Serum Albumin as an Early Predictor of Severity in Patients with Acute Pancreatitis
Abstract
1. Introduction
Predictive Factors of Severity in AP
2. Materials and Methods
Statistic Analysis
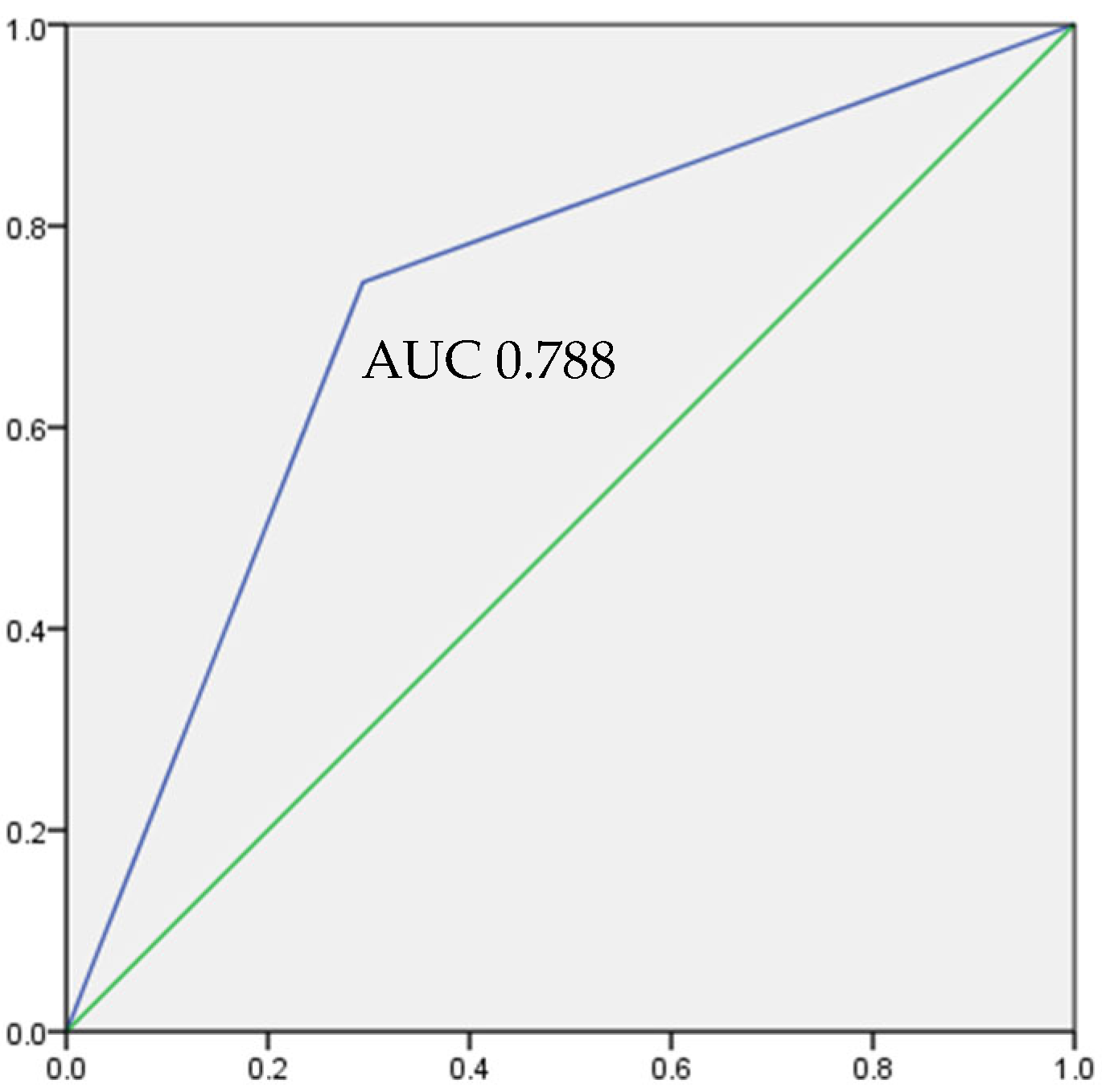
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Acute pancreatitis |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| AUC | Area under the curve |
| BISAP | Bedside Index for Severity of Acute Pancreatitis |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| CRP | C Reactive Protein |
| CTSI | Computed Tomography Severity Index |
| OF | Organ failure |
| PTa | Prothrombin Time activity |
| SIRS | Systemic inflammatory response syndrome |
References
- Bustamante, D.; Garcia, A.; Umanzor, W.; Leiva, L.; Barrientos, A.; Diek, L. Pancreatitis Aguda: Evidencia Actual. Arch. Med. 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Gapp, J.; Tariq, A.; Chandra, S. Acute Pancreatitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mutinga, M.; Rosenbluth, A.; Tenner, S.M.; Odze, R.R.; Sica, G.T.; Banks, P.A. Does mortality occur early or late in acute pancreatitis? Int. J. Pancreatol. 2000, 28, 91–95. [Google Scholar] [CrossRef]
- Dirección General de Estadística e Informática, Secretaría de Salud. Estadística de egresos hospitalarios de la Secretaría de Salud, 1999. Salud Pública México 2000, 42, 456–470. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Yamamoto, H.; Yazumi, S.; Watanabe, Y.; Matsueda, K.; Yamamoto, H.; Chiba, T. Perfusion computerized tomography can predict pancreatic necrosis in early stages of severe acute pancreatitis. Clin. Gastroenterol. Hepatol. 2007, 5, 1484–1492. [Google Scholar] [CrossRef]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Tabak, Y.; Conwell, D.L.; Banks, P.A. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut 2008, 57, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Mofidi, R.; Duff, M.D.; Wigmore, S.J.; Madhavan, K.K.; Garden, O.J.; Parks, R.W. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br. J. Surg. 2006, 93, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Buter, A.; Imrie, C.W.; Carter, C.R.; Evans, S.; McKay, C.J. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br. J. Surg. 2002, 89, 298–302. [Google Scholar] [CrossRef]
- Brown, A.; Orav, J.; Banks, P.A. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas 2000, 20, 367–372. [Google Scholar] [CrossRef]
- Wu, B.U.; Bakker, O.J.; Papachristou, G.I.; Besselink, M.G.; Repas, K.; van Santvoort, H.C.; Muddana, V.; Singh, V.K.; Whitcomb, D.C.; Gooszen, H.G.; et al. Blood urea nitrogen in the early assessment of acute pancreatitis: An international validation study. Arch. Intern. Med. 2011, 171, 669–676. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Mahlke, R.; Blum, T.; Bruns, A.; Bruns, D.; Maisonneuve, P.; Lowenfels, A.B. Hemoconcentration: An early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Am. J. Gastroenterol. 2001, 96, 2081–2085. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Ates, I.; Akpinar, M.Y.; Yuksel, M.; Kuzu, U.B.; Kacar, S.; Coskun, O.; Kayacetin, E. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Fernández, J. Bases fisiopatológicas del uso de la albúmina humana en la cirrosis hepática [Physiopathological bases for the use of human albumin in liver cirrhosis]. Gastroenterol. Hepatol. 2012, 35, 42–49. [Google Scholar] [CrossRef]
- Hong, W.; Lin, S.; Zippi, M.; Geng, W.; Stock, S.; Basharat, Z.; Cheng, B.; Pan, J.; Zhou, M. Serum Albumin Is Independently Associated with Persistent Organ Failure in Acute Pancreatitis. Can. J. Gastroenterol. Hepatol. 2017, 2017, 5297143. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Wu, H. The product value of serum albumin and prothrombin time activity could be a useful biomarker for severity prediction in AP: An ordinal retrospective study. Pancreatology 2019, 19, 230–236. [Google Scholar] [CrossRef]
- Singh, V.K.; Bollen, T.L.; Wu, B.U.; Repas, K.; Maurer, R.; Yu, S.; Mortele, K.J.; Conwell, D.L.; Banks, P.A. An assessment of the severity of interstitial pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.G.; van Santvoort, H.C.; Boermeester, M.A.; Nieuwenhuijs, V.B.; van Goor, H.; Dejong, C.H.; Schaapherder, A.F.; Gooszen, H.G.; Dutch Acute Pancreatitis Study Group. Timing and impact of infections in acute pancreatitis. Br. J. Surg. 2009, 96, 267–273. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S.; American College of Gastroenterology. American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Domínguez-Muñoz, J.E. Prognostic factors in acute pancreatitis. Int. J. Pancreatol. 1993, 14, 1–8. [Google Scholar] [CrossRef]
- Singh, V.K.; Wu, B.U.; Bollen, T.L.; Repas, K.; Maurer, R.; Johannes, R.S.; Mortele, K.J.; Conwell, D.L.; Banks, P.A. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am. J. Gastroenterol. 2009, 104, 966–971. [Google Scholar] [CrossRef]
- Papachristou, G.I.; Muddana, V.; Yadav, D.; O’Connell, M.; Sanders, M.K.; Slivka, A.; Whitcomb, D.C. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am. J. Gastroenterol. 2010, 105, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Harshit Kumar, A.; Singh Griwan, M. A comparison of APACHE II, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol. Rep. 2018, 6, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, E.J.; Robinson, D.L.; Megibow, A.J.; Ranson, J.H. Acute pancreatitis: Value of CT in establishing prognosis. Radiology 1990, 174, 331–336. [Google Scholar] [CrossRef]
- Mortele, K.J.; Wiesner, W.; Intriere, L.; Shankar, S.; Zou, K.H.; Kalantari, B.N.; Perez, A.; vanSonnenberg, E.; Ros, P.R.; Banks, P.A.; et al. A modified CT severity index for evaluating acute pancreatitis: Improved correlation with patient outcome. AJR Am. J. Roentgenol. 2004, 183, 1261–1265. [Google Scholar] [CrossRef]
- Bollen, T.L.; Singh, V.K.; Maurer, R.; Repas, K.; van Es, H.W.; Banks, P.A.; Mortele, K.J. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR Am. J. Roentgenol. 2011, 197, 386–392. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.P.; Pan, Y.M.; He, Z.J.; Tan, Y.P.; Wang, D.D.; Qu, X.G.; Zhang, Z.H. Predictive Value of C-Reactive Protein/Albumin Ratio for Acute Kidney Injury in Patients with Acute Pancreatitis. J. Inflamm. Res. 2024, 17, 5495–5507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xia, W.; Lu, Y.; Chen, W.; Zhao, Y.; Zhuang, Y. Predictive value of the C-reactive protein/albumin ratio in severity and prognosis of acute pancreatitis. Front. Surg. 2023, 9, 1026604. [Google Scholar] [CrossRef]
- Paye, F.; Presset, O.; Chariot, J.; Molas, G.; Rozé, C. Role of nonesterified fatty acids in necrotizing pancreatitis: An in vivo experimental study in rats. Pancreas 2001, 23, 341–348. [Google Scholar] [CrossRef]
- Sztefko, K.; Panek, J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology 2001, 1, 230–236. [Google Scholar] [CrossRef]
- Chung, B.H.; Tallis, G.A.; Cho, B.H.; Segrest, J.P.; Henkin, Y. Lipolysis-induced partitioning of free fatty acids to lipoproteins: Effect on the biological properties of free fatty acids. J. Lipid Res. 1995, 36, 1956–1970. [Google Scholar] [CrossRef]
- Bird, D.A.; Kabakibi, A.; Laposata, M. The distribution of fatty acid ethyl esters among lipoproteins and albumin in human serum. Alcohol. Clin. Exp. Res. 1997, 21, 602–605. [Google Scholar] [CrossRef] [PubMed]
| N = 103 | |
|---|---|
| Gender | |
| Men | 43 (41.7) |
| Women | 60 (58.3) |
| Etiology | |
| Biliary | 46 (44.7) |
| Hypertriglyceridemia | 11 (10.7) |
| Alcohol | 5 (4.9) |
| Post-ERCP | 15 (14.6) |
| Idiopathic | 21 (20.4) |
| Tumor | 4 (3.9) |
| Drug | 1 (1.0) |
| Body mass index | |
| Low | 5 (4.9) |
| Normal | 19 (18.4) |
| Overweight | 34 (33) |
| Obesity | 31 (30) |
| Diabetes mellitus | |
| No | 80 (77.7) |
| Yes | 23 (22.3) |
| Acute kidney injury | |
| No | 74 (71.8) |
| Yes | 29 (28.2) |
| SIRS | |
| No | 65 (63.1) |
| Yes | 38 (36.9) |
| APACHE score | |
| <8 | 58 (56.3) |
| >8 | 42 (40.8) |
| BISAP score | |
| ≤2 | 86 (82.7) |
| >2 | 17 (16.3) |
| MARSHALL score | |
| <2 | 46 (44.2) |
| >2 | 57 (54.8) |
| Age (years) | 47.76 (±15.38) ** |
| Number of days of hospital stay | 8.0 (6–12) * |
| Kirby | 301.37 (±75.81) ** |
| Albumin | |
| <3.5 g/dL | 34 (33) |
| >3.5 g/dL | 69 (67) |
| Albumin (g/dL) | 4.0 (3.3–4.40) * |
| Leukocytes (103) | 13.52 (±6.28) ** |
| Creatinine (mg/dL) | 0.94 (0.75–1.35) * |
| BUN (mg/dL) | 14 (11.2–28.0) * |
| Hematocrit (%) | 43 (33.9–48.3) * |
| Amilasa (U/L) | 786 (316–1521) * |
| Lipasa (U/L) | 845 (458–2447) * |
| Albúmina Normal N: 69 n (%) | Hipoalbuminemia N: 34 n (%) | p | |
|---|---|---|---|
| Gender | 0.934 * | ||
| Man | 29 (67.4) | 14 (32.6) | |
| Woman | 40 (66.7) | 20 (33.3) | |
| Etiology | 0.058 * | ||
| Biliary | 32 (69.6) | 14 (30.4) | |
| Hypertriglyceridemia | 4 (36.4) | 7 (63.6) | |
| Alcohol | 3 (60) | 2 (40) | |
| Post-CPRE | 14 (93.3) | 1 (6.7) | |
| Idiopathic | 14 (66.7) | 7 (33.3) | |
| Tumor | 2 (50) | 2 (50) | |
| Drugs | 0 (0) | 1 (100) | |
| Age | 0.131 * | ||
| <55 years | 50 (73.5) | 20 (58.8) | |
| >55 years | 18 (26.5) | 14 (41.2) | |
| Body mass index | 0.59 * | ||
| Low weight | 2 (40) | 3 (60) | |
| Normal | 13 (68.4) | 6 (31.6) | |
| Overweight | 24 (70.6) | 10 (29.4) | |
| Obesity | 20 (64.5) | 11 (35.5) | |
| Acute kidney injury | 0.001 * | ||
| No | 57 (77) | 17 (23) | |
| Yes | 12 (41.4) | 17 (58.6) | |
| APACHE score | 0.002 * | ||
| <8 | 47 (81) | 11 (19) | |
| >8 | 22 (52.4) | 20 (47.6) | |
| SIRS | 0.053 * | ||
| No | 48 (73.8) | 17 (26.2) | |
| Yes | 21 (55.3) | 17 (44.7) | |
| ATLANTA (entry) | 0.000 * | ||
| Mild | 40 (87) | 6 (13) | |
| Moderately severe | 29 (50.9) | 28 (49.1) | |
| Hematocrit < 44% | 68 (57.9) | 33 (36.7) | 0.001 ** |
| Creatinine | 69 (47.14) | 34 (61.85) | 0.019 ** |
| BUN < 25% | 69 (47.18) | 34 (61.7) | 0.020 ** |
| BUN | 0.001 * | ||
| <25% | 60 (53.6) | 20 (26.4) | |
| >25% | 9 (15.4) | 14 (7.6) | |
| BISAP score (>2) | 69 (44.75) | 34 (66.7) | 0.000 ** |
| a. | |||
| p | OR | IC 95% | |
| APACHE ≥ 8 | 0.000 | 5.19 | 2.14–12.58 |
| BISAP ≥ 2 | 0.001 | 6.98 | 2.21–22.05 |
| Atlanta M-S | 0.000 | 6.37 | 2.31–17.55 |
| Modified Marshall score ≥ 2 | 0.000 | 4.41 | 1.74–11.19 |
| SIRS ≥ 2 | 0.045 | 2.28 | 0.98–5.32 |
| b. | |||
| p | OR | IC 95% | |
| APACHE score > 8 | 0.000 | 5.40 | 2.19–13.32 |
| BISAP score > 2 | 0.006 | 5.85 | 1.65–20.72 |
| Atlanta (FOP) | 0.000 | 6.94 | 2.7–17.78 |
| Modified Marshall score > 2 | 0.000 | 5.43 | 2.18–13.53 |
| SIRS | 0.055 | 2.43 | 0.98–6.02 |
| APACHE Score | BISAP Score | Modified Atlanta | Modified Marshall | |||||
|---|---|---|---|---|---|---|---|---|
| Admission | 48 h | Admission | 48 h | Admission | 48 h | Admission | 48 h | |
| Se | 55 | 58 | 70 | 69 | 49 | 54 | 48 | 52 |
| S | 81 | 79 | 74 | 72 | 87 | 85 | 83 | 83 |
| PPV | 65 | 58 | 93 | 26 | 82 | 76 | 76 | 73 |
| NPV | 74 | 79 | 35 | 94 | 58 | 68 | 58 | 65 |
| LR | 2.89 | 2.82 | 2.76 | 2.49 | 3.77 | 3.72 | 2.77 | 3.13 |
| PTP | 65 | 59 | 35 | 26 | 82 | 76 | 76 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iniestra-Ayllón, O.F.; Morales-González, J.A.; Sánchez-Reyes, K.; Rodríguez-Negrete, E.V. Serum Albumin as an Early Predictor of Severity in Patients with Acute Pancreatitis. Gastroenterol. Insights 2025, 16, 17. https://doi.org/10.3390/gastroent16020017
Iniestra-Ayllón OF, Morales-González JA, Sánchez-Reyes K, Rodríguez-Negrete EV. Serum Albumin as an Early Predictor of Severity in Patients with Acute Pancreatitis. Gastroenterology Insights. 2025; 16(2):17. https://doi.org/10.3390/gastroent16020017
Chicago/Turabian StyleIniestra-Ayllón, Oscar Francisco, José Antonio Morales-González, Karina Sánchez-Reyes, and Elda Victoria Rodríguez-Negrete. 2025. "Serum Albumin as an Early Predictor of Severity in Patients with Acute Pancreatitis" Gastroenterology Insights 16, no. 2: 17. https://doi.org/10.3390/gastroent16020017
APA StyleIniestra-Ayllón, O. F., Morales-González, J. A., Sánchez-Reyes, K., & Rodríguez-Negrete, E. V. (2025). Serum Albumin as an Early Predictor of Severity in Patients with Acute Pancreatitis. Gastroenterology Insights, 16(2), 17. https://doi.org/10.3390/gastroent16020017





