Abstract
Background: Lysosomal acid lipase deficiency (LAL-D) is a rare genetic disease associated with the deregulation of lipid metabolism, leading to atherosclerosis, dyslipidemia, and hepatic steatosis, with potential progression to cirrhosis. Our study aims to assess the role of LAL-D in the setting of cryptogenic liver disease. Methods: A large multicenter cross-sectional study was conducted, which included 135 patients with cryptogenic liver disease from four liver centers in Brazil. All patients were submitted to the investigation of LAL enzyme activity on dried blood spots. Results: Three patients (two female) presented levels of LAL below the reference limit, compatible with LAL-D (2.2%). They had a mean age of 43.9 ± 10.1 years and a mean body-mass index (BMI) of 23.1 ± 1.7 kg/m2. The mean serum levels of glucose, HDL-cholesterol, and triglycerides were 89.7 ± 3.2, 21.7 ± 3.2, and 206.7 ± 25.5 mg/dL, respectively. All patients had duodenal polyposis with xanthomatous macrophages. LAL-D investigation should be considered for individuals with chronic liver disease of an unknown etiology, especially with a normal BMI, high triglycerides, and low-HDL-cholesterol levels. The identification of LAL-D patients is extremely important since enzyme replacement therapy with Sebelipase Alfa significantly increases their survival.
1. Introduction
Cryptogenic chronic hepatitis is a form of progressive liver disease that is characterized by a lack of explanation of its etiology. It is associated with the progression of end-stage liver disease, the development of hepatocellular carcinoma, and the need for liver transplantation [1,2]. It is estimated that 5.4% of patients with chronic liver disease present with an undefined etiology, while cryptogenic cirrhosis (CC) is diagnosed in 5–30% of patients with cirrhosis and 3–14% of those on the liver transplant list [1,2,3]. In most cases, potential underlying etiologies for CC include the progression of non-alcoholic steatohepatitis (NASH) to advanced fibrosis, clinically silent autoimmune hepatitis, occult viral hepatitis, alcohol-related liver injury, and alpha-1 antitrypsin deficiency [4,5,6]. In addition to these conditions, lysosomal acid lipase deficiency (LAL-D) can histologically mimic non-alcoholic fatty liver disease (NAFLD) and also falls within the spectrum of cryptogenic liver disease.
LAL-D is a rare autosomal recessive systemic lysosomal storage disorder caused by mutations to the LIPA gene, which is located on chromosome 10q23.2. These mutations result in the absence or partial deficiency of the lysosomal acid lipase (LAL) enzyme [7,8,9,10]. This enzyme plays a crucial role in lipid metabolism by hydrolyzing cholesterol esters and triglycerides in lysosomes, leading to the production of free cholesterol and free fatty acids. Due to impaired enzyme activity, cholesterol and triglyceride esters accumulate in the lysosomes of various cells throughout the body [7,11]. The disease spectrum ranges from the childhood phenotype, known as Wolman’s disease, which is the most severe form with a maximum one-year survival without treatment, to the less severe form affecting the young and adults, known as cholesterol ester storage disease (CESD) [12]. Clinically, the most significant manifestations include dyslipidemia, early atherosclerosis, liver disease that can progress to cirrhosis, and organ dysfunction [7,8,9,10,11,12].
Recently, the supplementation of Sebelipase Alfa has been associated with improved survival, growth, and liver function, offering a lifeline to children with rapidly progressive LAL-D [13]. The investigation of LAL-D in patients with chronic liver disease is not routinely performed in most centers, and there is a scarcity of data regarding the value of this strategy. Therefore, the aim of this study was to evaluate whether investigating LAL-D in patients with liver disease of unknown etiology is justified.
2. Materials and Methods
2.1. Clinical Design and Patients Selection
This is a Brazilian multicenter cross-sectional study which included patients with presumed cryptogenic liver disease who were followed at the outpatient liver units of the Hospital das Clínicas of the University of São Paulo School of Medicine (HCFMUSP), the Division of Gastroenterology (Gastrocentro) of the University of Campinas (UNICAMP), the School of Medicine of Ribeirão Preto of the University of Sao Paulo (FMRP-USP), and the Instituto Alfa de Gastroenterologia of the Hospital das Clínicas of the Federal University of Minas Gerais (UFMG) between October 2016 and November 2018. The STROBE statement for reporting observational studies was followed [14].
Patients ≥ 18 years old with presumed cryptogenic chronic hepatitis or who had undergone liver transplantation due to cryptogenic cirrhosis were evaluated for inclusion in this study. The exclusion criteria were any other potential cause of chronic liver disease, such as significant alcohol intake (>140 g/week), chronic hepatitis B or C, NASH seen on liver biopsy or the explant, human immunodeficiency virus (HIV) infection, autoimmune hepatitis, hemochromatosis, Wilson’s disease, alpha-1 antitrypsin deficiency, biliary liver diseases, previous exposure to potentially hepatotoxic medications and compounds, patients who did not undergo adequate laboratory and/or imaging and/or histological investigation, the absence of LAL activity measurement on a dried blood spot (DBS), or refusal to participate in the study.
Clinical and laboratory data, liver histology findings, and liver imaging tests were collected from medical records and reviewed by hepatologists in order to assess the presence of any exclusion criteria. Current and previous alcohol intake, as well as the use of potentially hepatotoxic medications, teas, herbs, and supplements, were assessed to exclude alcoholic liver disease and drug/herbal-induced liver injury, respectively. Serum markers of hepatitis B and C (anti-HCV, HBsAg, and anti-HBc), the anti-HIV antibody, serum anti-nuclear antibody, smooth muscle antibody, anti-mitochondrial antibody, anti-liver kidney microsomal antibody type 1, serum copper, ceruloplasmin, ferritin, and transferrin saturation, and alpha-1 antitrypsin levels were double-checked. Liver imaging tests were also evaluated for the exclusion of biliary and vascular etiology. The new definition of metabolic dysfunction-associated fatty liver disease (MAFLD) was accessed to establish “positive criteria” for the diagnosis of MAFLD [15]. Patients submitted to liver transplantation had their explant analyzed by liver pathologists in order to include only those whose histopathology review was unable to define the etiology of the liver disease that led to cirrhosis.
2.2. Study Variables
Demographic and anthropometric data were obtained (age, gender, weight, height, body mass index, BMI) as well as their comorbidities (diabetes mellitus, systemic arterial hypertension, and dyslipidemia).
Serum biochemistry included the following: fasting glucose, total cholesterol, and fractions, triglycerides. Liver histology (when available from liver biopsy or after liver transplantation) was evaluated by an experienced liver pathologist in order to clarify the etiology of liver disease. The diagnosis of LAL-D was made through deficient LAL activity, LIPA gene mutations, and/or pathologic liver biopsy findings [10,16,17,18].
2.3. Lysosomal Acid Lipase Enzyme Activity
A sample of about 5 mL was collected from peripheral venous access, pipetted (4 drops) on filter paper, and a dried blood spot (DBS). The material was referred randomly for analysis at Seattle Children’s Hospital, USA, or Afip (Research Incentive Fund Association), Brazil. LAL activity was measured via a highly specific test using lalistat 2, a specific LAL inhibitor, at both laboratories. In Seattle, an in-house methodology was considered. In the Afip laboratory, the methodology described by Hamilton was used [18]. The dosage of LAL activity was provided by Alexion Pharmaceuticals (New Haven, Connecticut, USA). Patients with LAL-D were submitted to the DNA sequencing of the acid lipase gene (LIPA) as follows: genomic DNA was isolated from the sample, and exons of the gene were amplified enzymatically and analyzed using automated sequencing. DNA segments were tested using multiple forward and reverse sequence runs to obtain a consensus sequence. The resulting sequence reads were blasted against the human LIPA genomic sequence (GenBank accession NG_008194). Multiple sequence runs of the same DNA strand were used to confirm positive or negative findings.
2.4. Statistical Analysis
Descriptive statistics (means, standard deviations, minimum, maximum, and median values) were computed. Given that, out of the 135 patients included in this study, only three had LAL-D detected, and no formal statistical analysis was considered for comparisons. The “R” software package (R Core Team-2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses.
3. Results
We evaluated 295 patients with presumed cryptogenic liver disease. A total of 160 patients met exclusion criteria and were removed from analyses (i.e., incomplete laboratory/imaging tests investigation: 43 patients; other liver diseases: 68 patients; lysosomal acid lipase activity not collected on DBS: 49 patients). The study population consisted of 135 patients, and 3 of them (2.2%) were diagnosed with LAL-D. Figure 1 shows the flowchart of the study population enrollment.
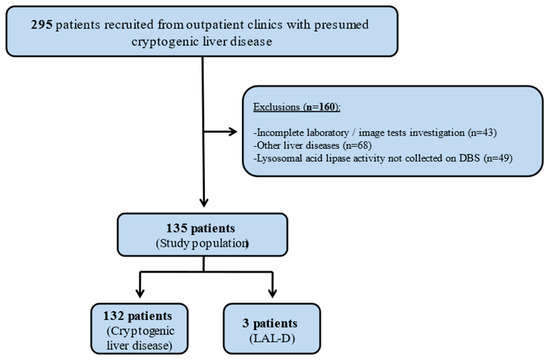
Figure 1.
Flowchart of study population enrollment. DBS: dried blood spot; LAL-D: lysosomal acid lipase deficiency.
Among the main causes of patient exclusion due to other diagnoses, non-alcoholic steatohepatitis was observed in 23 patients (33.8%) submitted to liver biopsy. Hereditary hemochromatosis was detected in 7 patients, biliary diseases in 7 patients, and excessive alcohol consumption in 7 patients. The most frequently omitted laboratory tests for investigating liver disease, which led to patient exclusion from the study, were alpha-1 antitrypsin/protein electrophoresis and ceruloplasmin dosage, respectively. These data are described in Figure 2.
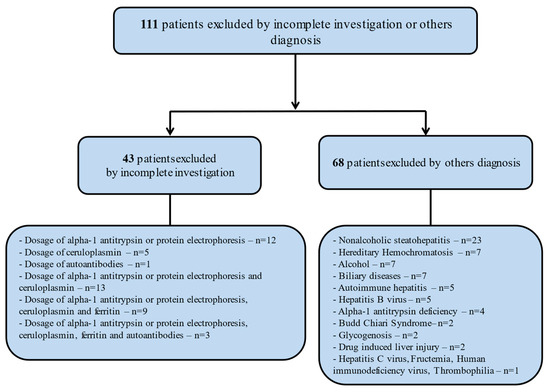
Figure 2.
Flowchart of patient exclusion.
The anthropometric, clinical, and biochemical characteristics of patients with LAL-D are shown in Table 1, and the data of patients with cryptogenic liver disease are shown in Table 2. Three patients (two female) presented values of LAL below the reference limit (2.2%) that were compatible with LAL-D. Their mean age was 43.9 ± 10.1 years, and their mean BMI was 23.1 ± 1.7 kg/m2. Regarding laboratory tests, their mean serum glucose, HDL-cholesterol, and triglycerides were 89.7 ± 3.2, 21.7 ± 3.2, and 206.7 ± 25.5 mg/dL, respectively. The mean values of total cholesterol and LDL-cholesterol were 165.6 ± 32.1 and 105 ± 33.9 mg/dL. They had normal blood pressure, and all of them had duodenal polyposis with xanthomatous macrophages observed at upper gastrointestinal endoscopy and histology. This finding was associated with malabsorption syndrome in one of the patients.

Table 1.
Clinical, laboratory analysis and characteristics of patients with LAL-D.

Table 2.
Clinical and laboratory analysis of patients with cryptogenic liver disease.
On the other hand, patients with cryptogenic liver disease (n = 132) were older (mean age 51.5 ± 14.6 years), with a higher weight (mean BMI 27.6 ± 5.9), higher serum glucose levels (110 ± 354.7 mg/dL) and HDL levels (48.1 ± 17 mg/dL) and lower triglycerides levels (114 ± 376.7 mg/dL). The mean values of total cholesterol and LDL-cholesterol were 165.3 ± 45.9 and 92.6 ± 38.4 mg/dL. In our study, 58 patients (43.9%) with cryptogenic liver disease underwent a liver biopsy for diagnostic clarification, with the majority of them already exhibiting advanced fibrosis (58.6%).
Among the patients with cryptogenic liver disease, thirty-seven patients met newly suggested criteria for metabolic dysfunction-associated steatotic liver disease (MASLD). The diagnosis of steatosis for 19 patients was based on a biopsy and for 18 on liver ultrasound. Among patients submitted to liver biopsy, all had steatosis in more than 5% of hepatocytes.
Comparison between Patients with MASLD and Non-MASLD Chronic Cryptogenic Hepatitis
One hundred and thirty-two patients were subdivided into two groups as follows: the MASLD group, consisting of 37 patients (28.1%), and the non-MASLD group, comprising 95 patients (71.9%). The mean age and gender distribution were similar between groups (51.7 ± 13.2 years, 67% female in the MASLD group, and 51.4 ± 15.1 years, 61% female in the non-MASLD group). Notably, there were significant differences in various metabolic markers between the two groups. These differences included BMI (31.5 ± 5.3 kg/m2 in the MASLD group vs. 26 ± 5.5 kg/m2 in the non-MASLD group, p < 0.001), glucose levels (109.9 ± 35.6 mg/dL in the MASLD group vs. 110 ± 60.5 mg/dL in the non-MASLD group, p = 0.04), HDL (41.4 ± 8.9 mg/dL in the MAFLD group vs. 50.8 ± 18.7 mg/dL in the non-MASLD group, p < 0.002), LDL (108.3 ± 44.2 mg/dL in the MAFLD group vs. 86.4 ± 34.1 mg/dL in the non-MASLD group), and triglycerides (165.3 ± 97.4 mg/dL in the MASLD group vs. 93.1 ± 54.5 mg/dL in the non-MASLD group, p < 0.001). Liver biopsies were performed on 18 patients (49%) in the MASLD group and 38 patients (40%) in the non-MASLD group (p = 0.48), with no significant difference in the rate of liver biopsies between the two groups.
4. Discussion
In our study, among the 135 patients with chronic liver disease of an unknown etiology, three adult patients (2.2%) receiving care at tertiary reference centers received a diagnosis of LAL-D. These patients were characterized by their youth, leanness, absence of systemic arterial hypertension or diabetes, and higher serum triglyceride values, as well as lower HDL levels when compared to the group of patients with cryptogenic liver disease. Notably, during study recruitment, we rigorously evaluated patients who were initially presumed to have cryptogenic liver disease and discovered that 68 (23%) of them had an underlying diagnosis of liver disease, which had not been initially detected. This finding emphasizes the importance of systematically excluding all potential etiologies of liver injury before classifying a patient as having cryptogenic liver disease. The primary diagnoses in these cases included non-alcoholic steatohepatitis, hereditary hemochromatosis, alcoholic liver disease, and biliary diseases.
The actual prevalence of LAL-D remains uncertain, with estimates ranging from 1 in 300,000 to 1 in 40,000, depending on ethnic and geographical factors [19,20]. A recent systematic review estimated the prevalence of LAL-D, using the allele frequency of c.894G>A in LIPA, as 1 in 160,000 (95% CI 1 in 65,025–761,652), although many other mutations have also been described [17,20]. Currently, the investigation of LAL-D is recommended in patients presenting with microvesicular or mixed hepatic steatosis, hepatomegaly, cholestasis, and those with dyslipidemia associated with elevated liver enzymes. LAL-D manifests as a rare disease with a broad spectrum of phenotypes, ranging from asymptomatic individuals with mildly elevated liver enzymes to those who have progressed to cirrhosis and portal hypertension. Due to its highly variable clinical presentation, physicians often overlook this diagnosis in cases of cryptogenic cirrhosis, potentially resulting in some patients undergoing liver transplantation or succumbing without a definitive diagnosis [7,19,21,22].
Data on patients with chronic cryptogenic hepatitis are often limited, making this a neglected population. Our results suggest that investigating LAL-D could enhance the etiological diagnosis of patients with cryptogenic liver disease, particularly in individuals with dyslipidemia (HDL < 40 mg/dL and triglycerides > 150 mg/dL) and a normal BMI. Furthermore, we demonstrated that the presence of duodenal polyposis, along with histological findings of xanthomatous macrophages, could raise suspicion of LAL-D, as it is associated with cholesterol deposition in the intestinal villi [7]. The primary rationale for including LAL-D screening in patients with cryptogenic liver disease is to enable early and appropriate treatment. The replacement of Sebelipase Alfa has been shown to improve liver inflammation and lipid profiles and likely slow progression to advanced liver fibrosis [23,24,25]. In real-life clinical practice, other rare liver diseases, such as alpha-1 antitrypsin deficiency and Wilson’s disease, are routinely investigated and treated under similar circumstances [1,26,27,28,29].
Interestingly, it is speculated that the majority of patients with cryptogenic cirrhosis are, in fact, individuals with NASH who have progressed to cirrhosis [4,5,30]. Despite some controversy regarding whether cryptogenic cirrhosis is similar to NASH cirrhosis [31,32,33], it is essential to recognize that many patients with cryptogenic cirrhosis share metabolic syndrome components with NASH patients [4,5,6,34]. In our study, we excluded 23 patients with a diagnosis of NASH based on liver biopsy evidence. Out of the 132 patients with cryptogenic liver disease, we observed that 21 patients presented with steatosis on liver biopsy, and 23 patients had hepatic steatosis on an abdominal ultrasound. If we adopt new MASLD criteria to classify these 132 patients with cryptogenic liver disease, 37 (28%) could be reclassified as having liver disease in relation to MASLD. This new proposal aims to standardize the diagnosis and facilitate the treatment of this disease, which has seen a significant increase in global incidence, both in young people and those over 60 years of age [35]. Our data align with the existing literature, as individuals classified as having cryptogenic cirrhosis often exhibit an altered metabolic profile, with most of them having MASLD as the cause of their liver disease [5,31,33,36,37].
Histological analysis plays a crucial role in etiological evaluation and serves as the gold standard examination for this purpose [30,31,36]. It can assist in the diagnostic confirmation and guide treatment in some cases of cryptogenic liver disease, elucidating up to 18% of cases without a defined etiology [36]. In our study, 58 patients (43.9%) with cryptogenic liver disease underwent a liver biopsy for diagnostic clarification. It is possible that some patients were not biopsied due to complications in advanced liver disease, such as ascites and an increased risk of bleeding [26]. Liver explant analysis may also aid in diagnosing patients with cryptogenic cirrhosis who have undergone liver transplantation, as most of them still exhibit residual MASLD.
This study has some limitations. The investigation of cryptogenic liver disease was not consistent across all liver centers, particularly with regard to liver biopsy, which may affect the percentage of patients classified as cryptogenic. A selection bias of diagnosed LAL-D patients cannot be ruled out, as all centers are referral healthcare facilities for hepatic diseases and liver transplantation. Therefore, these results should be validated in larger cohorts outside tertiary centers. Finally, it is difficult to address the role of genetic and ethnic backgrounds since the Brazilian population is highly mixed.
Histological analysis plays a crucial role in etiological evaluation and serves as the gold standard examination for this purpose [30,36,38]. It can assist in diagnostic confirmation and guide treatment in some cases of cryptogenic liver disease, elucidating up to 18% of cases without a defined etiology [36]. In our study, 58 patients (43.9%) with cryptogenic liver disease underwent a liver biopsy for diagnostic clarification. It is possible that some patients were not biopsied due to complications of advanced liver disease, such as ascites and an increased risk of bleeding [26]. Liver explant analysis may also aid in diagnosing patients with cryptogenic cirrhosis who have undergone liver transplantation, as most of them still exhibit residual steatosis.
This study has some limitations. The investigation of cryptogenic liver disease was not consistent across all liver centers, particularly with regard to liver biopsy, which could affect the percentage of patients classified as cryptogenic. A selection bias of diagnosed LAL-D patients cannot be ruled out, as all centers are referral healthcare facilities for hepatic diseases and liver transplantation. Therefore, these results should be validated in larger cohorts outside tertiary centers. Finally, it is difficult to address the role of genetic and ethnic backgrounds since the Brazilian population is highly miscegenated.
5. Conclusions
In conclusion, LAL-D is a rare and underdiagnosed condition. This study suggests that LAL activity should be assessed in patients with cryptogenic liver disease who exhibit dyslipidemia and a normal BMI. Our findings underscore the potential for establishing an etiological diagnosis in a significant proportion of patients with cryptogenic liver disease through a systematic evaluation protocol, with the majority of them having MASLD as their underlying cause.
Author Contributions
A.C.R.C., D.F.d.C.M., C.P.M.O. and M.G.P.: study concept and design, acquisition and interpretation of data, analysis drafting of the manuscript; P.M.Z.: acquisition of data and exam collection; M.C.-S., R.D.G., R.G.R.d.L., R.C.A., A.S.P.T.A., G.G.L.C., C.A.C. and M.J.N.: acquisition of data; A.Q.F. and F.J.C.: analysis drafting; M.C.-S. and R.D.G.: exam collection. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Clinics Hospitals of the University of Sao Paulo School of Medicine (HCFMUSP), the University of Campinas (UNICAMP), the Faculty of Medicine of Ribeirão Preto University of Sao Paulo (FMRP-USP), and the Federal University of Minas Gerais (UFMG) (numbers 1.754.690, 3.087.439, 2.485.354, 2.421.674, respectively).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are available upon request to the corresponding author.
Conflicts of Interest
The dosage of lysosomal acid lipase was provided by Alexion Pharmaceuticals, which had no role in the study design, data evaluation or manuscript writing. The authors declare no conflict of interest.
References
- Czaja, A.J. Cryptogenic chronic hepatitis and its changing guise in adults. Dig. Dis. Sci. 2011, 56, 3421–3438. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J.; Carpenter, H.A.; Santrach, P.J.; Moore, S.B.; Homburger, H.A. The nature and prognosis of severe cryptogenic chronic active hepatitis. Gastroenterology 1993, 104, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.B.; Walters, J.R.; Davies, A.P.; Paton, A. A 20-year prospective study of cirrhosis. Br. Med. J. (Clin. Res. Ed.) 1981, 282, 263–266. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Oelsner, D.H.; Iezzoni, J.C.; Hespenheide, E.E.; Battle, E.H.; Driscoll, C.J. Cryptogenic cirrhosis: Clinical characterization and risk factors for underlying disease. Hepatology 1999, 29, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.H.; Crespo, D.M. The spectrum expanded: Cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J. Hepatol. 2004, 40, 578–584. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Sanyal, A.J.; Harrison, S.A.; Ratziu, V.; Abdelmalek, M.F.; Diehl, A.M.; Caldwell, S.; Shiffman, M.L.; Schall, R.A.; et al. The conundrum of cryptogenic cirrhosis: Adverse outcomes without treatment options. J. Hepatol. 2018, 69, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.L.; Hülkova, H.; Bialer, M.G.; Desnick, R.J. Cholesteryl ester storage disease: Review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 2013, 58, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- Elleder, M.; Ledvinová, J.; Cieslar, P.; Kuhn, R. Subclinical course of cholesterol ester storage disease (CESD) diagnosed in adulthood—Report on two cases with remarks on the nature of the liver storage process. Virchows Arch. A Pathol. Anat. Histopathol. 1990, 416, 357–365. [Google Scholar] [CrossRef]
- Burke, J.A.; Schubert, W.K. Deficient activity of hepatic acid lipase in cholesterol ester storage disease. Science 1972, 176, 309–310. [Google Scholar] [CrossRef]
- Aslanidis, C.; Ries, S.; Fehringer, P.; Büchler, C.; Klima, H.; Schmitz, G. Genetic and biochemical evidence that CESD and Wolman disease are distinguished by residual lysosomal acid lipase activity. Genomics 1996, 33, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Dana, S.E.; Faust, J.R.; Beaudet, A.L. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J. Biol. Chem. 1975, 250, 8487–8496. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Breen, C.; Enns, G.M.; Deegan, P.B.; Honzík, T.; Jones, S.; Kane, J.P.; Malinova, V.; Sharma, R.; Stock, E.O.; et al. Clinical effect and safety profile of recombinant human lysosomal acid lipase in patients with cholesteryl ester storage disease. Hepatology 2013, 58, 950–957. [Google Scholar] [CrossRef]
- Frampton, J.E. Sebelipase Alfa: A Review in lysosomal acid lipase deficiency. Am. J. Cardiovasc. Drugs 2016, 16, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, F.; Vici, C.D.; Gentilucci, U.V.; Angelico, F.; Nobili, V.; Petta, S.; Valenti, L.; AISF Rare Diseases Committee. AISF update on the diagnosis and management of adult-onset lysosomal storage diseases with hepatic involvement. Dig. Liver Dis. 2020, 52, 359–367. [Google Scholar] [CrossRef]
- Cunha-Silva, M.; Mazo, D.F.C.; Corrêa, B.R.; Lopes, T.M.; Arrelaro, R.C.; Ferreira, G.L.; Rabello, M.I.; Sevá-Pereira, T.; Escanhoela, C.A.F.; Almeida, J.R.S. Lysosomal acid lipase deficiency leading to liver cirrhosis: A case report of a rare variant mutation. Ann. Hepatol. 2019, 18, 230–235. [Google Scholar] [CrossRef]
- Hamilton, J.; Jones, I.; Srivastava, R.; Galloway, P. A new method for the measurement of lysosomal acid lipase in dried blood spots using the inhibitor Lalistat 2. Clin. Chim. Acta 2012, 413, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Ž.; Guardamagna, O.; Nair, D.; Soran, H.; Hovingh, K.; Bertolini, S.; Jones, S.; Ćorić, M.; Calandra, S.; Hamilton, J.; et al. Lysosomal acid lipase deficiency—An under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis 2014, 235, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Brackley, S.M.; Gao, J.; Mann, J.P. The global prevalence and genetic spectrum of lysosomal acid lipase deficiency: A rare condition that mimics NAFLD. J. Hepatol. 2019, 70, 142–150. [Google Scholar] [CrossRef]
- Valayannopoulos, V.; Mengel, E.; Brassier, A.; Grabowski, G. Lysosomal acid lipase deficiency: Expanding differential diagnosis. Mol. Genet. Metab. 2016, 120, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Strebinger, G.; Müller, E.; Feldman, A.; Aigner, E. Lysosomal acid lipase deficiency—Early diagnosis is the key. Hepat. Med. 2019, 11, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Valayannopoulos, V.; Malinova, V.; Honzík, T.; Balwani, M.; Breen, C.; Deegan, P.B.; Enns, G.M.; Jones, S.A.; Kane, J.P.; Stock, E.O.; et al. Sebelipase alfa over 52 weeks reduces serum transaminases, liver volume and improves serum lipids in patients with lysosomal acid lipase deficiency. J. Hepatol. 2014, 61, 1135–1142. [Google Scholar] [CrossRef]
- Burton, B.K.; Balwani, M.; Feillet, F.; Barić, I.; Burrow, T.A.; Grande, C.C.; Coker, M.; Consuelo-Sánchez, A.; Deegan, P.; Di Rocco, M.; et al. A Phase 3 Trial of Sebelipase Alfa in lysosomal acid lipase deficiency. N. Engl. J. Med. 2015, 373, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Burton, B.K.; Marulkar, S.; Friedman, M.; Tripuraneni, R.; Furuya, K.N. [Poster] Long-term benefit of sebelipase alfa over 76 weeks in children and adults with lysosomal acid lipase deficiency (ARISE). Mol. Genet Metab. 2016, 120, S33. [Google Scholar] [CrossRef]
- Caldwell, S. Cryptogenic cirrhosis: What are we missing? Curr. Gastroenterol. Rep. 2010, 12, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.; Schilsky, M.L. Inherited metabolic disease of the liver. Curr. Opin. Gastroenterol. 2007, 23, 237–243. [Google Scholar] [CrossRef]
- Park, R.H.R.; Mccabe, P.; Fell, G.S.; Russell, R.I. Wilson’s disease in Scotland. Gut 1991, 32, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Hamesch, K.; Mandorfer, M.; Pereira, V.M.; Moeller, L.S.; Pons, M.; Dolman, G.E.; Reichert, M.C.; Schneider, C.V.; Woditsch, V.; Voss, J.; et al. Liver fibrosis and metabolic alterations in adults with alpha-1-antitrypsin deficiency caused by the Pi*ZZ Mutation. Gastroenterology 2019, 157, 705–719.e18. [Google Scholar] [CrossRef] [PubMed]
- De Lédinghen, V.; Combes, M.; Trouette, H.; Winnock, M.; Amouretti, M.; De Mascarel, A.; Couzigou, P. Should a liver biopsy be done in patients with subclinical chronically elevated transaminases? Eur. J. Gastroenterol. Hepatol. 2004, 16, 879–883. [Google Scholar] [CrossRef]
- Thuluvath, P.J.; Kantsevoy, S.; Thuluvath, A.J.; Savva, Y. Is cryptogenic cirrhosis different from NASH cirrhosis? J. Hepatol. 2018, 68, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.; Marchesini, G. Cryptogenic vs NASH-cirrhosis: The rose exists well before its name. J. Hepatol. 2018, 68, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Kantsevoy, S.; Thuluvath, A.J.; Savva, Y. Reply to: “NASH-related and cryptogenic cirrhosis similarities extend beyond cirrhosis”: Cryptogenic cirrhosis should not be equated with NASH cirrhosis based on UNOS data mining and Bayesian ‘doctrine of chances’. J. Hepatol. 2018, 69, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Cooksley, W.G.; Hanson, R.; Searle, J.; Halliday, J.W.; Powell, L.W. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatology 1990, 11, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Paik, J.; Reddy, R.; Bugianesi, E.; Trimble, G.; Younossi, Z.M. Prevalence and long-term outcomes of non-alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol. 2019, 19, 56. [Google Scholar] [CrossRef]
- Skelly, M.M.; James, P.D.; Ryder, S.D. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J. Hepatol. 2001, 35, 195–199. [Google Scholar] [CrossRef]
- Poonawala, A.; Nair, S.P.; Thuluvath, P.J. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: A case-control study. Hepatology 2000, 32, 689–692. [Google Scholar] [CrossRef]
- Czaja, A.J.; Carpenter, H.A. Optimizing diagnosis from the medical liver biopsy. Clin. Gastroenterol. Hepatol. 2007, 5, 898–907. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).