Abstract
Hepatitis C infection is a leading etiology of hepatic dysfunction and a major indication for liver transplantation due to the development of fibrosis, cirrhosis, and hepatocellular carcinoma. Nonalcoholic fatty liver disease (NAFLD) and, specifically, its subtype nonalcoholic steatohepatitis (NASH) is a rising cause of liver disease. It is predicted to surpass hepatitis C as a leading indication for transplant. The introduction of direct-acting antivirals (DAAs) decreased the prevalence of chronic hepatitis C infections, but the obesity epidemic and metabolic syndrome have increased the prevalence of NASH. Weight loss and dietary modifications are recommended NASH therapies, but unlike for hepatitis C, federally approved agents are lacking and currently under investigation. Clinical trials face many barriers in NASH treatment because of the difficulty of diagnosis and a lack of standardized and accurate clinical and histologic responses. Mortality and morbidity in NASH are heightened because of the presence of multiple comorbidities including cardiovascular disease, diabetes, and renal dysfunction. A liver transplant may be indicated, but a thorough screening of candidates, including a comprehensive cardiovascular assessment, is essential to ensuring successful outcomes pre- and post-transplant. Therapeutic agents for NASH are warranted before it becomes a significant and leading cause of morbidity and mortality worldwide.
1. Introduction
Chronic liver disease is estimated to affect 1.5 billion (109) people worldwide [1]. Etiologies include viral diseases, toxins, autoimmune conditions, and fatty liver diseases such as alcoholic liver disease and nonalcoholic fatty liver disease (NAFLD). Two of the most common causes of chronic liver disease are hepatitis C and NAFLD. These conditions have reversed in the last few years as the most prevalent indication for liver transplantation. This review focuses on the history, discovery, and development of hepatitis C and nonalcoholic steatohepatitis (NASH). It also includes current therapies; impacts on mortality and morbidity; the complexities of liver transplantation; and the concurrence of NASH, obesity, and metabolic syndrome.
2. Hepatitis C
2.1. Background
Hepatitis C is caused by infection with the hepatitis C virus (HCV), which is an RNA virus of the family Flaviviridae. HCV can lead to acute hepatitis C, from which 60–80% of patients develop a chronic form as the virus evades the host immune system [2]. Chronic hepatitis C infection provokes a chronic inflammatory process, which may lead to liver fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and death [3]. HCV is classified into 7 confirmed genotypes and 67 subtypes [4]. These genotypes influence the selection of appropriate antiviral therapies [5]. Based on 2015 data on the global HCV burden, it is estimated that about 71 million people worldwide are viremic, corresponding to a prevalence of 1% [6]. Public health awareness of HCV is low because many individuals with chronic HCV are unaware of their diagnosis. They are typically asymptomatic for an extended period and/or have not been tested for HCV [7]. HCV infection is a leading cause of chronic liver disease, is the main cause of HCC, and is a major indication for liver transplantation in Western countries [8]. Moreover, chronic HCV infections are linked to many extrahepatic manifestations (EHMs) [9].
2.2. Discovering HCV
In the mid-1970s, physicians documented cases of transfusion-associated hepatitis that were not due to hepatitis A, B, or any other known etiology. This phenomenon was termed non-A, non-B hepatitis (NANBH) [10,11,12]. Feinstone and colleagues were among the first to demonstrate this phenomenon in a group of patients with post-transfusion hepatitis [11]. Hepatitis A virus, hepatitis B virus, cytomegalovirus, and Epstein–Barr virus infections were excluded using serological markers. However, the authors suspected a then-unknown infectious etiology. Other investigators published similar findings during that time [12]. The newly identified disease was later identified as NANBH and was found to be accountable for up to 90% of post-transfusion hepatitis cases [13]. Studies continued to follow up on these patients, showing evidence that this disease caused chronic inflammatory changes at the histologic level in 50–60% of the patients, leading to liver fibrosis, cirrhosis, and HCC.
Over the following years, investigators provided increasing evidence that the infectious agent that caused NANBH was a small, enveloped viral agent. For example, they demonstrated that it induced specific changes in hepatocytes and was uninhibited by an 80 nm sized membrane filter, but it could be inactivated by chloroform [14]. The major breakthrough was in 1989 when Choo, Houghton, and colleagues discovered the genome of HCV [15]. They proposed that a low viral concentration was likely why prior studies failed to identify the HCV genome. With the use of a complementary DNA (cDNA) library, they were able to clone the viral agent responsible for NANBH. Then, they used Southern blot analysis to exclude genomic fragments from other species. With further experiments and confirmations, they reached a final proof, finding that the agent is indeed an RNA virus, which was later named HCV [15].
2.3. Genotypes
There are 7 known HCV genotypes, GT1, GT2, GT3, GT4, GT5, GT6, and GT7, and 67 confirmed subtypes [4]. A systematic review from 138 countries proposes that GT1 is the most predominant genotype globally at 49.1%, followed by GT3 at 17.9%, GT4 at 16.8%, GT2 at 11.0%, and both GT5 and GT6 at less than 5% [16]. However, relative prevalence differs. GT1 is predominant in North America, Latin America, and Europe, while GT4 is prevalent in Africa and the Middle East. GT7 was reported only once in four immigrants from the Democratic Republic of Congo in Canada [17]. In the Middle East, HCV GT4 is more widespread in Egypt, Iraq, Saudi Arabia, and Syria, while GT1a and GT1b are frequently seen subtypes in Turkey, Israel, Cyprus, and Iran [18].
2.4. Hepatitis C Treatment
The principal objective of HCV therapy is to obtain a sustained virological response (SVR), which is defined as undetectable levels of HCV RNA 12 (SVR12) after the end of treatment [19]. The modest efficacy of treatment and the resistance of certain genotypes, as well as the side effects of pegylated interferon ((peg-IFN)/ribavirin (RBV)), prompted the search for new HCV drugs and the development of direct-acting antiviral agents (DAAs) [20].
The discovery of the HCV life cycle paved the way for the invention of a new generation of HCV antiviral therapies: DAAs. DAAs directly interfere with a specific viral protein involved in the replication of HCV. Telaprevir and boceprevir were the first established DAAs, belonging to the protease inhibitor (PI) class. They prohibit the splicing of the HCV polyprotein between nonstructural proteins (NSs) 4A and NS3 via their respective NS4A proteases or HCV NS3.
The era of DAA began in 2011 when the two aforementioned PIs were approved for HCV antiviral therapy (Figure 1) [21,22,23,24]. This approval was one of the major steps in HCV history. Although single therapy with boceprevir or telaprevir led to a remarkable decrease in the replication of HCV, it led to the emergence of resistance-associated substitutions (RASs) and virological breakthroughs in all treated individuals [25]. Thus, a change in combination with peg-IFN–RBV was necessary. However, the limitations of this triple therapy were a major concern, from null responders to severe side effects [26]. The safety concerns were mainly in patients with advanced liver disease with albumin below the normal range and platelet counts under 100/μL [27].
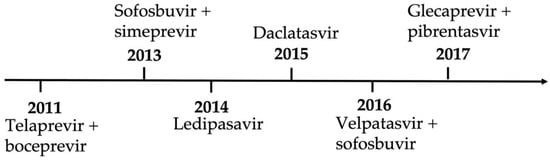
Figure 1.
Timeline of direct-acting antiviral regimens approved in the United States.
This led to an interferon-free concept because of side effects and efficacy concerns. IFN-free therapy became widely available because of the approval of the nucleotide analog sofosbuvir, an NS5B polymerase inhibitor. This was followed by simeprevir and daclatasvir. These drugs demonstrated high rates of SVRs, tolerability, and safety for certain genotypes. However, this regimen was terminated because of newer and more potent DAA combinations.
The next wave of DAAs brought the advantage of pan-genotypic regimens. This was the main accomplishment of sofosbuvir compared with its partner ledipasvir, which had lower efficacy against genotypes 2 and 3. Other studies reported on the proven efficacy of different DAAs against genotypes 1 and 2 [28,29].
In 2016, velpatasvir (the second-generation pan-genotypic NS5A inhibitor), in combination with sofosbuvir, was approved for chronic HCV treatment. This regimen achieved SVRs regardless of the HCV genotype in almost all patients (99%) [28]. However, a multicenter study demonstrated treatment failure with this combination therapy in individuals infected with genotype 3 and cirrhosis [28]. In light of this study, the European Association for the Study of the Liver (EASL) recommended against using sofosbuvir–velpatasvir as a first-line therapy in patients infected with genotype 3 and cirrhosis.
In 2017, further advancements in HCV therapy were accomplished using the new second-generation pan-genotypic PI glecaprevir in combination with pibrentasvir, an NS5A inhibitor. A study showed similar tolerability and response rates in cures. That trial concluded that an 8-week regimen in patients infected with genotypes 1, 2, 4, 5, or 6 is sufficient to achieve an SVR of 97–100% in patients without cirrhosis [28]. Further complex study models and trials were performed on patients infected with genotype 3, with and without cirrhosis, with durations ranging from 8 to 16 weeks, but it was later proven that this combination with an 8-week regimen is sufficient in the treatment of naïve patients with cirrhosis without the HCV genotype [30,31].
The impact of DAA regimens in the last decade has resulted in a significant reduction in the burden of chronic hepatitis C infection in the community. While only IFN and pegylated IFN with ribavirin achieved a virological cure of approximately 5% to 40–80% in chronic hepatitis C individuals of varying genotypes, the combination regimens of DAAs led to a higher SVR of greater than 95%, up to 100% [28,29]. The major pockets of HCV infection are now related to outbreaks in young people caused by substance use disorder and the opioid crisis [30]. As the development of DAAs and effective combination regimens decreased the incidence of hepatitis C and its complications, including cirrhosis and the need for liver transplantation, another liver disease began to gain increasing recognition. Infamously, NAFLD and its subtype, NASH, were becoming more prevalent worldwide.
3. Epidemiology of NASH and NAFLD
Nonalcoholic fatty liver disease, also known as hepatic steatosis, is a macrovesicular accumulation of triglycerides in hepatocytes. NAFLD affects 25% of the population worldwide, with a male predominance [31]. It is the most common cause of chronic liver disease and is rising in prevalence in the United States (US) as well as in developing economies such as those in the Middle East, Northern Africa, and Asia. Its rising trend coincides with the global epidemic of diabetes and obesity [32]. NAFLD is considered the hepatic manifestation of metabolic syndrome. Metabolic syndrome is characterized by the presence of three out of five metabolic abnormalities: hyperglycemia or insulin resistance, decreased high-density lipoprotein (HDL), high triglycerides, visceral obesity, and/or hypertension (Figure 2) [33].
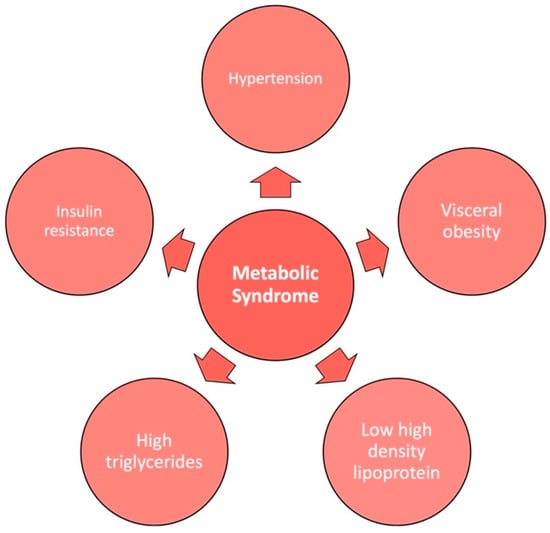
Figure 2.
The 5 factors of metabolic syndrome.
An inflammatory progressive subtype of NAFLD is known as NASH, which is defined by liver steatosis with hepatocyte injury (ballooning) and inflammation, with or without fibrosis [31]. It is estimated to affect 1.5–6.5% of individuals globally [34]. Of the US population, 3–5% of individuals have NASH. Risk factors for NASH include components of metabolic syndrome such as diabetes, dyslipidemia, hypertension, and obesity [35]. Similar to hepatitis C, the progression of NASH can lead to liver cirrhosis, end-stage liver disease (ESLD), and an increased risk of HCC. These conditions may qualify certain individuals for liver transplant (LT) if other therapeutic options are unsuccessful. Cardiovascular (CV) disease is the most common cause of death in NASH patients [31].
4. The Obesity Epidemic
NASH was first recognized in the 1980s, which correlates with the rising obesity epidemic [32]. Between 1975 and 2016, worldwide obesity almost tripled. The prevalence of obesity in children and adolescents between the ages of 5 to 19 years increased from 4% to 18%. The World Health Organization estimates 13% of adults are obese globally (11% of men and 15% of women), and this number will continue to increase [36]. This is due in part to the Western diet, which contains high amounts of saturated and trans fats, sodium, and refined and processed sugars with poor nutritional value. The Western diet promotes pro-inflammatory cytokines and is associated with an increased incidence of metabolic syndrome (MetS) [37]. Greater than two-thirds of NASH patients in the US have MetS, obesity, high triglycerides, dyslipidemia, and/or hypertension. T2DM is present in 44% of individuals with NASH. NASH is expected to increase by 56% by 2030, which will affect approximately 27 million people [31].
Currently, hepatitis C is the leading etiology of cirrhosis and HCC, comprising 40% of liver transplants in the US. However, the introduction of DAA therapy and asymptomatic screening are decreasing the incidence of HCV [38]. From the interferon era (2003–2010) to the DAA era (2014–2017), direct antiviral therapy led to a decrease in hepatitis C patients requiring transplants (35.3% to 23.6%), a decrease in listings (32%), and improved outcomes [39,40]. A European study found that 30.9% of HCV-decompensated cirrhosis patients were delisted after treatment with DAA because of clinical improvement [41]. These factors, coupled with a rise in obesity, indicate that NASH will surpass HCV and take over as the leading cause of liver transplantation in the future [38]. NASH is currently the leading indication for LT for women [31,42]. Among the baby boomer population, born between 1945 and 1965, NASH surpassed HCV infection as the leading indication for LT registrants in 2016. This is partly due to metabolic syndrome, which is associated with NASH, affecting 50% of those aged 60 years and older [38]. Between the years 2004 and 2013, there was a 170% increase in waitlist registrants for NASH, while there were only 45% and 14% increases in alcoholic liver disease and HCV infection, respectively [43]. Among LT candidates, NASH is the most rapidly growing etiology for HCC [44]. NASH is the second leading cause of HCC-related liver transplantation, which increased four-fold from 2002 to 2012 [45]. Wong and colleagues graphically demonstrated the rise in NASH compared with other liver disease etiologies in the United States in LT waitlist registrants between 2014 and 2019 [46].
5. NASH Therapies
Currently, there are no federally approved treatments for NASH. However, there are many potential pharmacological agents that are undergoing phase III clinical trials. Yet these clinical trials face many barriers. Barriers to this investigation include difficulty in diagnosis, staging difficulty, no good measurable endpoints, and accuracy in serum and imaging biomarkers [31,44].
5.1. NASH Diagnosis
The difficulty in diagnosing NASH is due to its invasive nature [31]. Liver biopsy is the gold standard for diagnosis, providing the grade of steatosis, degree of fibrosis, lobular inflammation, and hepatocyte ballooning. These factors will identify the NAFLD activity score (NAS). A NAS score of 5–8 is representative of NASH. Fibrosis staging is linked to clinical outcomes [47]. People with the F3 (severe fibrosis) and F4 (cirrhosis or advanced scarring) stages are predicted to have worse outcomes [44]. The disadvantages of liver biopsy are its invasive nature, expense, inter- and intra-observer variability, and high sampling error (1/5000 of the liver tissue is sampled). Although relatively safe, complication risks still exist. Morbidity can rise up to 1%, while mortality risk is estimated to be 0.2%, varying between disease states and the type of biopsy approach [48,49]. Therefore, noninvasive methods such as serum biomarkers and imaging are preferred to stage and quantify the disease, but accuracy and a lack of granularity can be problematic.
5.2. Biomarkers
Indirect and direct serum biomarkers are used for the diagnostic ability, risk stratification, and ability to assess treatment responses in NAFLD and NASH. They measure processes such as inflammation, necrosis, and cell death, which can lead to fibrosis [44]. Additional biomarkers and scores are still under investigation. Supported by American Association for the Study of Liver Diseases (AASLD) guidelines, commonly used panels of indirect serum biomarkers are the fibrosis-4 (FIB-4) index and the NAFLD fibrosis score (NFS), which are used for the initial evaluation of NAFLD and to assess fibrosis staging [50]. The FIB-4 index includes the platelet count, aspartate transaminase (AST), alanine transaminase (ALT), and age. The NFS score involves platelets, albumin, AST, ALT, age, body mass index (BMI), and insulin resistance. The BARD score is also often used in the clinic, along with FIB-4 and the NAFLD fibrosis scoring system [47]. In the general population, the sensitivity and specificity in detecting fibrosis (liver stiffness > 8 kPa) with FIB-4 are 37% and 69%, while for NFS, it is 52% and 69%, respectively. The accuracy of the biomarkers changes with varying thresholds [51]. In patients with NAFLD and NASH, FIB-4 at a threshold of 1.30 was shown to have a sensitivity and specificity of 84% and 69%, respectively. NFS was shown to have a sensitivity of 77% and a specificity of 70% at a cutoff of −1.455. BARD was found to have a sensitivity of 74% and a specificity of 66% [52].
Direct serum markers using collagen components or factors regulating fibrosis provide more granularity in fibrosis development. They can measure components of the liver matrix. Several scoring systems include PRO-C3 collagen neoepitope, which is a marker of extracellular matrix turnover and fibrogenesis. It can quantify liver fibrosis and help monitor progression and treatment responses [44]. Serum Pro-C3 levels have a positive correlation with NASH and cirrhosis, increasing with advanced fibrosis stages. Patients with severe lobular inflammation or liver ballooning degeneration have higher serum PRO-C3 levels, potentially demonstrating active disease. A decrease in these levels is associated with regression in fibrosis [53]. Mak and colleagues conducted a meta-analysis demonstrating patients with significant (≥2) and advanced (≥3) fibrosis had PRO-C3 sensitivities of 68% and 72%, respectively. The specificities were 79% and 73%, respectively [54].
Imaging biomarkers are also utilized, such as FibroScan and AST (FAST), which predicts NASH with fibrosis [47]. FibroScan, a type of elastography, measures high liver stiffness related to mortality. FibroScan has limitations in NAFLD because of patient characteristics, including obesity and severe hepatic steatosis; hence, individual risk assessments are required with this readily available test in hepatology clinics. It has been characterized as a prognostic biomarker in appropriate clinical scenarios [44]. In a meta-analysis, the pooled sensitivity and specificity of the FAST score were both 89% [55]. The gold standard of imaging in NAFLD is magnetic resonance imaging (MRI), including the proton density fat fraction (MRI-PDFF) and magnetic resonance elastography (MRE), which identify fat content and fibrosis staging, respectively [47]. Magnetic resonance methods were found to have high diagnostic accuracy with an area under the curve (AUC) of 0.90 (82% sensitivity and 87% specificity). They can differentiate NAFLD from NASH and monitor disease responses [56,57]. MRE has better diagnostic accuracy (AUC 0.90) than MRI-PDFF in identifying advanced fibrosis, whether F3 or F4, but its use as a screening test is limited because of costs. A combination of serum and imaging biomarkers is often used in clinical practice for screening, staging, and monitoring [44,58].
5.3. Treatment Response Endpoints
All these biomarkers can be utilized for the monitoring and treatment of NASH. There is no single noninvasive diagnostic test in NAFLD to identify NASH; hence, a composite approach with a longitudinal assessment is the favored approach, utilizing liver biopsies as the reference standard in selected cases and clinical trials. The FDA endpoints for treatment in clinical trials are defined as NASH resolutions without any worsening of fibrosis or the improvement of at least one fibrotic stage without any worsening of steatohepatitis. Repeated biopsies at the end of the treatment course will provide a definite response. However, given its invasive nature, serum and imaging biomarkers can be used as approved alternatives [47]. For example, a 30% decline in liver steatosis seen on an MRI-PDFF would be equivalent to regression in fibrosis [44]. Additional surrogate biomarkers used in clinical trials to assess responses to pharmacotherapy include Pro-C3, Enhanced Liver Fibrosis (ELF), Hemoglobin A1c (HbA1c), AST, cytokeratin 18 (CK-18), and MRI-biomarker-corrected T1, among others [47].
5.4. Lifestyle Measures
Weight loss from dietary changes and exercise is the mainstay treatment for NASH, as it is the most effective approach [31,37]. A body weight loss of 7% demonstrates histological improvement with a 90% steatohepatitis resolution. The NASH fibrosis score can be reduced by reducing calories by 500–750 kcal per day [32]. The Mediterranean diet, coupled with green plants rich in polyphenols, reveals significant benefits in reducing hepatic steatosis. The Mediterranean diet comprises olive oil, vegetables, legumes, whole grains, fruits, and nuts along with fish and seafood with minimal red and processed meats and low carbohydrates. Individuals who followed the Mediterranean diet with the addition of green plants experienced a 19% greater reduction in liver fat content compared with a Mediterranean diet without green plants, even though weight loss was similar between both groups [44,59]. The consumption of foods and beverages rich in the phenolic acids present in fruits, vegetables, nuts, green tea, and coffee demonstrates a lower prevalence of insulin resistance in addition to NAFLD and fibrosis [44]. Exercise is also an essential lifestyle approach to reducing the progression and severity of NAFLD and NASH [60]. A meta-analysis demonstrated physical exercise, despite minimal to no weight loss, led to a reduction in liver fat content [61]. Vigorous activity—defined as a metabolic equivalent value greater or equal to 6—compared with moderate intensity, decreased the odds of developing NASH (odds ratio (OR): 0.65; 95% confidence interval (CI): 0.43–0.98). In addition, doubling the time of vigorous activity (150 min or more per week) decreased the odds of progressing to fibrosis (OR: 0.53; 95% CI: 0.29–0.97) [60,62].
5.5. Pharmacological Agents
Besides conventional lifestyle changes, multiple pharmacological agents are undergoing evaluation. These include antioxidants such as vitamin E and routinely used medications in diabetes treatment such as pioglitazone and liraglutide. Potential targets in the microbiome are also under investigation, including fecal microbiota transplantation and pre- and probiotics [31,60]. Since cardiovascular disease is the number one cause of mortality in noncirrhotic NASH, new drugs must additionally be assessed for their impact on CV risk factors [47].
Vitamin E is proposed to play a role in NASH treatment, but there is a lack of efficacy in fibrosis resolution [47]. The American Association for the Study of Liver Diseases (AASLD) and EASL support strong consideration of pioglitazone in patients with NASH and diabetes. However, risk assessment in these individuals is critical given the adverse side effects of weight gain and heart failure [63]. Peroxisome proliferator-activated receptor (PPAR) agonists may also offer therapeutic benefits for NASH. In a phase IIb clinical trial, lanifibranor, a pan-PPAR agonist, demonstrated NASH resolution without worsening fibrosis. However, mild weight gain and peripheral edema occurred more frequently compared with the placebo group [64]. A phase III study is underway.
There are multiple phase III drugs that are under investigation. Obeticholic acid (OCA), a farnesoid X receptor (FXR) agonist, is a nuclear receptor that modulates bile acid synthesis, lipid and glucose homeostasis, and liver fibrosis. The REGENERATE trial discovered that the use of OCA led to improvement in fibrosis without worsening NASH. Some individuals experienced pruritis, increased LDL cholesterol, and decreased HDL and biliary events such as cholelithiasis and cholecystitis [47,63]. Resmetirom is a thyromimetic that targets hepatic thyroid hormone receptor β, which regulates hepatic triglycerides and cholesterol metabolism. Its phase IIb trial showed a significant reduction in relative liver fat content on MRI-PDFF and high rates of NASH resolution. Triglyceride levels and LDL cholesterol also improved. Fibrosis regression was, however, not significant. It is currently in a phase III trial, along with another drug candidate, aramchol. Aramchol is a bile acid and fatty acid conjugate that inhibits the stearoyl-CoA desaturase-1 enzyme, leading to the downregulation of liver steatosis. In its phase IIb trial, there was a greater decrease in hepatic fat, improvement in liver enzymes, and higher NASH resolution than in the placebo group. Additional drugs that demonstrated a histological response in clinical trials are shown in Table 1 [32,47]. Despite the multitude of developing drugs, liver transplantation remains the best therapeutic treatment for NASH-related advanced cirrhosis or HCC. Candidacy is problematic, as many individuals are morbidly obese, and it is even more problematic with the presence of cardiovascular risk factors. Despite liver transplantation, NASH can still recur post-transplant [32].

Table 1.
Pharmacological agents demonstrating histological responses.
6. Complexities of Treatment Due to Co-Morbidities Associated with NASH
Treatment is complex in NASH because of the common comorbidities of T2DM and CV disease (CVD). Dietary changes and exercise are first-line therapies in not only NASH but also cardiovascular disease.
6.1. Aspirin and Statins
Aspirin for the secondary prevention of CV disease is recommended, but its use in NASH is uncertain because of limited data. A prospective study demonstrated daily aspirin use exhibited less severe histological features in NASH and a lower risk of advanced fibrosis progression over time [72]. Patients with hyperlipidemia, T2DM, 10-year atherosclerotic cardiovascular disease (ASCVD) risk, or the clinical presence of CVD are strongly recommended to be on statins. Studies are emerging on its therapeutic role in NASH, as statins exert anti-inflammatory, proapoptotic, and antifibrotic properties [73]. In the GREACE study, statins reduced abnormal liver tests (AST, ALT, gamma-glutamyl transferase (GGT)) and decreased cardiovascular events by 68% in patients with likely NAFLD [74]. In a systemic review of 22 studies, statin therapy in interventional studies improved liver enzymes in NAFLD patients, but cross-sectional studies showed no difference between the treated and untreated groups [75]. Statins can benefit patients with a co-existing vascular risk with NASH but have no role in patients with advanced cirrhosis [76]. The American Gastroenterological Association (AGA) promotes the use of statins in NASH patients to reduce CVD risk given its association with hyperlipidemia. In our experience, statins are generally safe in NAFLD/NASH, except when prescribed to patients with Child–Pugh B/C cirrhosis and a MELD > 15, which requires a multidisciplinary risk-to-benefit assessment (primary-care physician/hepatologist/cardiologist) to avoid toxicity, including myositis and liver failure [57].
6.2. Diabetes Medications
Metformin is a first-line therapy in newly diagnosed individuals with T2DM. Although it improves CV outcomes, histological resolution in NAFLD has not been observed. Other diabetic agents, such as thiazolidinediones, glucagon-like peptide 1 (GLP-1) agonists, and sodium–glucose cotransporter-2 (SGLT2) inhibitors, have a potential therapeutic role in NASH. A meta-analysis of randomized controlled trials (RCTs) showed GLP-1 agonists, mainly liraglutide and semaglutide, decreased liver fat content on MRI imaging and improved liver enzymes with a histological resolution of NASH [77]. Multiple RCTs also demonstrated that SGLT2 inhibitors improved hepatic steatosis, fibrosis, and liver enzymes and reduced visceral fat and liver stiffness [78,79,80]. Additionally, GLP-1 agonists and SGLT2 inhibitors reduce CV risk [77]. Tirzepatide, a glucose-dependent insulinotropic polypeptide/GLP-1 agonist, is a novel agent approved in the US in 2022. It demonstrated significant body weight reduction (6.2–12.9 kg) and improvement in HgbA1c (1.87–2.59%) and may have a potential adjuvant role in NASH [81].
6.3. Polyunsaturated Fatty Acids
Polyunsaturated fatty acids (PUFA) can reduce lipid levels and improve liver steatosis, oxidative stress, and liver enzymes in NAFLD. However, it has a limited role in NASH [82]. N-3 PUFAs can bind and regulate PPARα receptors to control lipid metabolism. These fatty acids can reduce triglyceride levels and improve insulin sensitization, glucose, and fatty acid metabolism. Animal studies have demonstrated fish oil can reduce the very-low-density lipoprotein (VLDL), AST, ALT, alkaline phosphatase (ALP), GGT, bilirubin, and lipid content of obese animals. Its anti-inflammatory properties can reduce liver inflammation and, therefore, steatosis [83]. EASL-EASD-EASO guidelines endorse the role of PUFA in reducing plasma and liver lipids but do not support its use in NASH [84].
7. Impact on Morbidity and Mortality
Mortality and morbidity in NASH are unwavering because of limited treatment options. The major drivers of liver-related mortality and morbidity in NASH patients are cirrhosis and hepatocellular carcinoma. Cirrhosis is defined by compromised liver function and the presence of portal hypertension. Decompensated cirrhosis is a development of complications including ascites, gastrointestinal bleeding, hepatic encephalopathy, and/or jaundice. Ascites are the hallmark of decompensation [85]. Approximately 25% of NASH patients can progress to cirrhosis or experience complications such as portal hypertension, liver failure, and HCC. The major causes of mortality in patients with NAFLD are metabolic syndrome and CV disease [86].
7.1. Fibrosis and Cirrhosis
Advanced fibrosis or compensated cirrhosis is considered the most significant determinant of liver-related mortality and clinical outcomes [34,87]. It is estimated that 9.8 million NASH patients have stages F0–F2 fibrosis, 2 million have stage F3 fibrosis, and 1.3 million have stage F4 fibrosis [88]. Significant hepatic fibrosis greater than stage 2 is predictive of liver-related mortality, with exponential increases in mortality as fibrosis stages increase [35,89]. Mortality and the incidence of hepatic decompensation events are greatest among patients with stage F4 disease [88]. A large RCT involving 475 patients demonstrated that 22% of F3 fibrosis NASH patients progressed to cirrhosis. In total, 19% of cirrhosis patients with NASH experienced decompensating events over 2 years, such as hepatic encephalopathy, variceal bleeding, ascites, and even death in one individual [34]. Decompensating liver events were highest among patients with NASH and stages F3 and F4 fibrosis than those with nonalcoholic fatty liver and stages F0–F2. The mortality rate for stages F0–F2 was 0.32 deaths/100 person-years, which increased to 0.89 deaths/100 person-years for stage F3 and 1.76 deaths/100 person-years for stage F4 [88].
7.2. Hepatocellular Carcinoma
Although the incidence of HCV-related HCC is higher than in NASH cirrhosis, NASH is a rapidly emerging cause of HCC among LT-waitlisted individuals in the United States. The prevalence of hepatic malignancy in LT candidates with NASH increased 11.8-fold from 2002 to 2016 [90,91,92]. Interestingly, NASH can progress to hepatocellular carcinoma without cirrhosis [35]. In noncirrhotic NASH, a meta-analysis of 19 studies estimated the prevalence of HCC in 38% of patients. They had a higher likelihood of developing hepatic malignancy than noncirrhotic patients of other etiologies [93].
Hepatocellular carcinoma is a major contributor to poor outcomes in NASH individuals. The median survival of HCC is 6–20 months. In the US, the 2-year survival rate is estimated to be less than 50% and the 5-year survival rate decreases to 10% [94]. NASH-related HCCs have a high rate of mortality following HCC diagnosis [95]. This is partly due to the diagnosis of hepatic malignancy at later stages in comparison with other liver diseases such as HCV [44,96]. It is proposed that the presence of visceral obesity could potentially reduce detection rates in screening modalities [95]. Obesity also promotes NASH and HCC through independent mechanisms [97]. Compared with HCV, NAFLD individuals have shorter survival times and are more likely to die from primary liver malignancy [94,96]. The poor prognosis may also be associated with a lower likelihood of screening and more comorbidities, such as CV disease, which is problematic in transplant candidacy [96]. The AASLD recommends HCC screening in patients with NASH who have cirrhosis [97].
7.3. Non-Hepatic Events
Non-hepatic-related events also contribute to morbidity and mortality in NASH patients. Although the progression of NASH leads to fibrosis, cirrhosis, and/or HCC, the majority of deaths are cardiac-related. NASH patients are at an increased risk of CV disease, type 2 diabetes, and chronic kidney disease (CKD) [35].
NASH has been found to be an independent risk factor for venous thromboembolism in cirrhotic patients [98]. Patients with NASH also have higher rates of severe coronary artery disease (CAD) than those with hepatitis C. CV events increase in patients with NAFLD, particularly in advanced fibrosis, but an association with CV mortality is unclear [63]. One study found cardiac mortality was no different between the NASH and non-NASH subtypes of NAFLD. The presence of hepatic steatosis rather than NASH itself is a main risk factor for CV mortality [99]. However, the presence of CKD and diabetes in NAFLD increases CV mortality [100,101]. Increasing the severity of CKD is associated with overall mortality in NAFLD patients [100]. Additionally, the presence of both NASH and T2DM increases adverse clinical outcomes, morbidity, and liver-related and overall mortality [99,102]. Diabetes is an independent risk factor for death in LT candidates, and pre-existing diabetes is associated with inferior outcomes post-transplant [42]. It is proposed that over the next two decades, NASH with T2DM will be liable for 65,000 transplants, 812,000 liver-related deaths, and 1.37 million cardiovascular-related deaths [102]. T2DM promotes advanced fibrosis in overweight and obese individuals. This is significant because the presence of advanced fibrosis is associated with poor clinical outcomes [103]. Diabetes NAFLD also increases the risk of cerebrovascular accidents, CKD, and all-cause and CVD mortality compared with nondiabetic NAFLD. Monitoring hemoglobin A1c may be crucial in NAFLD patients without diabetes. This will allow for the prompt initiation of lifestyle measures to reduce the risk of developing diabetes, which can, therefore, reduce morbidity and mortality [101]. Diabetic NAFLD increases the all-cause mortality (hazard ratio (HR): 1.60; 95% CI: 1.38–1.85; p < 0.01) compared with nondiabetic NAFLD [101].
The presence of metabolic syndrome is also associated with adverse outcomes in NAFLD. Compared with those without NAFLD, individuals with NAFLD typically have increased metabolic syndrome severity scores due to co-existing comorbidities. The higher the MetS severity scores in NAFLD, the higher risk of all-cause, cardiac-related, diabetes-related, and hypertension-related mortality [33].
8. Liver Transplant Evaluation
The mainstay treatment option to reduce morbidity and mortality in NASH is liver transplantation. Liver transplant evaluation is a complex process that involves appropriate patient selection, risk assessments, and the prediction of outcomes. A multidisciplinary team is needed given the complexities of the selection process. The Model for End-Stage Liver Disease (MELD) score is used to assess the severity and urgency of liver transplants, but revised MELD models and other predictive models of mortality are being developed.
Transplant evaluation for NASH is even more complex than HCV because of the presence of metabolic comorbidities associated with inferior patient and graft survival outcomes. Waitlist registrants with NASH are likely to deteriorate or die from cardiac or liver disease before transplants [104]. During the modern DAA era between January 2014 to May 2017, NASH was found to have lower 1- and 3-year graft survival rates compared with HCV recipients [40]. NASH patients also have an increased risk of post-transplant mortality compared with those with hepatitis C. Between 2016 and 2017, the one-year patient survival rate was 90.4% for NASH and 92.8% for hepatitis C. There was a higher risk of mortality associated with cardiovascular and cerebrovascular disease in the NASH group (11.5% of deaths) than in the hepatitis C group (7.0% of deaths). It is proposed that diabetes and obesity contribute to poor outcomes [105]. Both pretransplant diabetes and obesity lead to early postoperative complications compared with obesity alone or with other CV risk factors. Individuals have a longer length of stay and a higher risk of infections and/or CV events. Pre-existing diabetes is associated with all-cause mortality (61.68 per 1000 person-years versus 47.80 per 1000 person-years) and death due to cardiac and renal diseases post-liver transplant in NASH patients [106]. Pretransplant renal dysfunction predicts increased post-transplant CV disease mortality. Pretransplantation, severe renal dysfunction is associated with a higher risk of death and lower graft survival in NASH [42]. Obesity is also an independent risk factor for developing pretransplant portal venous thrombosis (PVT). Compared with those without PVT, NASH transplant recipients with PVT demonstrated 37% and 31% increased risks of graft failure and overall death, respectively [107]. One year of enoxaparin can prevent PVT in cirrhosis. TIPS is another option, but the data are limited [42].
CVD continues to be the leading cause of mortality in NASH patients within one year of a liver transplant. In total, 70% of cardiac-related events arose in the perioperative period, and the occurrence of CV events was associated with a 50% mortality rate [108]. Individuals may face arrhythmia, sudden cardiac death, and acute pulmonary edema [109]. Perioperative risk stratification is essential because liver transplantation involves acute changes in cardiovascular hemodynamics intraoperatively [42]. Screening NASH patients for cardiac and renal diseases is critical to improving outcomes [106]. Cardiovascular disease in liver candidates has become more recognized because of the increasing prevalence of NASH. NASH cirrhosis is a strong determinant of the presence of CAD [110].
Cardiovascular Assessment
The optimal approach to detecting CAD has not yet been clearly established and continues to evolve (Figure 3). Screening and management in LT candidates with coronary heart disease should be individualized based on local expertise and the severity of CAD and liver dysfunction [111]. NASH cirrhosis has a higher prevalence of single- and three-vessel cardiac disease compared with HCV patients, indicating a more comprehensive cardiac evaluation. For all LT candidates without known coronary heart disease (CHD), the American Heart Association recommends cardiac physical examination, electrocardiogram (ECG), and resting transthoracic echocardiogram (TTE) [108,111]. Stress testing is needed if a patient has more than two cardiac risk factors, such as age greater than 50, hypertension, hyperlipidemia, and obesity [108]. The 2013 practice guidelines from the AASLD and American Society of Transplantation also recommend initial noninvasive evaluation with TTE, noninvasive stress testing, and cardiology evaluation if cardiac risk factors are present [112]. Stress testing can be performed with dobutamine stress echocardiography (DSE) or nuclear perfusion stress testing (single-photon emission computed tomography (SPECT)) [108]. Dobutamine stress testing has a high predictive value in identifying low-risk groups but has suboptimal results in cirrhotic patients. Patients with decompensated cirrhosis cannot reach a maximal heart rate for accurate results. CT coronary artery calcification scoring and cardiac MRI may be valuable in assessing CAD in advanced cirrhosis, but additional studies are needed for confirmation. If stress testing results are abnormal, coronary angiography is warranted [109]. Additionally, anatomic coronary imaging is warranted in candidates with a high risk of significant CHD, including those with NASH, diabetes, or with two or more cardiac risk factors. Cardiac risk factors include hyperlipidemia/dyslipidemia, hypertension, CKD, left-ventricular hypertrophy, a family history of premature CHD, a history of smoking, and/or a coronary artery calcification score greater than zero [111]. A noninvasive form of coronary angiography is coronary computed tomography angiography (CCTA), which can evaluate CHD risk in LT candidates. It has a high negative predictive value in excluding clinically significant CHD [110,111]. It can be supplemented with CT-derived fractional flow reserve (FFR) to provide supplementary information on coronary blood flow and ischemia-causing lesions [111].
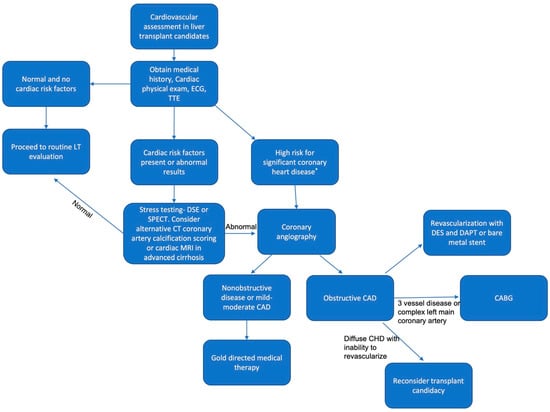
Figure 3.
Algorithm for cardiovascular evaluation in liver transplant candidates. * Nonalcoholic steatohepatitis, diabetes, or 2+ cardiac disease risk factors. Abbreviations: LT, liver transplant; ECG, electrocardiogram; TTE, transesophageal echocardiogram; DSE, dobutamine stress echocardiogram; SPECT, single-photon emission computed tomography; MRI; magnetic resonance imaging; CAD, coronary artery disease; DES, drug-eluting stent; DAPT, dual antiplatelet therapy; CABG, coronary artery bypass graft.
Gold-directed medical therapies, such as beta blockers and statins, are appropriate for individuals with mild-to-moderate or nonobstructive CAD. If a patient is found to have obstructive CAD—defined as a greater than 50% reduction in the diameter of major coronaries—percutaneous intervention (PCI) and revascularization should be considered before the evaluation of transplant candidacy, although the severity and extent of CAD have no impact on post-transplant survival with revascularization, and it shows little evidence of benefit [108,111]. Bare-metal stenting is preferred over dual antiplatelet therapy (DAPT), but LT must be delayed by at least 6 weeks [108,112]. PCI, with a drug-eluting stent (DES) and a short course of DAPT of 3–6 months, is appropriate in patients with stable end-stage liver disease [111]. However, there is an increased risk of postoperative morbidity and mortality, especially in advanced decompensated cirrhosis [111]. A multidisciplinary team should review various options for potential transplant candidates considering a high short-term risk of hepatic decompensation or death. The risks and benefits of DES and DAPT or combined coronary artery bypass graft (CABG) prior to liver transplantation should be discussed and weighed. Candidates with a complex left main- or three-vessel disease may benefit from undergoing a CABG and liver transplant at the same time. CABG alone is a contraindication for patients with a MELD score greater than 13 or a Child–Pugh Score of B or C cirrhosis. Individuals with diffuse CHD who cannot be revascularized should be reconsidered for a liver transplant because of high perioperative mortality and adverse outcomes [111].
9. Post-Transplant Complications in NASH and Hepatitis C
9.1. Obesity
Obesity is a concern in NASH pre- and post-transplant because of clinical complications. The AASLD states that a BMI greater than 40 is a relative contraindication because of a high risk of post-transplant complications and death. EASL guidelines recommend carefully evaluating patients who have a BMI greater than 35 before considering candidacy [42]. Interestingly, a retrospective study demonstrated NASH patients with a higher BMI had better survival outcomes than those with a normal BMI (18.5 to <25 kg/m2). This may be due to the higher waitlist dropout of morbidly obese individuals and rigid selection criteria for high-BMI patients. This supports the successful selection of NASH patients with elevated BMIs [113]. Although studies conflict regarding the effect of pretransplant obesity on mortality, a meta-analysis demonstrated inferior survival outcomes in obese patients when compared with nonobese individuals with similar causes of liver diseases, yet no difference in mortality was found in overall liver diseases [114]. One study demonstrated that pretransplant obesity with a BMI > 30 predicted post-LT death with an OR of 2.921 (95% CI: 1.216–7.854; p = 0.01) [115]. Obese patients following LT were associated with higher morbidity such as an increased length of stay and infectious complications [116].
In a post-transplant setting, up to 42% of patients develop obesity [117]. Obesity rates post-transplant in all LT patients were 33.7% and 40.3% after 1 and 5 years, respectively [118] These rates are highest in all liver disease patients who have had transplants over age 50, were obese prior to transplant, and were on high-dose steroids. The risk of developing diabetes and metabolic syndrome increases along with associated complications, including CVD, renal disease, and de novo NASH [119,120]. The catabolic state in cirrhotic patients and pretransplant sarcopenia, hypoalbuminemia, and protein–calorie malnutrition increase the risk of rapid weight gain post-transplant. There is increased fat consumption, hyperphagia, and a loss of thermogenesis even with rising body mass and a reduction in resting energy expenditure. This may be explained by the transection of autonomic nerves in the native liver. The liver regulates feeding behavior because of the humoral and neuronal signaling relayed between the brainstem and hypothalamus. Postoperatively, the loss of afferent and efferent neural input from the native liver can alter the energy homeostasis of the recipient, contributing to obesity [117]. In a study of primary liver diseases, BMI values post-transplant were inversely associated with long-term survival. High-BMI recipients after one-year post-transplant demonstrated a two-fold increased risk of mortality compared with normal-weight recipients. Interestingly, there was no significant association between BMI and CV mortality [121]. Weight-lowering agents can be considered. Orlistat, a reversible inhibitor of pancreatic lipase, was studied in a prospective open-label trial and demonstrated a significant reduction in weight circumference in the post-LT setting without interference from tacrolimus. Orlistat appears to be safe if immunosuppression levels are closely monitored, but more studies are warranted [122]. Bariatric surgery is also a consideration.
9.2. Bariatric Surgery
The gold standard for bariatric surgery (BS) has traditionally been Roux-en-Y gastric bypass in the United States, but laparoscopic sleeve gastrectomy is gaining popularity [123]. For pretransplant obesity, bariatric surgery is considered an option, but the associated malnutrition can lead to delisting and death on the waitlist [42]. In those with a prior history of bariatric surgery, most commonly Roux-en-Y, delisting/death on the waitlist was higher among patients with BS (33.3% vs. 10.1%, p = 0.002), and transplant rates were lower (48.9% vs. 65.2%, p = 0.03) compared with those without surgery. The increased risk of death in patients with a history of BS on the waitlist increased with the presence of malnutrition (HR 4.9; 95% CI: 1.8–13.4). Sarcopenia was higher among individuals who were delisted (71.4% vs. 16.7%; p = 0.04) [124]. Laparoscopic sleeve gastrectomy is now a preferred method for long-term weight loss and is a safer alternative to Roux-en-Y [42,123]. Its popularity is due to multiple advantages, including shorter operating time, endoscopic access to bile ducts, a lower likelihood of interfering with immunosuppression reabsorption, and a lower malabsorption risk [123]. The timing of bariatric surgery is significant, as it can be performed before, during, or after a liver transplant. In a pre-LT setting, cirrhosis patients who underwent bariatric surgery demonstrated a 5% major complication risk and a 1-year post-LT mortality rate of 7% compared with those without cirrhosis [125]. In BS after LT, there was a three-fold increase in postoperative major complications, postulated to be heightened by impaired healing processes and immune responses in fragile patients [125]. Bariatric surgery pre-LT should be considered in the non-decompensated cirrhosis group, while BS after LT should be considered after the failure of other therapies if morbid obesity occurs/recurs [125]. In patients with concomitant BS and LT, postoperative major morbidity and 1-year overall survival were lower but were evaluated in individuals with compensated liver disease. Sleeve gastrectomy during LT is complex but can reduce comorbidities with significant weight loss and stable weight loss even 3 years post-transplant [123,126].
9.3. Recurrent NASH
Unfortunately, it is common to develop hepatic steatosis post-transplant. Although cirrhosis-related complications resolve in NASH patients, the metabolic factors persist and can increase with steroids and immunosuppressive therapy [127]. In a period of less than 6 months to 10 years, the recurrence of NAFLD occurred in 8.2% to 62.5% of recipients [128]. Bhati and colleagues demonstrated, over a median time of 47 months after NASH transplant, that liver biopsies revealed 88.2% of patients had recurrent NAFLD while 41.2% had recurrent NASH. One-quarter of individuals had advanced fibrosis. Leading causes of mortality post-LT were non-hepatic malignancy (25%), infectious (25%), and CV complications (21.9%) [115]. Recurrent graft cirrhosis led to three patients’ deaths over a mean of approximately 3 years [115]. Metabolic factors associated with recurrent NASH include obesity, weight gain, hypertension, and dyslipidemia [129]. Some studies have also found a genetic link with recurrent NAFLD in recipient and donor grafts [128]. Lifestyle modifications are the cornerstone of preventing and/or reducing the incidence of metabolic syndrome factors. Avoiding excess weight gain via dietary control and exercise can prevent NASH recurrence post-transplant [127]. Pharmacotherapy can be considered if lifestyle measures fail [119]. Weight-loss agents such as orlistat, liraglutide, naltrexone-bupropion, and phentermine–topiramate can be considered, but there are limited data to support its use in a post-LT setting [128]. Corticosteroids and calcineurin inhibitors (CNIs) are utilized to prevent allograft rejection but also have an increased risk of metabolic complications, including weight gain, diabetes mellitus, and hyperlipidemia. Immunosuppressive regimen protocols that decrease the dosage of tacrolimus may be impactful [130]. A prospective multicenter study found the use of everolimus with a reduced dose of tacrolimus decreased weight gain compared with the standard tacrolimus regimen at 1- and 2-year follow-ups [131]. Avoiding steroids or early discontinuation within 3 months and minimizing CNIs during the maintenance phase of immunosuppression can prevent metabolic syndrome components [127,132]. Studies on steroid-free versus steroid-based immunosuppression are too indeterminate to make concrete conclusions about the proposed benefits or harms of mortality and graft loss or infection rates. Although the steroid-free regimen has lower rates of hypertension, cholesterol, and diabetes, there are higher rates of acute rejection, steroid resistance rejection, and increased creatinine levels [132].
9.4. Recurrent Infection in HCV
Metabolic syndrome, obesity, and recurrent NASH are a concern in NASH individuals post-transplant. However, recurrent infection is a concern in hepatitis C LT recipients, but it is a rare occurrence in individuals who achieved an SVR prior to enlistment. The recurrence of the infection of the allograft is universal in hepatitis C patients with detectable HCV viremia at the time of transplant, occurring within hours of transplant. It unavoidably progresses to chronic liver infection since viral clearance cannot be achieved in recurrent HCV [133]. Compared with NASH, the recurrence of HCV is associated with worse survival outcomes post-transplant [40]. Approximately one-third of HCV-infected allografts will progress to cirrhosis within 5 years. Once cirrhosis arises, survival decreases to 41% and 10% after 1 and 3 years, respectively, most frequently because of graft failure [134,135]. Preventative measures include identifying early histological damage in protocol liver biopsies after transplant to identify individuals at a high risk of severe hepatitis C recurrence. Transient elastography and hepatic venous pressure gradients can be used to identify fibrosis and identify those at risk of decompensation. Antiviral therapy is indicated for those who have grade 3–4 inflammation or stage 2 fibrosis [136]. The AASLD and EASL have developed guidelines for DAA regimens that can be used for recurrent HCV [133]. DAAs can lead to SVRs in recurrent HCV infections, demonstrating their high efficacy. A prospective study found that, at week 12 after DAA therapy, the SVR rate in HCV-recurrent individuals was 95.8% [137]. A greater than 90% SVR rate was achieved at week 12 in multiple studies but was evaluated in those with compensated cirrhosis [138]. Further studies of DAAs are needed in the prevention of HCV recurrence [139]. Re-transplantation is the only therapeutic option for graft cirrhosis, especially for those in clinical decompensation [136].
10. Conclusions
Hepatitis C and NASH in the 21st century have had a significant impact on the health of the global population. The introduction of DAAs, coupled with the obesity epidemic, has led to a steady decline in HCV and an exponential rise in NASH. NASH will soon become the leading cause of hepatic disease and an indication for transplantation in the coming decades. Pharmacological treatments are still under investigation, and in the interim, clinicians should recommend a structured program through a nutritionist and exercise therapist for the treatment of metabolic syndrome and NASH. In patients with severe obesity who failed their lifestyle modifications and pharmacologic treatments, bariatric surgery should be considered to treat NASH. For individuals who will benefit from liver transplants, thorough screening and the involvement of a multidisciplinary team are necessary to ensure successful outcomes. Moreover, there should be regular monitoring post-transplant, as complications, including recurrent NASH, can arise if patients are not guided appropriately, which can lead to increased morbidity and mortality. NASH is a rising epidemic in the 21st century that requires regular global public health initiatives in addition to national, regional, and local medical management protocols.
Author Contributions
Conceptualization, R.M.; writing—original draft preparation, S.S. and A.A.; visualization, S.S.; writing—review and editing, R.M., S.S. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; AGA, American Gastroenterological Association; AASLD, American Association for the Study of Liver Diseases; ASCVD, atherosclerotic cardiovascular disease; BS, bariatric surgery; BMI, body mass index; CV, cardiovascular; CVD, cardiovascular disease; cDNA, complementary DNA; CKD, chronic kidney disease; CABG, coronary artery bypass graft; CCTA, coronary computed tomography angiography; CAD, coronary artery disease; CHD, coronary heart disease; CK-18, cytokeratin 18; DAAs, direct-acting antiviral agents; DSE, dobutamine stress echocardiography; DES, drug-eluting stent; DAPT, dual antiplatelet therapy; ECG, electrocardiogram; EHMs, extrahepatic manifestations; ESLD, end-stage liver disease; ELF, Enhanced Liver Fibrosis; FXR, farnesoid X receptor; FAST, FibroScan and aspartate transaminase; FIB-4, fibrosis-4; FFR, fractional flow reserve; GGT, gamma-glutamyl transferase; GLP-1, glucagon-like peptide 1; HbA1c, hemoglobin A1c; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDL, high-density lipoprotein; LT, liver transplant; MRE, magnetic resonance elastography; MRI, magnetic resonance imaging; MetS, metabolic syndrome; MELD, Model for End-Stage Liver Disease; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NANBH, non-A, non-B hepatitis; NS, nonstructural protein; NAS, NAFLD activity score; NFS, NAFLD fibrosis score; OCA, obeticholic acid; peg-IFN, pegylated interferon; PCI, percutaneous intervention; PPAR, peroxisome proliferator-activated receptor; PUFAs, polyunsaturated fatty acids; PVT, portal venous thrombosis; PDFF, proton density fat fraction; RCTs, randomized controlled trials; RASs, resistance-associated substitutions; RBV, ribavirin; SPECT, single-photon emission computed tomography; SGLT2, sodium–glucose cotransporter-2; TTE, transthoracic echocardiogram; TG, triglycerides; T2DM, type 2 diabetes mellitus, US, United States; VLDL, very-low-density lipoprotein.
References
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.-R.; Elmasry, S.; Saito, T. Organ system view of the hepatic innate immunity in HCV infection. J. Med. Virol. 2016, 88, 2025–2037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stanaway, J.D.; Flaxman, A.D.; Naghavi, M.; Fitzmaurice, C.; Vos, T.; Abubakar, I.; Abu-Raddad, L.J.; Assadi, R.; Bhala, N.; Cowie, B.; et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet 2016, 388, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2013, 59, 318–327. [Google Scholar] [CrossRef]
- Rosen, H.R. Clinical practice. Chronic hepatitis C infection. N. Engl. J. Med. 2011, 364, 2429–2438. [Google Scholar] [CrossRef]
- Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Modi, A.; Liang, T. Hepatitis C: A clinical review. Oral Dis. 2007, 14, 10–14. [Google Scholar] [CrossRef]
- Panel A-IHG. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018, 67, 1477–1492. [Google Scholar] [CrossRef]
- El-Zayadi, A.R.; Anis, M. Hepatitis C virus induced insulin resistance impairs response to anti viral therapy. World J. Gastroenterol. 2012, 18, 212–224. [Google Scholar] [CrossRef]
- Alter, H.J.; Holland, P.V.; Purcell, R.H.; Lander, J.J.; Feinstone, S.M.; Morrow, A.G.; Schmidt, P.J. Posttransfusion Hepatitis After Exclusion of Commercial and Hepatitis-B Antigen-Positive Donors. Ann. Intern. Med. 1972, 77, 691–699. [Google Scholar] [CrossRef]
- Feinstone, S.M.; Kapikian, A.Z.; Purcell, R.H.; Alter, H.J.; Holland, P.V. Transfusion-Associated Hepatitis Not Due to Viral Hepatitis Type A or B. N. Engl. J. Med. 1975, 292, 767–770. [Google Scholar] [CrossRef]
- Prince, A.; Grady, G.; Hazzi, C.; Brotman, B.; Kuhns, W.; Levine, R.; Millian, S. Long-incubation post-transfusion hepatitis without serological evidence of exposure to hepatitis-B virus. Lancet 1974, 2, 241–246. [Google Scholar] [CrossRef]
- Alter, H.; Holland, P.; Purcell, R.; Popper, H. Transmissible Agent in Non-A, Non-B Hepatitis. Lancet 1978, 311, 459–463. [Google Scholar] [CrossRef]
- Bradley, D.W.; Maynard, J.E.; Popper, H.; Cook, E.H.; Ebert, J.W.; McCaustland, K.A.; Schable, C.A.; Fields, H.A. Posttransfusion Non-A, Non-B Hepatitis: Physicochemical Properties of Two Distinct Agents. J. Infect. Dis. 1983, 148, 254–265. [Google Scholar] [CrossRef]
- Choo, Q.-L.; Kuo, G.; Weiner, A.J.; Overby, L.R.; Bradley, D.W.; Houghton, M. Isolation of a cDNA cLone Derived from a Blood-Borne Non-A, Non-B Viral Hepatitis Genome. Science 1989, 244, 359–362. [Google Scholar] [CrossRef]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Murphy, D.G.; Sablon, E.; Chamberland, J.; Fournier, E.; Dandavino, R.; Tremblay, C.L. Hepatitis C Virus Genotype 7, a New Genotype Originating from Central Africa. J. Clin. Microbiol. 2015, 53, 967–972. [Google Scholar] [CrossRef]
- Ghaderi-Zefrehi, H.; Gholami-Fesharaki, M.; Sharafi, H.; Sadeghi, F.; Alavian, S.M. The Distribution of Hepatitis C Virus Genotypes in Middle Eastern Countries: A Systematic Review and Meta-Analysis. Hepat. Mon. 2016, 16, e40357. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee, Clinical Practice Guidelines Panel C, representative EGB, Panel m. EASL recommendations on treatment of hepatitis C: Final update of the series (☆). J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Jazwinski, A.B.; Muir, A.J. Direct-acting antiviral medications for chronic hepatitis C virus infection. Gastroenterol. Hepatol. 2011, 7, 154–162. [Google Scholar]
- Bacon, B.R.; Gordon, S.C.; Lawitz, E.; Marcellin, P.; Vierling, J.M.; Zeuzem, S.; Poordad, F.; Goodman, Z.D.; Sings, H.L.; Boparai, N.; et al. Boceprevir for Previously Treated Chronic HCV Genotype 1 Infection. N. Engl. J. Med. 2011, 364, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; McCone, J., Jr.; Bacon, B.R.; Bruno, S.; Manns, M.P.; Sulkowski, M.S.; Jacobson, I.M.; Reddy, K.R.; Goodman, Z.D.; Boparai, N.; et al. Boceprevir for Untreated Chronic HCV Genotype 1 Infection. N. Engl. J. Med. 2011, 364, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.E.; Flamm, S.L.; Afdhal, N.H.; Nelson, D.R.; Sulkowski, M.S.; Everson, G.T.; Fried, M.W.; Adler, M.; Reesink, H.W.; Martin, M.; et al. Response-Guided Telaprevir Combination Treatment for Hepatitis C Virus Infection. N. Engl. J. Med. 2011, 365, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, I.M.; McHutchison, J.G.; Dusheiko, G.; Di Bisceglie, A.M.; Reddy, K.R.; Bzowej, N.H.; Marcellin, P.; Muir, A.J.; Ferenci, P.; Flisiak, R.; et al. Telaprevir for Previously Untreated Chronic Hepatitis C Virus Infection. N. Engl. J. Med. 2011, 364, 2405–2416. [Google Scholar] [CrossRef]
- Romano, K.P.; Ali, A.; Royer, W.E.; Schiffer, C.A. Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc. Natl. Acad. Sci. USA 2010, 107, 20986–20991. [Google Scholar] [CrossRef]
- Zeuzem, S.; Andreone, P.; Pol, S.; Lawitz, E.; Diago, M.; Roberts, S.; Focaccia, R.; Younossi, Z.; Foster, G.R.; Horban, A.; et al. Telaprevir for Retreatment of HCV Infection. N. Engl. J. Med. 2011, 364, 2417–2428. [Google Scholar] [CrossRef]
- Gordon, S.C.; Muir, A.J.; Lim, J.K.; Pearlman, B.; Argo, C.K.; Ramani, A.; Maliakkal, B.; Alam, I.; Stewart, T.G.; Vainorius, M.; et al. Safety profile of boceprevir and telaprevir in chronic hepatitis C: Real world experience from HCV-TARGET. J. Hepatol. 2014, 62, 286–293. [Google Scholar] [CrossRef]
- Poordad, F.; Dieterich, D. Treating hepatitis C: Current standard of care and emerging direct-acting antiviral agents. J. Viral Hepat. 2012, 19, 449–464. [Google Scholar] [CrossRef]
- Asselah, T.; Marcellin, P.; Schinazi, R.F. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018, 38 (Suppl. S1), 7–13. [Google Scholar] [CrossRef]
- Zibbell, J.E.; Asher, A.K.; Patel, R.C.; Kupronis, B.; Iqbal, K.; Ward, J.W.; Holtzman, D. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am. J. Public Health 2018, 108, 175–181. [Google Scholar] [CrossRef]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Kabarra, K.; Golabi, P.; Younossi, Z.M. Nonalcoholic steatohepatitis: Global impact and clinical consequences. Endocr. Connect. 2021, 10, R240–R247. [Google Scholar] [CrossRef]
- Elsaid, M.I.; Bridges, J.F.; Li, N.; Rustgi, V.K. Metabolic Syndrome Severity Predicts Mortality in Nonalcoholic Fatty Liver Disease. Gastro. Hep. Adv. 2022, 1, 445–456. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Harrison, S.A.; Ratziu, V.; Abdelmalek, M.; Diehl, A.M.; Caldwell, S.; Shiffman, M.L.; Schall, R.A.; Jia, C.; McColgan, B.; et al. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data from the Simtuzumab Trials. Hepatology 2019, 70, 1913–1927. [Google Scholar] [CrossRef]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 July 2021).
- Kim, J.Y.; He, F.; Karin, M. From Liver Fat to Cancer: Perils of the Western Diet. Cancers 2021, 13, 1095. [Google Scholar] [CrossRef]
- Shirazi, F.; Wang, J.; Wong, R.J. Nonalcoholic Steatohepatitis Becomes the Leading Indication for Liver Transplant Registrants Among US Adults Born Between 1945 and 1965. J. Clin. Exp. Hepatol. 2019, 10, 30–36. [Google Scholar] [CrossRef]
- Flemming, J.A.; Kim, W.R.; Brosgart, C.L.; Terrault, N.A. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 2016, 65, 804–812. [Google Scholar] [CrossRef]
- Parrish, N.F.; Feurer, I.D.; Matsuoka, L.K.; Rega, S.A.; Perri, R.; Alexopoulos, S.P. The Changing Face of Liver Transplantation in the United States: The Effect of HCV Antiviral Eras on Transplantation Trends and Outcomes. Transpl. Direct 2019, 5, e427. [Google Scholar] [CrossRef]
- Perricone, G.; Duvoux, C.; Berenguer, M.; Cortesi, P.A.; Vinaixa, C.; Facchetti, R.; Mazzarelli, C.; Rockenschaub, S.-R.; Martini, S.; Morelli, C.; et al. Delisting HCV-infected liver transplant candidates who improved after viral eradication: Outcome 2 years after delisting. Liver Int. 2018, 38, 2170–2177. [Google Scholar] [CrossRef]
- Samji, N.S.; Heda, R.; Satapathy, S.K. Peri-transplant management of nonalcoholic fatty liver disease in liver transplant candidates. Transl. Gastroenterol. Hepatol. 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.-F.; Anstee, Q.M.; Bugianesi, E.; Harrison, S.; Loomba, R.; Paradis, V.; Tilg, H.; Wong, V.W.-S.; Zelber-Sagi, S. Current therapies and new developments in NASH. Gut 2022, 71, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef]
- Wong, R.J.; Singal, A.K. Trends in Liver Disease Etiology Among Adults Awaiting Liver Transplantation in the United States, 2014-2019. JAMA Netw. Open 2020, 3, e1920294. [Google Scholar] [CrossRef]
- Alkhouri, N.; Tincopa, M.; Loomba, R.; Harrison, S.A. What Does the Future Hold for Patients with Nonalcoholic Steatohepatitis: Diagnostic Strategies and Treatment Options in 2021 and Beyond? Hepatol. Commun. 2021, 5, 1810–1823. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef]
- Pandey, N.; Hoilat, G.J.; John, S. Liver Biopsy; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ando, Y.; Jou, J.H. Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin. Liver Dis. 2021, 17, 23–28. [Google Scholar] [CrossRef]
- Graupera, I.; Thiele, M.; Serra-Burriel, M.; Caballeria, L.; Roulot, D.; Wong, G.L.-H.; Fabrellas, N.; Guha, I.N.; Arslanow, A.; Expósito, C.; et al. Low Accuracy of FIB-4 and NAFLD Fibrosis Scores for Screening for Liver Fibrosis in the Population. Clin. Gastroenterol. Hepatol. 2021, 20, 2567–2576.e6. [Google Scholar] [CrossRef]
- Sun, W.; Cui, H.; Hongli, C.; Wei, Y.; Lai, S.; Yang, Y.; Yin, X.; Chen, D.-F. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol. Res. 2016, 46, 862–870. [Google Scholar] [CrossRef]
- Luo, Y.; Oseini, A.; Gagnon, R.; Charles, E.D.; Sidik, K.; Vincent, R.; Collen, R.; Idowu, M.; Contos, M.J.; Mirshahi, F.; et al. An Evaluation of the Collagen Fragments Related to Fibrogenesis and Fibrolysis in Nonalcoholic Steatohepatitis. Sci. Rep. 2018, 8, 12414. [Google Scholar] [CrossRef]
- Mak, A.L.; Lee, J.; van Dijk, A.-M.; Vali, Y.; Aithal, G.P.; Schattenberg, J.M.; Anstee, Q.M.; Brosnan, M.J.; Zafarmand, M.H.; Ramsoekh, D.; et al. Systematic Review with Meta-Analysis: Diagnostic Accuracy of Pro-C3 for Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines 2021, 9, 1920. [Google Scholar] [CrossRef]
- Ravaioli, F.; Dajti, E.; Mantovani, A.; Newsome, P.N.; Targher, G.; Colecchia, A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: A systematic review and meta-analysis. Gut 2023, 72, 1399–1409. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, X.J.; Ma, L. Diagnostic performance of magnetic resonance technology in detecting steatosis or fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Medicine 2018, 97, e10605. [Google Scholar] [CrossRef]
- Noureddin, N.; Schattenberg, J.M.; Alkhouri, N.; Noureddin, M. Noninvasive Testing Using Magnetic Resonance Imaging Techniques as Outcomes in Nonalcoholic Steatohepatitis Clinical Trials: How Full Is the Glass? Hepatol. Commun. 2020, 4, 141–144. [Google Scholar] [CrossRef]
- Dulai, P.S.; Sirlin, C.B.; Loomba, R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J. Hepatol. 2016, 65, 1006–1016. [Google Scholar] [CrossRef]
- Meir, A.Y.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Anthony Sinha, R. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front. Biosci. (Landmark Ed.) 2021, 26, 206–237. [Google Scholar] [CrossRef]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef]
- Kistler, K.D.; Brunt, E.M.; Clark, J.M.; Diehl, A.M.; Sallis, J.F.; Schwimmer, J.B.; NASH CRN Research Group. Physical Activity Recommendations, Exercise Intensity, and Histological Severity of Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2011, 106, 460–468. [Google Scholar] [CrossRef]
- Shroff, H.; VanWagner, L.B. Cardiovascular Disease in Nonalcoholic Steatohepatitis: Screening and Management. Curr. Hepatol. Rep. 2020, 19, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Sanyal, A.J.; Loomba, R.; Rinella, M.; Harrison, S.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; MacConell, L.; Shringarpure, R.; et al. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp. Clin. Trials 2019, 84, 105803. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 394, 2012–2024. [Google Scholar] [CrossRef]
- Ratziu, V.; de Guevara, L.; Safadi, R.; Poordad, F.; Fuster, F.; Flores-Figueroa, J.; Arrese, M.; Fracanzani, A.L.; Ben Bashat, D.; Lackner, K.; et al. Aramchol in patients with nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 2021, 27, 1825–1835. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef]
- Harrison, S.A.; Abdelmalek, M.F.; Neff, G.; Gunn, N.; Guy, C.D.; Alkhouri, N.; Bashir, M.R.; Freilich, B.; Kohli, A.; Khazanchi, A.; et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 2022, 7, 603–616. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Alkhouri, N.; Herring, R.; Kabler, H.; Kayali, Z.; Hassanein, T.; Kohli, A.; Huss, R.S.; Zhu, Y.; Billin, A.N.; Damgaard, L.H.; et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial. J. Hepatol. 2022, 77, 607–618. [Google Scholar] [CrossRef]
- Simon, T.G.; Henson, J.; Osganian, S.; Masia, R.; Chan, A.T.; Chung, R.T.; Corey, K.E. Daily Aspirin Use Associated with Reduced Risk for Fibrosis Progression In Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 2776–2784.e4. [Google Scholar] [CrossRef]
- Torres-Peña, J.D.; Martín-Piedra, L.; Fuentes-Jiménez, F. Statins in Non-alcoholic Steatohepatitis. Front. Cardiovasc. Med. 2021, 8, 777131. [Google Scholar] [CrossRef]
- Athyros, V.G.; Tziomalos, K.; Gossios, T.D.; Griva, T.; Anagnostis, P.; Kargiotis, K.; Pagourelias, E.D.; Theocharidou, E.; Karagiannis, A.; Mikhailidis, D.P. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: A post-hoc analysis. Lancet 2010, 376, 1916–1922. [Google Scholar] [CrossRef]
- Pastori, D.; Pani, A.; Di Rocco, A.; Menichelli, D.; Gazzaniga, G.; Farcomeni, A.; D’Erasmo, L.; Angelico, F.; Del Ben, M.; Baratta, F. Statin liver safety in non-alcoholic fatty liver disease: A systematic review and metanalysis. Br. J. Clin. Pharmacol. 2021, 88, 441–451. [Google Scholar] [CrossRef]
- Caldwell, S. NASH Therapy: Omega 3 supplementation, vitamin E, insulin sensitizers and statin drugs. Clin. Mol. Hepatol. 2017, 23, 103–108. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Targher, G. Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials. Metabolites 2021, 11, 73. [Google Scholar] [CrossRef]
- Lai, L.L.; Vethakkan, S.R.; Nik Mustapha, N.R.; Mahadeva, S.; Chan, W.K. Empagliflozin for the Treatment of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes Mellitus. Dig. Dis. Sci. 2020, 65, 623–631. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2018, 21, 285–292. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, X.; Guo, L.; Li, J.; Li, L. Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus with Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2021, 12, 635556. [Google Scholar] [CrossRef]
- De Block, C.; Bailey, C.; Wysham, C.; Hemmingway, A.; Allen, S.E.; Peleshok, J. Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obes. Metab. 2022, 25, 3–17. [Google Scholar] [CrossRef]
- Valenzuela, R.; Ortiz, M.; Hernández-Rodas, M.C.; Echeverría, F.; Videla, L.A. Targeting n-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease. Curr. Med. Chem. 2020, 27, 5250–5272. [Google Scholar] [CrossRef]
- Antraco, V.J.; Hirata, B.K.S.; de Jesus Simão, J.; Cruz, M.M.; da Silva, V.S.; da Cunha de Sá, R.D.C.; Abdala, F.M.; Armelin-Correa, L.M.; Alonso-Vale, M.I.C. Omega-3 polyunsaturated fatty acids prevent nonalcoholic steatohepatitis (NASH) and stimulate adipogenesis. Nutrients 2021, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Bernardi, M.; Angeli, P. Towards a new definition of decompensated cirrhosis. J. Hepatol. 2022, 76, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Feldstein, A.E. Diagnosis of Nonalcoholic Fatty Liver Disease: Invasive versus Noninvasive. Semin. Liver Dis. 2008, 28, 386–395. [Google Scholar] [CrossRef]
- Boursier, J.; Shreay, S.; Fabron, C.; Torreton, E.; Fraysse, J. Hospitalization costs and risk of mortality in adults with nonalcoholic steatohepatitis: Analysis of a French national hospital database. Eclinicalmedicine 2020, 25, 100445. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Wang, S.; Toy, M.; Pham, T.T.H.; So, S. Causes and trends in liver disease and hepatocellular carcinoma among men and women who received liver transplants in the U.S., 2010–2019. PLoS ONE 2020, 15, e0239393. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2018, 17, 748–755.e3. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Stine, J.G.; Wentworth, B.J.; Zimmet, A.; Rinella, M.E.; Loomba, R.; Caldwell, S.H.; Argo, C.K. Systematic review with meta-analysis: Risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment. Pharmacol. Ther. 2018, 48, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Fazel, S.; Otgonsuren, M.; Sayiner, M.; Locklear, C.T.; Younossi, Z.M. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine 2017, 96, e5904. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Henry, L.; De Avila, L.; Younossi, E.; Racila, A.; Younossi, Z.M. Mortality Related to Nonalcoholic Fatty Liver Disease Is Increasing in the United States. Hepatol. Commun. 2019, 3, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Felsher, D.W. A Tale of Two Complications of Obesity: NASH and Hepatocellular Carcinoma. Hepatology 2019, 70, 1056–1058. [Google Scholar] [CrossRef]
- Stine, J.G.; Niccum, B.A.; Zimmet, A.N.; Intagliata, N.; Caldwell, S.H.; Argo, C.K.; Northup, P.G. Increased risk of venous thromboembolism in hospitalized patients with cirrhosis due to non-alcoholic steatohepatitis. Clin. Transl. Gastroenterol. 2018, 9, e140. [Google Scholar] [CrossRef]
- Stepanova, M.; Rafiq, N.; Makhlouf, H.; Agrawal, R.; Kaur, I.; Younoszai, Z.; McCullough, A.; Goodman, Z.; Younossi, Z.M. Predictors of All-Cause Mortality and Liver-Related Mortality in Patients with Non-Alcoholic Fatty Liver Disease (NAFLD). Dig. Dis. Sci. 2013, 58, 3017–3023. [Google Scholar] [CrossRef]
- Paik, J.; Golabi, P.; Younoszai, Z.; Mishra, A.; Trimble, G.; Younossi, Z.M. Chronic kidney disease is independently associated with increased mortality in patients with nonalcoholic fatty liver disease. Liver Int. 2018, 39, 342–352. [Google Scholar] [CrossRef]
- Ng, C.H.; Chan, K.E.; Chin, Y.H.; Zeng, R.W.; Tsai, P.C.; Lim, W.H.; Tan, D.J.H.; Khoo, C.M.; Goh, L.H.; Ling, Z.J.; et al. The effect of diabetes and prediabetes on the prevalence, complications and mortality in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2022, 28, 565–574. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Tampi, R.P.; Racila, A.; Qiu, Y.; Burns, L.; Younossi, I.; Nader, F. Economic and Clinical Burden of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes in the U.S. Diabetes Care 2019, 43, 283–289. [Google Scholar] [CrossRef]
- Barb, D.; Repetto, E.M.; Stokes, M.E.; Shankar, S.S.; Cusi, K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity 2021, 29, 1950–1960. [Google Scholar] [CrossRef]
- Golabi, P.; Bush, H.; Stepanova, M.; Locklear, C.T.; Jacobson, I.M.; Mishra, A.; Trimble, G.; Erario, M.; Venkatesan, C.; Younossi, I.; et al. Liver Transplantation (LT) for Cryptogenic Cirrhosis (CC) and Nonalcoholic Steatohepatitis (NASH) Cirrhosis: Data from the Scientific Registry of Transplant Recipients (SRTR): 1994 to 2016. Medicine 2018, 97, e11518. [Google Scholar] [CrossRef]
- Nagai, S.; Collins, K.; Chau, L.C.; Safwan, M.; Rizzari, M.; Yoshida, A.; Abouljoud, M.S.; Moonka, D. Increased Risk of Death in First Year After Liver Transplantation Among Patients with Nonalcoholic Steatohepatitis vs Liver Disease of Other Etiologies. Clin. Gastroenterol. Hepatol. 2019, 17, 2759–2768.e5. [Google Scholar] [CrossRef]
- Lee, D.U.; Han, J.; Lee, K.J.; Kwon, J.; Fan, G.H.; Jung, D.; Urrunaga, N.H. The clinical implications of pre-liver transplant diabetes on post-liver transplant outcomes in patients with NASH: Analysis of the UNOS database. Hepatol. Int. 2022, 16, 1448–1457. [Google Scholar] [CrossRef]
- Agbim, U.; Jiang, Y.; Kedia, S.K.; Singal, A.K.; Ahmed, A.; Bhamidimarri, K.R.; Bernstein, D.E.; Harrison, S.A.; Younossi, Z.M.; Satapathy, S.K. Impact of Nonmalignant Portal Vein Thrombosis in Transplant Recipients with Nonalcoholic Steatohepatitis. Liver Transpl. 2019, 25, 68–78. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Bhave, M.; Te, H.S.; Feinglass, J.; Alvarez, L.; Rinella, M.E. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology 2012, 56, 1741–1750. [Google Scholar] [CrossRef]
- Malhi, H.; Allen, A.M.; Watt, K.D. Nonalcoholic fatty liver: Optimizing pretransplant selection and posttransplant care to maximize survival. Curr. Opin. Organ Transplant. 2016, 21, 99–106. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Duseja, A. Screening of Cardiovascular Disease in Nonalcoholic Fatty Liver Disease: Whom and How? J. Clin. Exp. Hepatol. 2019, 9, 506–514. [Google Scholar] [CrossRef]
- Cheng, X.S.; VanWagner, L.B.; Costa, S.P.; Axelrod, D.A.; Bangalore, S.; Norman, S.P.; Herzog, C.A.; Lentine, K.L.; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Cardiovascular Radiology and Intervention. Emerging Evidence on Coronary Heart Disease Screening in Kidney and Liver Transplantation Candidates: A Scientific Statement from the American Heart Association: Endorsed by the American Society of Transplantation. Circulation 2022, 146, e299–e324. [Google Scholar] [CrossRef]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R., Jr.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2013, 59, 1144–1165. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Jiang, Y.; Agbim, U.; Wu, C.; Bernstein, D.E.; Teperman, L.W.; Kedia, S.K.; Aithal, G.P.; Bhamidimarri, K.R.; Duseja, A.; et al. Posttransplant Outcome of Lean Compared with Obese Nonalcoholic Steatohepatitis in the United States: The Obesity Paradox. Liver Transpl. 2019, 26, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Lalezari, D.; Pruthi, P.; Alper, T.; Tong, M.J. The impact of obesity on patient survival in liver transplant recipients: A meta-analysis. Liver Int. 2014, 35, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Bhati, C.; Idowu, M.O.; Sanyal, A.J.; Rivera, M.; Driscoll, C.; Stravitz, R.T.; Kohli, D.R.; Matherly, S.; Puri, P.; Gilles, H.; et al. Long-term Outcomes in Patients Undergoing Liver Transplantation for Nonalcoholic Steatohepatitis-Related Cirrhosis. Transplantation 2017, 101, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.R.; Cockbain, A.J.; Raza, S.S.; Pollard, S.G.; Toogood, G.J.; Attia, M.A.; Ahmad, N.; Hidalgo, E.L.; Prasad, K.R.; Menon, K.V. Increased morbidity in overweight and obese liver transplant recipients: A single-center experience of 1325 patients from the United Kingdom. Liver Transpl. 2013, 19, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Spiritos, Z.; Abdelmalek, M.F. Metabolic syndrome following liver transplantation in nonalcoholic steatohepatitis. Transl. Gastroenterol. Hepatol. 2021, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Gunson, B.; Johnson, J.; Neuberger, J. Weight gain and obesity after liver transplantation. Transpl. Int. 2005, 18, 461–466. [Google Scholar] [CrossRef]
- De Luca, L.; Westbrook, R.; Tsochatzis, E.A. Metabolic and cardiovascular complications in the liver transplant recipient. Ann. Gastroenterol. 2015, 28, 183–192. [Google Scholar]
- Malik, S.M.; Devera, M.E.; Fontes, P.; Shaikh, O.; Sasatomi, E.; Ahmad, J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009, 15, 1843–1851. [Google Scholar] [CrossRef]
- van Son, J.; Stam, S.P.; Gomes-Neto, A.W.; Osté, M.C.; Blokzijl, H.; van den Berg, A.P.; Porte, R.J.; Bakker, S.J.; de Meijer, V.E. Post-transplant obesity impacts long-term survival after liver transplantation. Metabolism 2020, 106, 154204. [Google Scholar] [CrossRef]
- Cassiman, D.; Roelants, M.; Vandenplas, G.; Van der Merwe, S.W.; Mertens, A.; Libbrecht, L.; Verslype, C.; Fevery, J.; Aerts, R.; Pirenne, J.; et al. Orlistat treatment is safe in overweight and obese liver transplant recipients: A prospective, open label trial. Transpl. Int. 2006, 19, 1000–1005. [Google Scholar] [CrossRef]
- Ahmed, Z.; Khan, M.A.; Vazquez-Montesino, L.M.; Ahmed, A. Bariatric surgery, obesity and liver transplantation. Transl. Gastroenterol. Hepatol. 2022, 7, 25. [Google Scholar] [CrossRef]
- Idriss, R.; Hasse, J.; Wu, T.; Khan, F.; Saracino, G.; McKenna, G.; Testa, G.; Trotter, J.; Klintmalm, G.; Asrani, S.K. Impact of Prior Bariatric Surgery on Perioperative Liver Transplant Outcomes. Liver Transpl. 2019, 25, 217–227. [Google Scholar] [CrossRef]
- Chierici, A.; Alromayan, M.; Defatico, S.; Céline, D.R.A.I.; Vinci, D.; Rodolphe, A.N.T.Y.; Schiavo, L.; Iannelli, A. Is bariatric surgery safer before, during, or after liver transplantation? A systematic review and meta-analysis. J. Liver Transpl. 2023, 9, 100139. [Google Scholar] [CrossRef]
- Zamora-Valdes, D.; Watt, K.D.; Kellogg, T.A.; Poterucha, J.J.; Di Cecco, S.R.; Francisco-Ziller, N.M.; Taner, T.; Rosen, C.B.; Heimbach, J.K. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology 2018, 68, 485–495. [Google Scholar] [CrossRef]
- Sharma, P.; Arora, A. Approach to prevention of non-alcoholic fatty liver disease after liver transplantation. Transl. Gastroenterol. Hepatol. 2020, 5, 51. [Google Scholar] [CrossRef]
- Iacob, S.; Beckebaum, S.; Iacob, R.; Gheorghe, C.; Cicinnati, V.; Popescu, I.; Gheorghe, L. Genetic and Life Style Risk Factors for Recurrent Non-alcoholic Fatty Liver Disease Following Liver Transplantation. Front. Nutr. 2022, 8, 787430. [Google Scholar] [CrossRef]
- Taneja, S.; Roy, A. Nonalcoholic steatohepatitis recurrence after liver transplant. Transl. Gastroenterol. Hepatol. 2020, 5, 24. [Google Scholar] [CrossRef]
- Burra, P.; Becchetti, C.; Germani, G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. 2020, 2, 100192. [Google Scholar] [CrossRef]
- Charlton, M.; Rinella, M.; Patel, D.; McCague, K.; Heimbach, J.; Watt, K. Everolimus Is Associated with Less Weight Gain Than Tacrolimus 2 Years After Liver Transplantation: Results of a Randomized Multicenter Study. Transplantation 2017, 101, 2873–2882. [Google Scholar] [CrossRef]
- Fairfield, C.J.; Harrison, E.M.; Wigmore, S.J.; VanWagner, L.B. Steroid-Free Versus Steroid-Containing Immunosuppression for Liver Transplant Recipients. Clin. Liver Dis. 2020, 16, 193–197. [Google Scholar] [CrossRef]
- Mitchell, O.; Gurakar, A. Management of Hepatitis C Post-liver Transplantation: A Comprehensive Review. J. Clin. Transl. Hepatol. 2015, 3, 140–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vinaixa, C.; Rubin, A.; Aguilera, V.; Berenguer, M. Recurrence of hepatitis C after liver transplantation. Ann. Gastroenterol. 2013, 26, 304–313. [Google Scholar] [PubMed]
- Berenguer, M.; Prieto, M.; Rayón, J.M.; Mora, J.; Pastor, M.; Ortiz, V.; Carrasco, D.; Juan, F.S.; Burgueño, M.; Mir, J.; et al. Natural History of Clinically Compensated Hepatitis C Virus–Related Graft Cirrhosis After Liver Transplantation. Hepatology 2000, 32, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Carrión, J.A.; Navasa, M.; Forns, X. Retransplantation in patients with hepatitis C recurrence after liver transplantation. J. Hepatol. 2010, 53, 962–970. [Google Scholar] [CrossRef]
- Cieciura, T.; Hryniewiecka, E.; Foroncewicz, B.; Strzelczyk, Z.; Ciszek, M.; Paczek, L. Long-Term Follow-up of Liver Transplant Recipients Treated with Direct-Acting Antiviral Agents for Hepatitis C Recurrence After Transplantation. Transpl. Proc. 2020, 52, 2468–2471. [Google Scholar] [CrossRef]
- Suraweera, D.; Sundaram, V.; Saab, S. Treatment of Hepatitis C Virus Infection in Liver Transplant Recipients. Gastroenterol. Hepatol. 2016, 12, 23–30. [Google Scholar]
- Grassi, A.; Ballardini, G. Post-liver transplant hepatitis C virus recurrence: An unresolved thorny problem. World J. Gastroenterol. 2014, 20, 11095–11115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).