoprazan bismuth-containing triple therapy with quadruple therapy in Helicobacter pylori-infected treatment-naive patients: A prospective multicenter randomized controlled trial. J. Gastroenterol. Hepatol. 2024, 39, 2293–2298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.; Zhang, D.; Huang, S.; Zeng, F.; Li, D.; Zhang, X.; Chen, R.; Chen, S.; Wang, J.; Bai, F. Comparison of vonoprazan dual therapy, quadruple therapy and standard quadruple therapy for Helicobacter pylori infection in Hainan: A single-center, open-label, non-inferiority, randomized controlled trial. BMC Gastroenterol. 2024, 24, 131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, L.; Wang, Y.; Ye, J.; Han, Y.; Lou, G.; Li, Y.; Yan, H.; Du, Q. Quadruple therapy with vonoprazan 20 mg daily as a first-line treatment for Helicobacter pylori infection: A single-center, open-label, noninferiority, randomized controlled trial. Helicobacter 2023, 28, e12940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huh, K.Y.; Chung, H.; Kim, Y.K.; Lee, S.; Bhatia, S.; Takanami, Y.; Nakaya, R.; Yu, K.S. Evaluation of safety and pharmacokinetics of bismuth-containing quadruple therapy with either vonoprazan or lansoprazole for Helicobacter pylori eradication. Br. J. Clin. Pharmacol. 2022, 88, 138–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tungtrongchitr, N.; Bongkotvirawan, P.; Ratana-Amornpin, S.; Siramolpiwat, S.; Eiamsitrakoon, T.; Gamnarai, P.; Wongcha-Um, A.; Yamaoka, Y.; Pawa, K.K.; Vilaichone, R.K. Fourteen-day vonoprazan-based bismuth quadruple therapy for H. pylori eradication in an area with high clarithromycin and levofloxacin resistance: A prospective randomized study (VQ-HP trial). Sci. Rep. 2024, 14, 8986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.; Shen, W.; Liu, Y.; Dong, Q.; Shi, Y. Efficacy and safety of triple therapy containing berberine, amoxicillin, and vonoprazan for Helicobacter pylori initial treatment: A randomized controlled trial. Chin. Med. J. 2023, 136, 1690–1698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shekeban, Y.M.; Hamdy, N.A.; Header, D.A.; Ahmed, S.M.; Helmy, M.M. Vonoprazan-based therapy versus standard regimen for Helicobacter pylori infection management in Egypt: An open-label randomized controlled trial. Sci. Rep. 2025, 15, 15989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Zhang, Z.Y.; Wang, F.; Zhuang, K.; Xu, X.; Liu, D.S.; Fan, H.Z.; Yang, L.; Jiang, K.; Zhang, D.K.; et al. Effects of amoxicillin dosage on cure rate, gut microbiota, and antibiotic resistome in vonoprazan and amoxicillin dual therapy for Helicobacter pylori: A multicentre, open-label, non-inferiority randomized controlled trial. Lancet Microbe. 2025, 6, 100975. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Suzuki, S.; Kusano, C.; Ikehara, H.; Ichijima, R.; Ohyauchi, M.; Kawamura, M.; Yoda, Y.; Nakahara, M.; Kawabe, K.; et al. High Body Mass Index Is Correlated with the Success of Vonoprazan-Based Second-Line Therapy for Helicobacter Pylori Infection. Tohoku J. Exp. Med. 2021, 253, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, X.; Ouyang, Y.B.; He, C.; Li, N.S.; Xie, C.; Peng, C.; Zhu, Z.H.; Shu, X.; Xie, Y.; et al. Altered Gut Microbiota and Short-Chain Fatty Acids After Vonoprazan- Amoxicillin Dual Therapy for Helicobacter pylori Eradication. Front. Cell. Infect. Microbiol. 2022, 12, 881968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, L.; Lu, H.; Song, Z.; Lyu, B.; Chen, Y.; Wang, J.; Xia, J.; Zhao, Z.; on behalf of Helicobacter Pylori Study Group of Chinese Society of Gastroenterology. 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin. Med. J. 2022, 135, 2899–2910, Erratum in Chin. Med. J. 2024, 137, 1068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).