Abstract
Inflammatory bowel diseases (IBDs) represent multifactorial chronic inflammatory conditions of the gastrointestinal tract. The main IBDs are Crohn’s disease (CD) and ulcerative colitis (UC). CD may cause perforation, stricture or transmural inflammation, which can occur discontinuously in the entire gastrointestinal tract (GIT). UC leads to mucosal inflammation as well as mucosal atrophy in the rectum and the colon. Innate immunity is considered the first line of defense against microbial invasion; among Toll-like receptors, TLR2 is the most important for defense against mycobacterial infection. TLR2 has been reported to have a lot of functions in infectious diseases and in other pathologies, such as chronic and acute inflammatory diseases. Alfa-Smooth Muscle Actin (α-SMA) is an important biomarker in IBDs. All myofibroblasts express α-SMA, which has been found to be upregulated in CD and UC. Paraformaldehyde-fixed intestinal tissues, from patients with CD and patients with UC, were analyzed by immunostaining for TLR2 and α-SMA. Our results showed that, in the samples obtained from UC patients with inflamed mucosa, TLR2-positive epithelial cells concentrated on the mucosal surface and scattered immune cells in the connective tissue; furthermore, numerous α-SMA-positive cells (subepithelial myofibroblasts) were detected in the lamina propria and around glands, while some myofibroblasts co-localizing with α-SMA and TLR2 could be inflammatory macrophages. In CD patients, TLR2-positive enterocytes and α-SMA-positive myofibroblasts in the lamina propria of the villus have been observed. In control samples, a low positivity to α-SMA and TLR2 was observed in subepithelial myofibroblasts and scattered immune cells of the lamina propria. These data showed the recall of α-SMA-positive myofibroblasts during the inflammatory state; in addition, TLR2 expression has been observed to change in the intestinal epithelium in IBDs, demonstrating that alterations in the innate system response may contribute to the pathogenesis of these diseases.
1. Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are two main pathologies of inflammatory bowel diseases (IBDs). IBDs affect a broad population, including pediatrics, adults, and seniors worldwide [1].
The IBDs are still increasing worldwide, with higher prevalence and incidence rates observed in westernized and industrialized countries [2].
Numerous studies show the presence of other chronic immune disorders associated with IBDs, such as arthritis, ankylosing spondylitis, erythema nodosum, inflammatory ocular disorders, psoriasis, and primary sclerosing cholangitis [3,4].
Crohn’s disease is characterized by transmural inflammation, involving any part of the gastrointestinal tract from the mouth to the anus. It most commonly affects the small intestine and the proximal part of the large intestine [5].
Ulcerative colitis only affects the innermost lining of the colon. The inflammation usually begins in the rectum and distal colon, but it may involve the entire colon up to the cecum and is called pancolitis [6].
IBDs’ main symptoms are abdominal pain, diarrhea, and rectal bleeding and are often incorrectly associated with irritable bowel syndrome. Complications may include stricture and blockage (bowel obstruction) and perforation [7] in CD, or megacolon in UC.
Although they both have an undetermined etiology, there are some factors, such as smoking, medications, diet, host microbiota, genetic susceptibility, environmental factors, and dysregulated immune system, that can increase the risk of developing the disease [8,9,10].
The intestinal immune system corresponds to the largest compartment of the innate and adaptive immune system, coming constantly in contact with microbes and food-derived antigens [11,12,13]. The intestinal mucosa lined by a layer of epithelial cells represents an interface between the organism and its environment [14].
The intestinal epithelium consists of different types of cells, such as enterocytes, the main and most frequent, goblet cells producing both mucus and peptides for epithelial growth and repair [15,16], neuroendocrine cells coordinating the neuro-immune response [17,18,19], and the immune innate cells, which protect the exposure to infection [20].
Intestinal epithelial cells (IECs) are maintained on a network of interconnected myofibroblasts, which produce molecules necessary for the basal membrane in addition to factors required for epithelial growth.
All these cells communicate and collaborate to regulate epithelial function and integrity and keep this barrier intact [12,16,21,22,23].
In chronic inflammatory diseases, there is a morphological, structural, and functional change in the cells themselves that are activated to defend the body.
Pattern recognition receptors (PRRs) are a key element of the immune system, including Toll-like receptors (TLRs). TLRs belong to the family of integral membrane glycoproteins type I [24,25,26]. The most important Toll-like receptor for defense against mycobacterial infection is Toll-like 2 (TLR2), which has been shown to have several functions in infectious diseases and other pathologies, such as chronic and acute inflammatory diseases, and it is also phylogenetically conserved [27,28,29,30,31]. Alfa-Smooth Muscle Actin (α-SMA) is an important biomarker in IBDs [32,33,34]. All myofibroblasts express α-Smooth Muscle Actin (α-SMA), which is a protein used for the assessment of activated fibroblasts in several tissues and organs. This study aimed to characterize α-SMA- and TLR2-expressing cells in the pathogenesis of IBD, such as CD and UC, to diagnose these diseases and give us information on their progression.
2. Materials and Methods
2.1. Endoscopy in IBD Diagnosis
In patients with clinical presentations suggesting IBD, the initial evaluation should include a colonoscopy with intubation and examination of the terminal ileum [35]. Colonoscopy with ileoscopy not only allows for direct visualization of the colon and terminal ileum but also allows for necessary biopsies to be performed. When IBD is suspected, two biopsy specimens from five sites, including the ileum and rectum, are recommended [36,37,38]. Biopsy specimens (2 mm diameter) should be obtained from both affected and normal-appearing mucosa in order to assess the severity of disease. Specimens from different locations should be labeled and submitted separately [35]. The combination of endoscopic and histologic features assists in IBD diagnosis, the differentiation of CD vs. UC, as well as in the exclusion of other disease entities with similar presentations (e.g., drug-induced colitis, infectious colitis, ischemic colitis, and segmental colitis-associated with diverticulosis). Classically described endoscopic findings in UC include edema, loss of vascularity, erythema, mucosal granularity and friability, erosions and ulcers, and pseudopolyps. Additionally, approximately 5% of patients may also have an area of isolated peri-appendiceal inflammation, commonly known as a cecal patch, which does not correlate with disease activity or clinical course [39,40]. While many of the classic findings of UC can also be seen in CD, three major endoscopic findings that can aid in distinguishing CD from UC are the presence of aphthous ulcers, cobblestoning, and discontinuous or “skip” lesions [41]. Although isolated involvement of the terminal ileum is highly suggestive of CD, “backwash ileitis” can occur in UC in the setting of pancolitis [42]. Mucosal biopsies with histologic examination, upper gastrointestinal (GI) and small bowel endoscopy, small bowel imaging, and serologic markers can further assist when diagnostic uncertainty remains (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6).
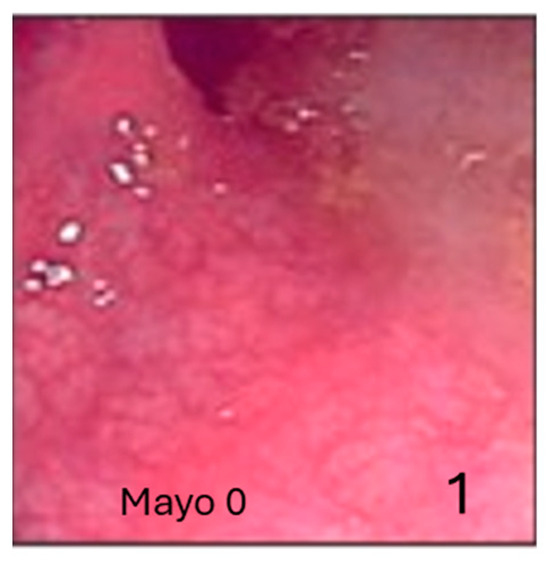
Figure 1.
The Mayo Endoscopic Score (ESM) is the most widely used endoscopic index to evaluate the stage of ulcerative colitis. Normal mucosa (1).
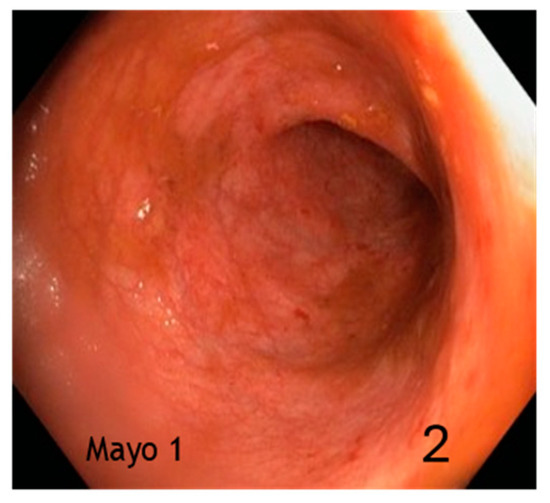
Figure 2.
Mild activity (erythema, thinned/distorted vascular reticulum) (2).
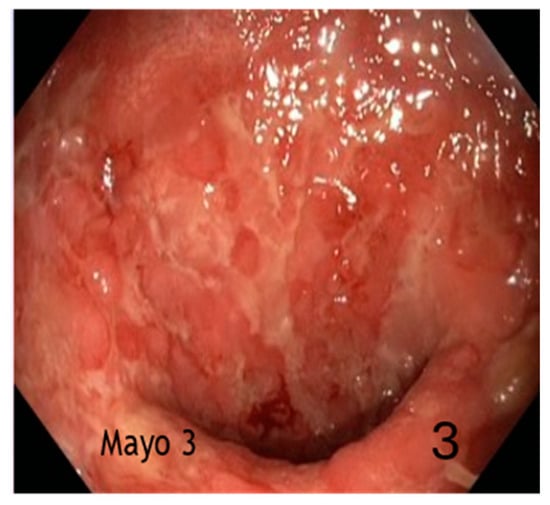
Figure 3.
Severe activity (spontaneous bleeding, extensive ulceration) (3).
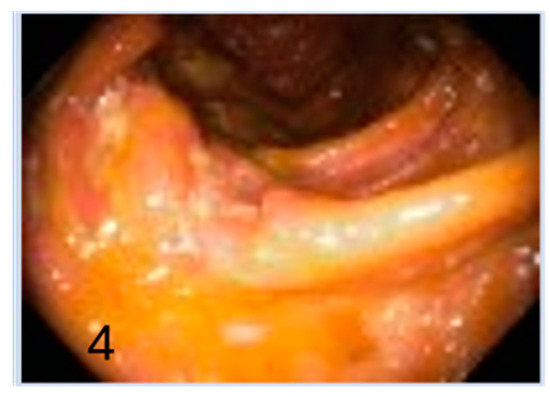
Figure 4.
Normal mucosa (4).
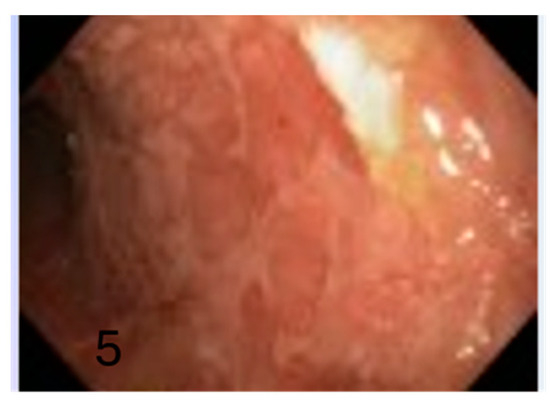
Figure 5.
Mild activity with ulcerated surfaces (5).
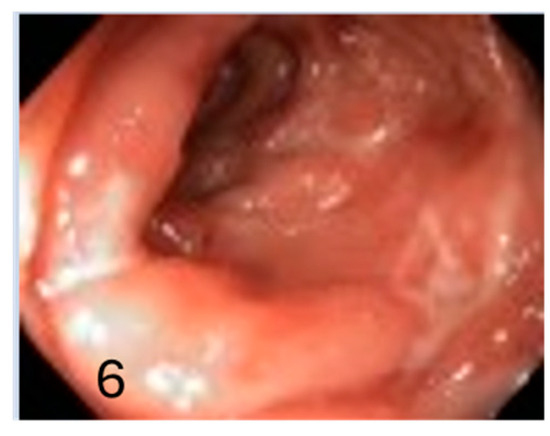
Figure 6.
Severe activity with inflamed mucosa due to Crohn’s disease (6).
2.2. Samples and Tissue Treatment
Using ileocolonoscopy, biopsies of inflamed ileum from 30 patients with CD and samples from the sigmoid colon of 30 patients with UC were taken. Furthermore, 15 controls underwent screening for family history of inflammatory bowel disease. The individuals ranged in age from 18 to 25 years old, with a mean age of 21.5 years. Microbiological, clinical, laboratory, and endoscopic results were used to diagnose CD and UC. Six months following the original diagnosis, these patients had another evaluation. Pathology’s duration and severity are comparable because patients are observed in a follow-up setting for a minimum of three years. Every sample, in both healthy and diseased circumstances, was taken precisely every 10 cm during the endoscopic biopsies that were conducted in the final 30 cm of the ileum and rectum. The Helsinki Declaration was followed when conducting the research. Each subject gave consent during the first consultation, all samples were gathered at the University Policlinic in Messina, Italy, and the study was approved by the ethical council under C.E. prot. 103/19.
2.3. Immunofluorescence
The 5–10 μm sections underwent deparaffinization, rehydration, and PBS rinsing [43]. Fetal bovine serum (F7524, Sigma-Aldrich, St. Louis, Missouri, USA) was added to the washed sections for 30 min to prevent nonspecific binding [44]. Toll-like receptor 2 (TLR2) monoclonal antibody was used together with anti-α-Smooth Muscle Actin (α-SMA) monoclonal antibody, and the sections were incubated overnight at 4 °C in a humid chamber. Following PBS washing, the sections were incubated for one hour with secondary antisera: Alexa Fluor 488 donkey anti-mouse IgG conjugated with FITC and Alexa Fluor 594 donkey anti-rabbit IgG conjugated with TRITC (Table 1). To prevent photobleaching, the sections were further cleaned, mounted using FluoromountTM Aqueous Mounting Medium (F4680 Sigma-Aldrich, St. Louis, MO, USA), and then coated with a coverslip. Experiments without primary antibodies were conducted as controls.

Table 1.
Summary of primary and secondary antibodies.
2.4. Laser Confocal Immunofluorescence
Zeiss LSMDUO confocal laser scanning microscopy with META module (Carl Zeiss Micro Imaging GmbH, Oberkochen, Germany) microscope LSM700 AxioObserver was used to analyze the sections and take pictures. The expression of both antibody signals was highlighted using Zen 2011 1.0 (LSM 700 Zeiss software) [45,46,47] built-in “colocalization view” to create a “colocalization” signal, which was then utilized to generate measurements for the fluorescent signal and scatter plot. Ten fields and five sections were investigated for every subject in order to gather data for statistical analysis. The ImageJ program was used to examine each field. After converting the acquired image to 8-bit, the background was removed, and the cellular positivity was highlighted using a mask and a “Threshold” filter. The cells were then counted using the “Analyze particles” plug-in.
2.5. Quantitative Analysis
Ten sections and twenty fields per sample were examined to collect data for the quantitative analysis. Using ImageJ software 1.53e, the cell positivity and quantity were evaluated [48]. Sigma Plot version 14.0 (Systat Software, San Jose, CA, USA) was used to count the number of cells positive for the tested antibodies. One-way ANOVA and Student’s t-test were used to assess the normally distributed data (Monforte et al., 2012). The mean values and standard deviations (SD) of the number of immunoreactive cells are reported: ** p ≤ 0.01 (Table 2). To assess the power analysis of the sample size, Jamovi version 2.4.7 (The jamovi project, 2024) was used, and, in particular, the extension “jpower”. A significance level of 0.01 (α) and a minimum desired power of 0.8 were set. Figure 7 shows the minimum sample size to confirm the hypothesis (n = 61), so our sample of 75 individuals can be considered valid.

Table 2.
Quantitative analysis results (mean values ± standard deviations; n = 75).
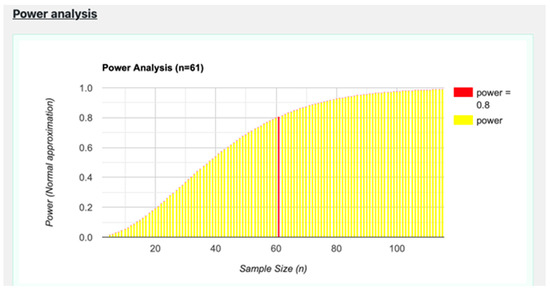
Figure 7.
Graph of the power analysis data.
3. Results
3.1. UC Disease
Our results showed, in the samples obtained from patients affected by UC disease, an inflamed mucosa, with TLR2-positive epithelial cells between goblet cells and scattered immune cells in the connective tissue (Figure 8); furthermore, numerous α-SMA-positive cells (subepithelial myofibroblasts) were found in lamina propria (Figure 8) and around glands, forming a pericryptal fibroblast sheath (PCFS) that surrounds the epithelial cells (Figure 9), with some connective myofibroblasts co-localizing with α-SMA and TLR2 (Figure 8). The control sample show a healthy colonic mucosa (Figure 10).
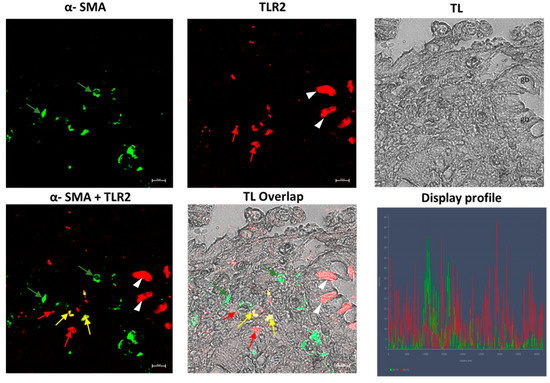
Figure 8.
UC human colon biopsy samples, 40×, scale bar 20 µm. TLR2-positive epithelial cells (arrowheads), between goblet cells (gb) and scattered immune cells (red arrows) in the connective tissue are present. Numerous positive cells (subepithelial myofibroblasts α-SMA positive (green arrows) in the lamina propria and in the connective tissue can be noted. Some myofibroblasts in the connective tissue (yellow arrows) co-localize with TLR2 and α-SMA. TL = transmitted light, to highlight tissue morphology.
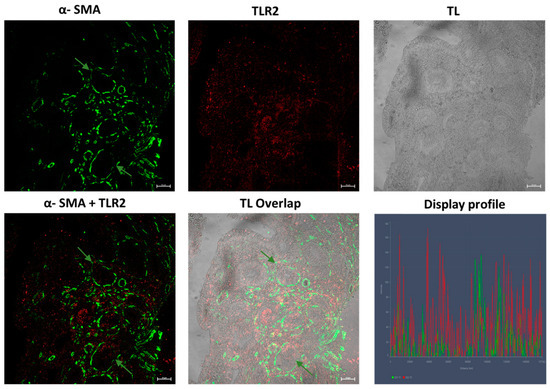
Figure 9.
UC human colon biopsy samples, 40×, scale bar 20 µm. α-SMA positive myofibroblasts (green arrows) forming a pericryptal fibroblast sheath around glands (green arrows) can be seen. TL = transmitted light, to highlight tissue morphology.
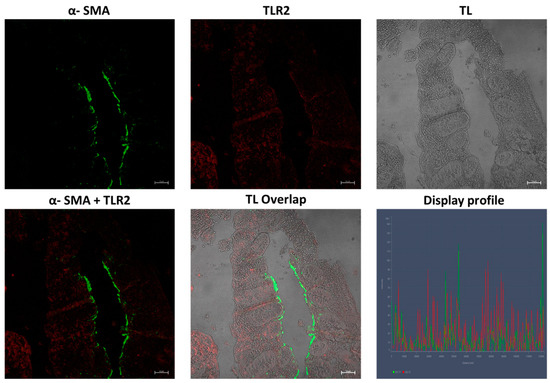
Figure 10.
Control group colon, 40×, scale bar 20 µm. Low reactivity to TLR2 and α-SMA is shown in healthy colonic mucosa. TL = transmitted light, to highlight tissue morphology.
3.2. Crohn’s Disease
In the samples obtained from Crohn’s disease patients, we observed TLR2-positive epithelial cells with greater positivity in the apical part of the cells (Figure 11); α-SMA-positive myofibroblasts were present in the lamina propria of the villus (Figure 11 and Figure 12) and were very numerous in the muscularis mucosae, forming a continuous layer (Figure 11). In control samples regarding both pathologies, a low positivity to α-SMA and TLR2 was observed, respectively, in subepithelial myofibroblasts, in scattered immune cells of the lamina propria and in epithelial cells. Furthermore, the fibroblasts were not seen forming a sheath around the glands (Figure 11 and Figure 12). The healthy control group show a low reactivity to the antibodies (Figure 13).
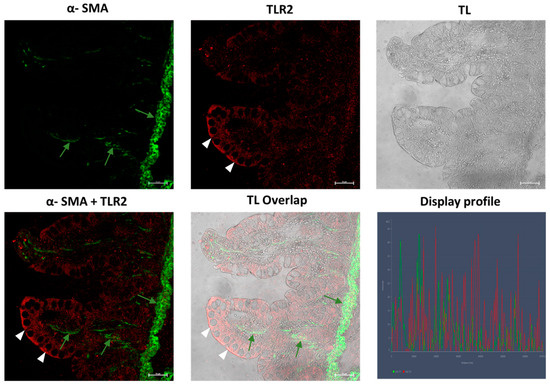
Figure 11.
Samples of inflamed ileum in CD patients, 40×, scale bar 20 µm. TLR2-positive enterocytes (arrowheads) are present. α-SMA-positive myofibroblasts are present in the lamina propria of the villus (green arrows), which form a continuous layer in the muscularis mucosae. TL = transmitted light, to highlight tissue morphology.
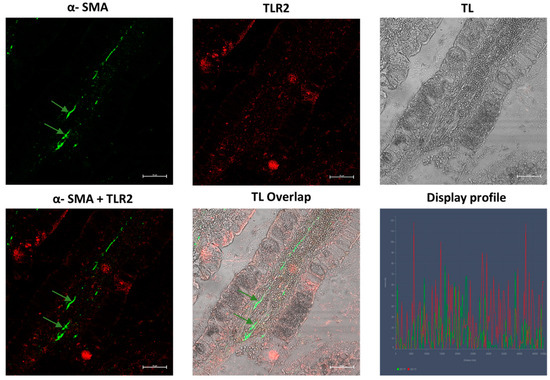
Figure 12.
Samples of inflamed ileum in CD patients, 40×, scale bar 20 µm. α-SMA-positive myofibroblasts are present in the lamina propria of the villus (green arrows). TL = transmitted light, to highlight tissue morphology.
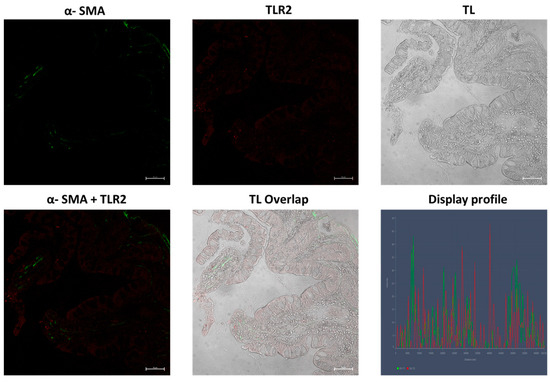
Figure 13.
Control group ileum, 40×, scale bar 20 µm. Low reactivity to TLR2 and α-SMA is shown in samples of the healthy gut. TL = transmitted light, to highlight tissue morphology.
4. Discussion
The results demonstrated that TLR2 and α-SMA were highly expressed in IBDs, respectively, in epithelial and stromal cells, if compared to intestinal control samples.
The intestinal epithelium acts as a barrier regulating interactions between the luminal contents and the underlying immune system.
During IBD pathogenesis, the intestinal epithelial barrier (IEB) is damaged by injuries and infections, which can give rise to chronic inflammation [49].
The epithelial cells that participate in mucosal barrier function are goblet cells (GCs) producing mucus, enterochromaffin cells secreting neuropeptides, and the main and most frequent cells, enterocytes, which may be involved in immune defense [50].
In our previous studies, we demonstrated the interaction between these different types of cells with the immune cells, to maintain both the integrity of the intestinal mucosa and, in some cases, the inflammatory state [12,16].
These interactions are complicated due to the numerous molecules that come into play, and many mechanisms are still poorly understood (Rescigno 2011; Allaire et al., 2018; Kayama et al., 2020).
The intestinal epithelial cells perform numerous functions, such as absorption, digestion, and secretion; in addition, these cells are directly involved in various immune processes, regulating the mucosal immune responses.
Indeed, numerous studies demonstrated that IECs produce constitutively, or by induction, cytokines, chemokines, and immunologically active mediators [51,52]
Helena Tlaskalová-Hogenová et al. (2004) showed the expression of CD14 and Toll-like receptors in the intestinal epithelial cells, thus demonstrating their active participation in the intestinal immune defense [53].
Several Toll-like receptors have been detected in both IECs and stromal tissue cells in the small and large intestines of mice and humans [54]. Moreover, it has been observed that the expression of TLR2, 4, 8, and 9 genes is upregulated in patients with active UC [27].
In a mouse model, Inoue et al., 2017 found that the postnatal expression of TLR2 and TLR4 in intestinal epithelial cells (IECs) is dynamic and depends on the presence of commensal intestinal microbiota [55].
Numerous studies report changes in the gut microbiome in IBD, such as increases in the major phylotype Proteobacteria and a decrease in Firmicutes [27,56].
Microbiota could regulate TLR expression [57], stimulating IECs to produce antimicrobial proteins that can kill Gram-positive bacteria. Indeed, the activated TLR signaling pathways can have a protective function against the invasion of intestinal pathogens.
Investigating this link between the microbiome, TLR2 expression and intestinal inflammation could be useful for improving the health status of IBD patients. In this study, TLR2-positive IECs have been shown in both IBDs; it has been observed that in UC, the positivity to TLR2 is expressed in the entire cell, while in CD, the cells present an apical positivity.
We can hypothesize that the expression of TLR2 may depend on the cells’ involvement in the disease at that moment. In our previous research on the rabbit corneal epithelium, we demonstrated that the increased expression of TLR2 on the epithelial cell surface supports the hypothesis that TLR2 may help to regulate immune responses by acting as the first line of defense [58].
Furthermore, TLR2-positive stromal cells can be considered immunologically active cells, such as macrophages producing mediators that are involved in epithelial physiology and dendritic cells, which represent the link between innate and adaptive immunity [59].
Intestinal mucosa subepithelial myofibroblasts are interconnected with epithelial cells; indeed, these cells produce molecules necessary for epithelial growth such as cytokines and chemokines [51,60].
Our results showed numerous α-SMA-positive subepithelial myofibroblasts in lamina propria of the samples obtained by IBD patients; α-SMA is a marker of activated myofibroblasts that presents a hybrid phenotype between fibroblasts and smooth muscle cells (SMCs) [61,62,63,64,65].
Also, in this case, we found positivity distributed differently in the two pathologies. In Crohn’s disease, α-SMA-positive myofibroblasts were present in the lamina propria of the villus and in the muscularis mucosae, forming a continuous layer.
This strong positivity in the muscularis mucosae can demonstrate the presence of chronic transmural inflammation due to excessive and abnormal deposition of extracellular matrix produced by activated myofibroblasts [19,32].
Chronic transmural inflammation can lead to the formation of strictures determining clinical intestinal obstructions [66].
The positivity to α-SMA found in patients suffering from UC disease is noteworthy. In this case, we demonstrated the presence of a pericryptal fibroblast sheath (PCFS) surrounding the intestinal glands.
Mutoh, in 2004, studied PCFS in colorectal carcinoma using an antibody against α-SMA, thus demonstrating that the occurrence of PCFS may represent the beginning of the formation of intestinal-type gastric carcinoma [67].
In agreement with Mutoh and colleagues, we believe that there is a strong interaction between epithelial and mesenchymal cells; extracellular matrix molecules play a fundamental role in epithelial cell behavior and proliferation [68,69,70].
Furthermore, the presence of some connective myofibroblasts co-localizing with α-SMA and TLR2 confirms the great dynamism of these cells that can differentiate in myofibroblasts or inflammatory macrophages in chronic inflammatory diseases [8,32].
Recent data estimate that, in Italy, about 250,000 people are suffering from inflammatory bowel disease. Unfortunately, there are no specific treatments for these diseases; therefore, basic research aimed at characterizing cells and molecules involved in the inflammatory state is particularly interesting for expanding knowledge regarding new therapeutic approaches. This study shows the molecular interactions between different cells such as epithelial, mesenchymal, and immune cells. In the case of intestinal damage, fibroblasts, for example, are activated and support the infiltration and function of immune cells, while during repair, they re-epithelialize the tissue. For this reason, further studies should clarify their plasticity, their role during inflammation and regeneration, and their potential usefulness in the diagnosis and/or therapy of intestinal disorders.
Author Contributions
Conceptualization, A.M. (Anthea Miller) and S.P. (Simona Pergolizzi); formal analysis, A.M. (Anthea Miller) and G.P.L.; investigation, A.M. (Anthea Miller) and G.P.L.; data curation, A.M. (Anthea Miller) and G.P.L.; writing—original draft preparation, A.M. (Anthea Miller); writing—review and editing, A.M. (Anthea Miller), G.R., M.K., G.M., S.P. (Socrate Pallio), A.M. (Alba Migliorato), G.C. and S.P. (Simona Pergolizzi); visualization, A.M. (Anthea Miller) and G.P.L.; supervision S.P. (Simona Pergolizzi). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University Policlinic (Messina, Italy) (C.E. prot. 103/19, 13 December 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Buie, M.J.; Quan, J.; Windsor, J.W.; Coward, S.; Hansen, T.M.; King, J.A.; Kotze, P.G.; Gearry, R.B.; Ng, S.C.; Mak, J.W. Global hospitalization trends for crohn’s disease and ulcerative colitis in the 21st century: A systematic review with temporal analyses. Clin. Gastroenterol. Hepatol. 2023, 21, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Jess, T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. UEG J. 2022, 10, 1113–1120. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Wajda, A.; Blanchard, J.F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: A population-based study. Gastroenterology 2005, 129, 827–836. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal manifestations of inflammatory bowel disease: Current concepts, treatment, and implications for disease management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Yangyang, R.Y.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. In Proceedings of the Seminars in Pediatric Surgery; Elsevier: Amsterdam, The Netherlands, 2017; Volume 26, pp. 349–355. [Google Scholar]
- McDowell, C.; Farooq, U.; Haseeb, M. Continuing Education Activity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470312/ (accessed on 1 July 2023).
- Ardizzone, S.; Armuzzi, A.; Caprioli, F.; Castiglione, F.; Danese, S.; Daperno, M.; Fantini, M.C.; Fries, W.; Principi, M.B.; Savarino, E. Timing of proper introduction, optimization and maintenance of anti-TNF therapy in IBD: Results from a Delphi consensus. Dig. Liver Dis. 2024, 56, 98–105. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Chierici, M.; Puica, N.; Pozzi, M.; Capistrano, A.; Donzella, M.D.; Colangelo, A.; Osmani, V.; Jurman, G. Automatically detecting Crohn’s disease and Ulcerative Colitis from endoscopic imaging. BMC Med. Inform. Decis. Mak. 2022, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Rochev, Y. IBD disease-modifying therapies: Insights from emerging therapeutics. Trends Mol. Med. 2023, 29, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Rizzo, G.; Favaloro, A.; Alesci, A.; Pallio, S.; Melita, G.; Cutroneo, G.; Lauriano, E.R. Expression of VAChT and 5-HT in Ulcerative colitis dendritic cells. Acta Histochem. 2021, 123, 151715. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, J. Innate lymphoid cells and intestinal inflammatory disorders. Int. J. Mol. Sci. 2022, 23, 1856. [Google Scholar] [CrossRef] [PubMed]
- Tlaskalová-Hogenová, H.; Štěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, Y. ENTERIC PARASITE INFECTION-INDUCED ALTERATION OF THE GUT MICROBIOTA REGULATES INTESTINAL GOBLET CELL BIOLOGY AND MUCIN PRODUCTION VIA TLR2 SIGNALLING. PhD Thesis, McMaster University, Hamilton, ON, Canada, 2022. [Google Scholar]
- Miller, A.; Cutroneo, G.; Lombardo, G.P.; D’Angelo, R.; Pallio, S.; Migliorato, A.; Fumia, A.; Favaloro, A.; Lauriano, E.R.; Pergolizzi, S. Association between neuropeptides and mucins in Crohn’s disease mucous cells. Acta Histochem. 2023, 125, 152115. [Google Scholar] [CrossRef] [PubMed]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.O.; Murugan, S.; Klose, C.S. Neuro-immune circuits regulate immune responses in tissues and organ homeostasis. Front. Immunol. 2020, 11, 511994. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Alesci, A.; Centofanti, A.; Aragona, M.; Pallio, S.; Magaudda, L.; Cutroneo, G.; Lauriano, E.R. Role of serotonin in the maintenance of inflammatory state in crohn’s disease. Biomedicines 2022, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Kałużna, A.; Olczyk, P.; Komosińska-Vassev, K. The role of innate and adaptive immune cells in the pathogenesis and development of the inflammatory response in ulcerative colitis. J. Clin. Med. 2022, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of intestinal epithelial cells properties and functions by amino acids. BioMed Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef] [PubMed]
- Ternet, C.; Kiel, C. Signaling pathways in intestinal homeostasis and colorectal cancer: KRAS at centre stage. Cell Commun. Signal 2021, 19, 31. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Santacroce, G.; Rossi, C.M.; Broglio, G.; Lenti, M.V. Role of mucosal immunity and epithelial–vascular barrier in modulating gut homeostasis. Intern. Emerg. Med. 2023, 18, 1635–1646. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Silvestri, G.; Kuciel, M.; Zuwała, K.; Zaccone, D.; Palombieri, D.; Alesci, A.; Pergolizzi, S. Immunohistochemical localization of Toll-like receptor 2 in skin Langerhans’ cells of striped dolphin (Stenella coeruleoalba). Tissue Cell 2014, 46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Fumia, A.; D’Angelo, R.; Mangano, A.; Lombardo, G.P.; Giliberti, A.; Messina, E.; Alesci, A.; Lauriano, E.R. Expression and function of toll-like receptor 2 in vertebrate. Acta Histochem. 2023, 125, 152028. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like receptors and inflammatory bowel disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Fumia, A.; Lauriano, E.R. Neuronal regeneration: Vertebrates comparative overview and new perspectives for neurodegenerative diseases. Acta Zool. 2022, 103, 129–140. [Google Scholar] [CrossRef]
- Alesci, A.; Cicero, N.; Fumia, A.; Petrarca, C.; Mangifesta, R.; Nava, V.; Lo Cascio, P.; Gangemi, S.; Di Gioacchino, M.; Lauriano, E.R. Histological and chemical analysis of heavy metals in kidney and gills of boops boops: Melanomacrophages centers and rodlet cells as environmental biomarkers. Toxics 2022, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Spaink, H.P. The role of TLR2 in infectious diseases caused by mycobacteria: From cell biology to therapeutic target. Biology 2022, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Fumia, A.; Calabrò, C.; Lo Cascio, P.; Lauriano, E.R. Mast cells in goldfish (Carassius auratus) gut: Immunohistochemical characterization. Acta Zool. 2023, 104, 366–379. [Google Scholar] [CrossRef]
- Severi, C.; Sferra, R.; Scirocco, A.; Vetuschi, A.; Pallotta, N.; Pronio, A.; Caronna, R.; Di Rocco, G.; Gaudio, E.; Corazziari, E. Contribution of intestinal smooth muscle to Crohn’s disease fibrogenesis. Eur. J. Histochem. EJH 2014, 58, 2457. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Lin, S.-N.; Rieder, F.; Chen, M.-H.; Zhang, S.-H.; Mao, R. Noncoding RNAs as promising diagnostic biomarkers and therapeutic targets in intestinal fibrosis of Crohn’s disease: The path from bench to bedside. Inflamm. Bowel Dis. 2021, 27, 971–982. [Google Scholar] [CrossRef]
- Tavares de Sousa, H.; Magro, F. How to Evaluate Fibrosis in IBD? Diagnostics 2023, 13, 2188. [Google Scholar] [CrossRef] [PubMed]
- Shergill, A.K.; Lightdale, J.R.; Bruining, D.H.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 2015, 81, 1101–1121. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Shanahan, F.; Anton, P.A.; Weinstein, W.M. Patchiness of mucosal inflammation in treated ulcerative colitis: A prospective study. Gastrointest. Endosc. 1995, 42, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Mowat, C.; Cole, A.; Windsor, A.L.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571–607. [Google Scholar] [CrossRef] [PubMed]
- Waye, J.D. The role of colonoscopy in the differential diagnosis of inflammatory bowel disease. Gastrointest. Endosc. 1977, 23, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Bakman, Y.; Katz, J.; Shepela, C. Clinical significance of isolated peri-appendiceal lesions in patients with left sided ulcerative colitis. Gastroenterol. Res. 2011, 4, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Haens, G.; Geboes, K.; Peeters, M.; Baert, F.; Ectors, N.; Rutgeerts, P. Patchy cecal inflammation associated with distal ulcerative colitis: A prospective endoscopic study. Am. J. Gastroenterol. 1997, 92, 1275–1279. [Google Scholar] [PubMed]
- Pera, A.; Bellando, P.; Caldera, D.; Ponti, V.; Astegiano, M.; Barletti, C.; David, E.; Arrigoni, A.; Rocca, G.; Verme, G. Colonoscopy in inflammatory bowel disease: Diagnostic accuracy and proposal of an endoscopic score. Gastroenterology 1987, 92, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Haskell, H.; Andrews Jr, C.W.; Reddy, S.I.; Dendrinos, K.; Farraye, F.A.; Stucchi, A.F.; Becker, J.M.; Odze, R.D. Pathologic features and clinical significance of “backwash” ileitis in ulcerative colitis. Am. J. Surg. Pathol. 2005, 29, 1472–1481. [Google Scholar] [CrossRef]
- Di Mauro, D.; Gaeta, R.; Arco, A.; Milardi, D.; Lentini, S.; Runci, M.; Rizzo, G.; Magaudda, L. Distribution of costameric proteins in normal human ventricular and atrial cardiac muscle. Folia Histochem. Cytobiol. 2009, 47, 605–608. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Aragona, M.; Alesci, A.; Cascio, P.L.; Pergolizzi, S. Toll-Like Receptor 2 and α-Smooth Muscle Actin expressed in the tunica of a urochordate, Styela plicata. Tissue Cell 2021, 71, 101584. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.R.; Żuwała, K.; Kuciel, M.; Budzik, K.A.; Capillo, G.; Alesci, A.; Pergolizzi, S.; Dugo, G.; Zaccone, G. Confocal immunohistochemistry of the dermal glands and evolutionary considerations in the caecilian, Typhlonectes natans (Amphibia: Gymnophiona). Acta Zool. 2016, 97, 154–164. [Google Scholar] [CrossRef]
- Ventura Spagnolo, E.; Mondello, C.; Di Mauro, D.; Vermiglio, G.; Asmundo, A.; Filippini, E.; Alibrandi, A.; Rizzo, G. Analysis on sarcoglycans expression as markers of septic cardiomyopathy in sepsis-related death. Int. J. Leg. Med. 2018, 132, 1685–1692. [Google Scholar] [CrossRef]
- Zaccone, G.; Lauriano, E.R.; Capillo, G.; Kuciel, M. Air-breathing in fish: Air-breathing organs and control of respiration: Nerves and neurotransmitters in the air-breathing organs and the skin. Acta Histochem. 2018, 120, 630–641. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Capillo, G.; Cascio, P.L.; Lauriano, E.R. Rodlet cells in kidney of goldfish (Carassius auratus, Linnaeus 1758): A light and confocal microscopy study. Acta Histochem. 2022, 124, 151876. [Google Scholar] [CrossRef]
- Otte, M.L.; Tamang, R.L.; Papapanagiotou, J.; Ahmad, R.; Dhawan, P.; Singh, A.B. Mucosal healing and inflammatory bowel disease: Therapeutic implications and new targets. World J. Gastroenterol. 2023, 29, 1157. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [PubMed]
- McGhee, J.R.; Strober, W. Mucosal Immune Responses: An Overview. Mucosal Immunology 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27169/ (accessed on 1 July 2023).
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The Immunology of Mucosal Models of Inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef] [PubMed]
- Tlaskalová-Hogenová, H.; Štěpánková, R.; Hudcovic, T.; Tučková, L.; Cukrowska, B.; Lodinová-Žádnıková, R.; Kozáková, H.; Rossmann, P.; Bártová, J.; Sokol, D. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 2004, 93, 97–108. [Google Scholar] [CrossRef]
- Moossavi, S.; Rezaei, N. Toll-like receptor signalling and their therapeutic targeting in colorectal cancer. Int. Immunopharmacol. 2013, 16, 199–209. [Google Scholar] [CrossRef]
- Inoue, R.; Yajima, T.; Tsukahara, T. Expression of TLR2 and TLR4 in murine small intestine during postnatal development. Biosci. Biotechnol. Biochem. 2017, 81, 350–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Grasa, L.; Abecia, L.; Forcén, R.; Castro, M.; De Jalón, J.A.G.; Latorre, E.; Alcalde, A.I.; Murillo, M.D. Antibiotic-Induced Depletion of Murine Microbiota Induces Mild Inflammation and Changes in Toll-Like Receptor Patterns and Intestinal Motility. Microb. Ecol. 2015, 70, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Pergolizzi, S.; Lauriano, E.R.; Santoro, G.; Spataro, F.; Cimino, F.; Speciale, A.; Nostro, A.; Bisignano, G. TLR 2 activation in corneal stromal cells by Staphylococcus aureus-induced keratitis. APMIS 2015, 123, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Bland, P.W. Mucosal T cell-epithelial cell interactions. Mucosal T Cells 1998, 71, 40–63. [Google Scholar] [CrossRef]
- Walker, W.A. Development of the intestinal mucosal barrier. J. Pediatr. Gastroenterol. Nutr. 2002, 34, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Tsuneyoshi, M. Significance of pericryptal fibroblasts in colorectal epithelial tumors: A special reference to the histologic features and growth patterns. Hum. Pathol. 1993, 24, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W.; Mifflin, R.C.; Valentich, J.D.; Crowe, S.E.; Saada, J.I.; West, A.B. Myofibroblasts. I. Paracrine cells important in health and disease. Am. J. Physiol.-Cell Physiol. 1999, 277, C1–C19. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W.; Mifflin, R.C.; Valentich, J.D.; Crowe, S.E.; Saada, J.I.; West, A.B. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am. J. Physiol.-Cell Physiol. 1999, 277, C183–C201. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Perspective Article: Tissue repair, contraction, and the myofibroblast. Wound Repair. Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Geboes, K.; Rutgeerts, P. Medical therapy for Crohn’s disease strictures. Inflamm. Bowel Dis. 2004, 10, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, H.; Sakurai, S.; Satoh, K.; Tamada, K.; Kita, H.; Osawa, H.; Tomiyama, T.; Sato, Y.; Yamamoto, H.; Isoda, N. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004, 64, 7740–7747. [Google Scholar] [CrossRef] [PubMed]
- Simo, P.; Simon-Assmann, P.; Arnold, C.; Kedinger, M. Mesenchyme-mediated effect of dexamethasone on laminin in cocultures of embryonic gut epithelial cells and mesenchyme-derived cells. J. Cell Sci. 1992, 101, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Birchmeier, C.; Birchmeier, W. Molecular Aspects of Mesenchymal-Epithelial Interactions. Annu. Rev. Cell. Biol. 1993, 9, 511–540. [Google Scholar] [CrossRef]
- Kedinger, M.; Freund, J.-N.; Launay, J.F.; Simon-Assmann, P.; Sanderson, I.R.; Walker, W.A. Cell interactions through the basement membrane in intestinal development and differentiation. Dev. Gastrointest. Tract. 2000, 83–102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).