A Systematic Review and Meta-Analysis on the Role of Somatostatin Therapy in Non-Variceal Gastrointestinal Bleeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Selection Process
2.4. Data Extraction
2.5. Assessment of Bias Risk
2.6. Statistical Analysis
3. Results
3.1. Study Screening
3.2. Study Characteristics
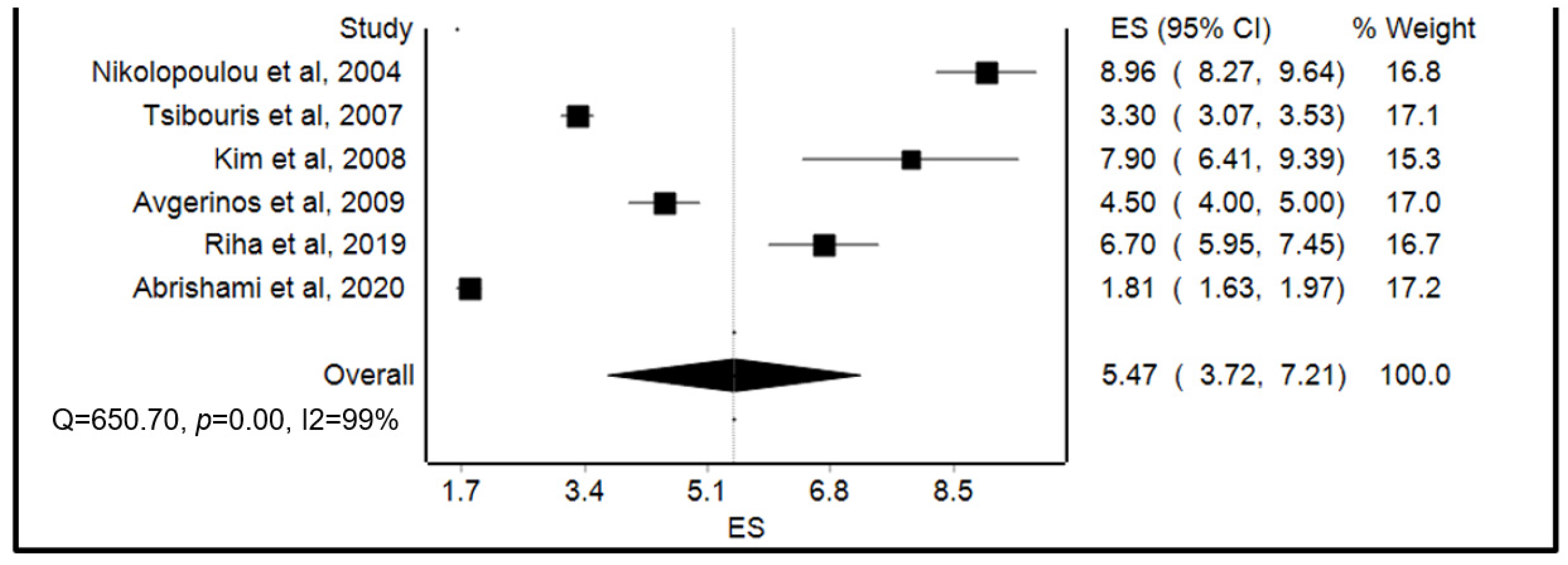
3.3. Meta-Analysis of Primary Outcomes
3.4. Meta-Analysis of Secondary Outcomes
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelms, D.W.; Pelaez, C.A. The Acute Upper Gastrointestinal Bleed. Surg. Clin. N. Am. 2018, 98, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, G.J.; Thomopoulos, K.C.; Sakellaropoulos, G.; Katsakoulis, E.; Nikolopoulou, V. Changing trends in the epidemiology and clinical outcome of acute upper gastrointestinal bleeding in a defined geographical area in Greece. J. Clin. Gastroenterol. 2008, 42, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Kang, D.H.; Kim, H.W.; Park, S.B.; Park, K.T.; Kim, G.H.; Song, G.A.; Cho, M. Somatostatin adjunctive therapy for non-variceal upper gastrointestinal rebleeding after endoscopic therapy. World J. Gastroenterol. 2011, 17, 3441–3447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wara, P. Incidence, diagnosis, and natural course of upper gastrointestinal hemorrhage. Prognostic value of clinical factors and endoscopy. Scand. J. Gastroenterol. Suppl. 1987, 137, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.; Peymani, P.; Zare, M.; Lankarani, K.B. The Effect of Octreotide in Acute Nonvariceal Upper Gastrointestinal Bleeding: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Res. Pharm. Pract. 2020, 9, 94–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barkun, A.N.; Almadi, M.; Kuipers, E.J.; Laine, L.; Sung, J.; Tse, F.; Leontiadis, G.I.; Abraham, N.S.; Calvet, X.; Chan, F.K.L.; et al. Management of Nonvariceal Upper Gastrointestinal Bleeding: Guideline Recommendations From the International Consensus Group. Ann. Intern. Med. 2019, 171, 805–822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barkun, A.; Sabbah, S.; Enns, R.; Armstrong, D.; Gregor, J.; Fedorak, R.N.; Rahme, E.; Toubouti, Y.; Martel, M.; Chiba, N.; et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): Endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am. J. Gastroenterol. 2004, 99, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Birgisson, S. Somatostatin or octreotide compared with H2 antagonists and placebo in the management of acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Ann. Intern. Med. 1997, 127, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Goltstein, L.C.M.J.; Grooteman, K.V.; Bernts, L.H.P.; Scheffer, R.C.H.; Laheij, R.J.F.; Gilissen, L.P.L.; Schrauwen, R.W.M.; Talstra, N.C.; Zuur, A.T.; Braat, H.; et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology 2024, 166, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, Z.; Du, B.; Pang, Y. PRISMA—Practical meta-analysis of applying local triamcinolone acetonide injection for stenosis after esophageal cancer surgery. Cancer Manag. Res. 2018, 10, 6327–6338. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Cochrane. Risk of Bias 2 (RoB 2) Tool. Available online: https://methods.cochrane.org/risk-bias-2 (accessed on 2 July 2024).
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A.; DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.A.; Dwivedi, P.; Padhi, B.K. Beyond the funnel plot: The advantages of Doi plots and prediction intervals in meta-analyses. Asian J. Psychiatr. 2023, 84, 103550. [Google Scholar] [CrossRef] [PubMed]
- Lin, L. Use of Prediction Intervals in Network Meta-analysis. JAMA Netw. Open 2019, 2, e199735. [Google Scholar] [CrossRef]
- Fagan, T. Exact 95% confidence intervals for differences in binomial proportions. Comput. Biol. Med. 1999, 29, 83–87. [Google Scholar] [CrossRef]
- Riha, H.M.; Wilkinson, R.; Twilla, J.; Harris, L.J., Jr.; Kimmons, L.A.; Kocak, M.; Van Berkel, M.A. Octreotide Added to a Proton Pump Inhibitor Versus a Proton Pump Inhibitor Alone in Nonvariceal Upper-Gastrointestinal Bleeds. Ann. Pharmacother. 2019, 53, 794–800. [Google Scholar] [CrossRef]
- Avgerinos, A.; Sgouros, S.; Viazis, N.; Vlachogiannakos, J.; Papaxoinis, K.; Bergele, C.; Sklavos, P.; Raptis, S.A. Somatostatin inhibits gastric acid secretion more effectively than pantoprazole in patients with peptic ulcer bleeding: A prospective, randomized, placebo-controlled trial. Scand. J. Gastroenterol. 2005, 40, 515–522. [Google Scholar] [CrossRef]
- Tsibouris, P.; Zintzaras, E.; Lappas, C.; Moussia, M.; Tsianos, G.; Galeas, T.; Potamianos, S. High-dose pantoprazole continuous infusion is superior to somatostatin after endoscopic hemostasis in patients with peptic ulcer bleeding. Am. J. Gastroenterol. 2007, 102, 1192–1199. [Google Scholar] [CrossRef]
- Kim, I.; Lee, Y.-S.; Koh, B.S.; Kim, W.; Lim, K.S. Does Adding Somatostatin to Proton Pump Inhibitor Improve the Outcome of Peptic Ulcer Bleeding? Acute Crit. Care 2008, 23, 75–78. [Google Scholar] [CrossRef]
- Nikolopoulou, V.N.; Thomopoulos, K.C.; Katsakoulis, E.C.; Vasilopoulos, A.G.; Margaritis, V.G.; Vagianos, C.E. The effect of octreotide as an adjunct treatment in active nonvariceal upper gastrointestinal bleeding. J. Clin. Gastroenterol. 2004, 38, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Rehman, S.T.; Zulfiqar, S.; Awan, S.; Abid, S. Real-world comparison of terlipressin vs. octreotide as an adjuvant treatment in the management of variceal bleeding. Sci. Rep. 2024, 14, 6692. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, A.Q.; Garcia-Tsao, G. The Role of Medical Therapy for Variceal Bleeding. Gastrointest. Endosc. Clin. N. Am. 2015, 25, 479–490. [Google Scholar] [CrossRef]
- Mullady, D.K.; Wang, A.Y.; Waschke, K.A. AGA Clinical Practice Update on Endoscopic Therapies for Non-Variceal Upper Gastrointestinal Bleeding: Expert Review. Gastroenterology 2020, 159, 1120–1128. [Google Scholar] [CrossRef]
- Khedr, A.; Mahmoud, E.E.; Attallah, N.; Mir, M.; Boike, S.; Rauf, I.; Jama, A.B.; Mushtaq, H.; Surani, S.; Khan, S.A. Role of octreotide in small bowel bleeding. World J. Clin. Cases 2022, 10, 9192–9206. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Murad, M.H.; Montori, V.M.; Ioannidis, J.P.; Jaeschke, R.; Devereaux, P.J.; Prasad, K.; Neumann, I.; Carrasco-Labra, A.; Agoritsas, T.; Hatala, R.; et al. How to read a systematic review and meta-analysis and apply the results to patient care: Users’ guides to the medical literature. JAMA 2014, 312, 171–179. [Google Scholar] [CrossRef]
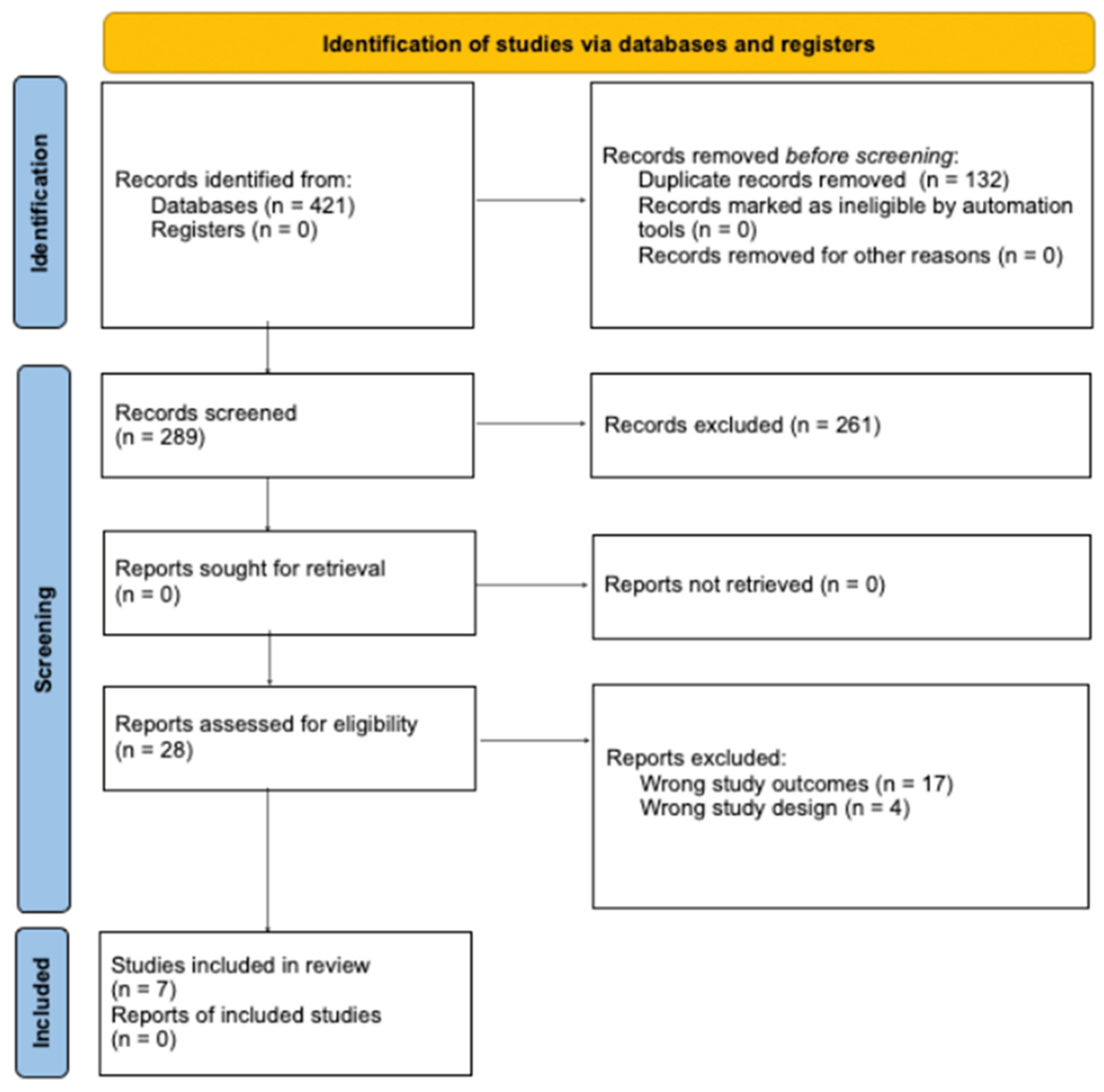

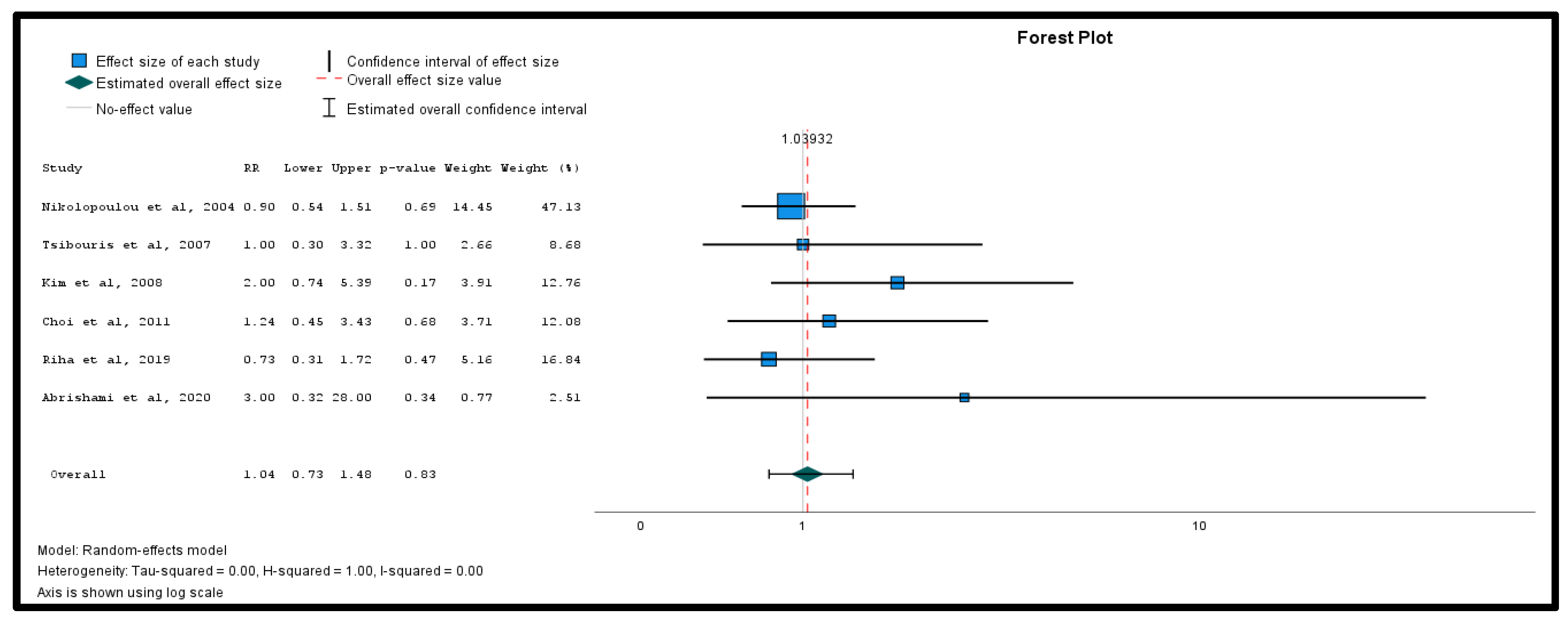
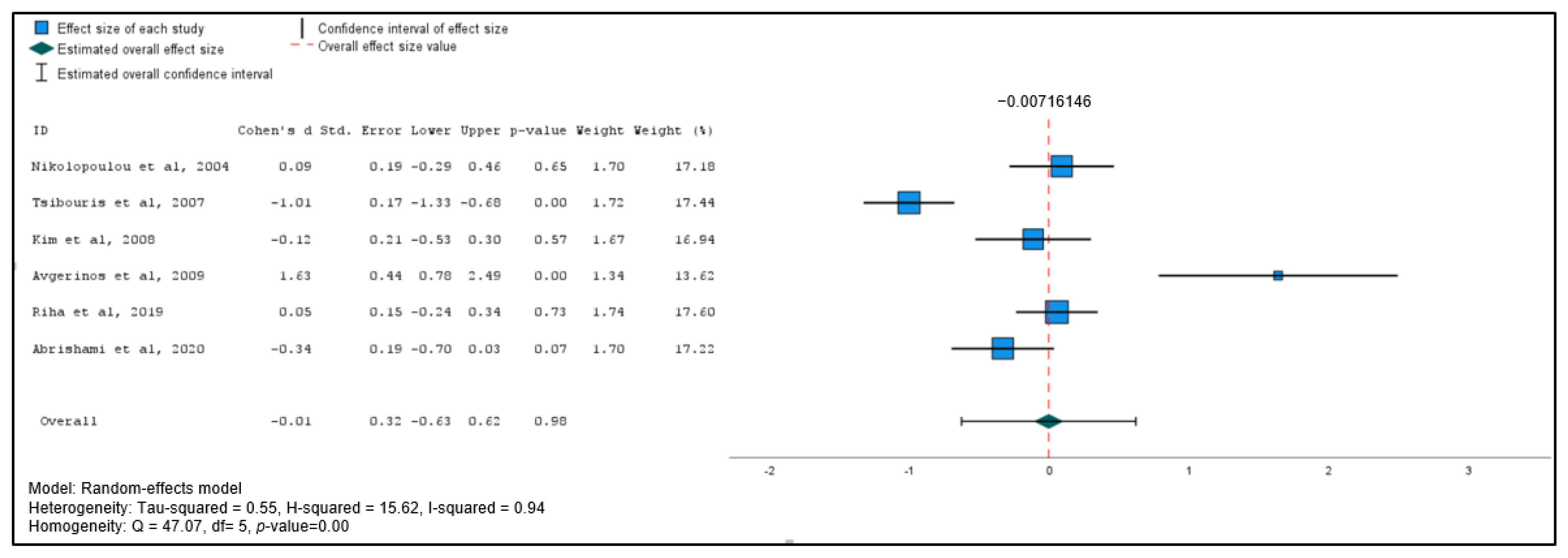


| Study | Design | Country | Sample Size, N | Age, y (Mean SD *) | Males/ Females, N | Average Hemoglobin on Admission, y (Mean SD *) | Study Quality (MINORS) | Study Quality (Cochrane Risk of Bias) |
|---|---|---|---|---|---|---|---|---|
| Nikolopoulou et al., 2004 [23] | Randomized double-blinded placebo- controlled trial | Greece | 55 | 64.4 (16.1) | 34/21 | 9.0 (2) | Low | |
| 55 | 62.4 (14.1) | 32/23 | 9.2 (1.5) | |||||
| Riha et al., 2019 [19] | Retrospective cohort study | US | 90 | 62 (median range, 53–69) | 58/32 | 7.6 (median range, 6.1–9.0) | Fair | |
| 90 | 60 (median range, 53–69) | 55/35 | 6.8 (median range, 5.9–9.2) | |||||
| Abrishami et al., 2020 [5] | Randomized double-blinded placebo- controlled trial | Iran | 58 | 56 (1.59) | 38/20 | 11.0 (3.1) | Low | |
| 58 | 55 (1.85) | 38/20 | 11.0 (3.5) | |||||
| Choi et al., 2011 [3] | Retrospective cohort study | Korea | 52 | 65.4 (19.5) | 19/33 | 8.6 (2.8) | Fair | |
| 49 | 64.2 (14.2) | 32/17 | 8.3 (2.6) | |||||
| Avgerinos et al., 2009 [20] | Randomized double-blinded placebo-controlled trial | Greece | 14 | 53.2 (4.9) | 11/3 | 11.5 (0.4) | Low | |
| 14 | 45.1 (2.8) | 13/1 | 11.3 (0.3) | |||||
| Tsibouris et al., 2007 [21] | Randomized double-blinded placebo- controlled trial | Greece | 82 | 67.8 (13.1) | 60/22 | NR | Low | |
| 82 | 66.4 (13.0) | 60/22 | NR | |||||
| Kim et al., 2008 [22] | Retrospective cohort study | Korea | 45 | 60.2 (15.8) | 36/9 | 9.3 (2.7) | Fair | |
| 45 | 62.4 (15.2) | 33/12 | 9.2 (2.8) |
| Study | Treatment Group | Invasive Treatment with Endoscopy, n (%) | Comorbidities | Etiology of Bleed, N | Blood Transfusions Received, Units of pRBCs (Mean SD *) | Shock on Admission, N |
|---|---|---|---|---|---|---|
| Nikolopoulou et al., 2004 [23] | PPI monotherapy | 13 (24) | Alcohol user: 7 Smoker: 18 CHF: NR Blood thinner use: 37 History of H. pylori: 36 | Gastric ulcer: 22 Duodenal ulcer: 26 Other: 7 | 3.7 (2.04) | 20 |
| PPIs + octreotide | 11 (20) | Alcohol user: 7 Smoker: 14 CHF: NR Blood thinner use: 33 History of H. pylori: 34 | Gastric ulcer: 21 Duodenal ulcer: 26 Other: 8 | 2.5 (2.4) | 35 | |
| Riha et al., 2019 [19] | PPI monotherapy | 37 (41) | Alcohol user: 15 Smoker: NR CHF: 18 Blood thinner use: 89 History of H. pylori: 17 | Gastric ulcer: 41 Duodenal ulcer: 38 Other: 11 | 2.0 (median range, 0–5) | 37 |
| PPIs + octreotide | 28 (31) | Alcohol user: 44 Smoker: NR CHF: 17 Blood thinner use: 63 History of H. pylori: 15 | Gastric ulcer: 53 Duodenal ulcer: 19 Other: 18 | 3 (median range, 0–5) | 47 | |
| Abrishami et al., 2020 [5] | PPI monotherapy | 1 (1.72) | Alcohol user: 4 Smoker: 14 CHF: NR Blood thinner use: 34 History of H. pylori: 39 | Gastric ulcer: 20 Duodenal ulcer: 27 Other: 10 | 1.7 (0.5) | NR |
| PPIs + octreotide | 3 (5.17 | Alcohol user: 2 Smoker: 8 CHF: NR Blood thinner use: 43 History of H. pylori: 32 | Gastric ulcer: 21 Duodenal ulcer: 26 Other: 11 | 1.7 (0.5) | NR | |
| Choi et al., 2011 [3] | PPI monotherapy | NR | Alcohol user: NR Smoker: NRCHF: 7 Blood thinner use: 30 History of H. pylori: 14 | Gastric ulcer: 27 Duodenal ulcer: 11 Other: 14 | NR | 26 |
| PPIs + somatostatin analog | NR | Alcohol user: NR Smoker: NR CHF: 4 Blood thinner use: 23 History of H. pylori: 8 | Gastric ulcer: 29 Duodenal ulcer: 8 Other: 12 | NR | 27 | |
| Avgerinos et al., 2009 [20] | PPI monotherapy | NR | Alcohol user: NR Smoker: NR CHF: NR Blood thinner use: NR History of H. pylori: NR | Gastric ulcer: NR Duodenal ulcer: NR Other: | 1.0 | NR |
| PPIs + somatostatin | NR | Alcohol user: NR Smoker: NR CHF: NR Blood thinner use: NR History of H. pylori: NR | Gastric ulcer: NR Duodenal ulcer: NROther: | 2.0 | NR | |
| Tsibouris et al., 2007 [21] | PPI monotherapy | NR | Alcohol user: 43 Smoker: 82 CHF: 22 Blood thinner use: 57 History of H. pylori: 60 | Gastric ulcer: NR Duodenal ulcer: NROther: | 3.3 (1.4) | 36 |
| PPIs + somatostatin | NR | Alcohol user: 43 Smoker: 82 CHF: 23 Blood thinner use: 47 History of H. pylori: 60 | Gastric ulcer: NR Duodenal ulcer: NROther: | 3.2 (1.6) | 36 | |
| Kim et al., 2008 [22] | PPI monotherapy | NR | Alcohol user: NR Smoker: NR CHF: NR Blood thinner use: 19 History of H. pylori: 16 | Gastric ulcer: 29 Duodenal ulcer: 13 Other: 3 | 3.2 (2.8) | NR |
| PPIs + somatostatin | NR | Alcohol user: NR Smoker: NR CHF: NR Blood thinner use: 10 History of H. pylori: 14 | Gastric ulcer: 24 Duodenal ulcer: 1 Other: 20 | 4.5 (4.4) | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, M.; Vongsavath, T.; Sonaiya, S.; Liu, L.; Tun, K.M.; Batra, K.; Gish, R.G. A Systematic Review and Meta-Analysis on the Role of Somatostatin Therapy in Non-Variceal Gastrointestinal Bleeding. Gastroenterol. Insights 2025, 16, 18. https://doi.org/10.3390/gastroent16020018
Chun M, Vongsavath T, Sonaiya S, Liu L, Tun KM, Batra K, Gish RG. A Systematic Review and Meta-Analysis on the Role of Somatostatin Therapy in Non-Variceal Gastrointestinal Bleeding. Gastroenterology Insights. 2025; 16(2):18. https://doi.org/10.3390/gastroent16020018
Chicago/Turabian StyleChun, Magnus, Tahne Vongsavath, Sneh Sonaiya, Lily Liu, Kyaw Min Tun, Kavita Batra, and Robert G. Gish. 2025. "A Systematic Review and Meta-Analysis on the Role of Somatostatin Therapy in Non-Variceal Gastrointestinal Bleeding" Gastroenterology Insights 16, no. 2: 18. https://doi.org/10.3390/gastroent16020018
APA StyleChun, M., Vongsavath, T., Sonaiya, S., Liu, L., Tun, K. M., Batra, K., & Gish, R. G. (2025). A Systematic Review and Meta-Analysis on the Role of Somatostatin Therapy in Non-Variceal Gastrointestinal Bleeding. Gastroenterology Insights, 16(2), 18. https://doi.org/10.3390/gastroent16020018