Abstract
The aim of the present study was to test a new oral contrast medium composed of natural components for the magnetic resonance (MR) imaging of small bowel diseases. Between January 2018 and June 2019, 35 patients affected by ileocolic Crohn’s disease (CD) were enrolled in the present study. Each patient underwent two sequential MR enterographies, first with the standard polyethylene glycol (PEG) water solution and, after 3 weeks, with the new natural beverage designed by our team. At the end of the administration of each oral contrast, a satisfaction survey was given to the patients to assess the palatability of both beverages. The intestinal distention and the quality of images were evaluated by two expert radiologists for both studies and the interreader agreement was calculated. According to the satisfaction questionnaire, 97.1% of patients expressed positive judgments regarding the natural beverage (71.4% very good and 25.7% good) whereas only 8.6% of them appreciated the PEG water solution (8.6% good) (p = 0.0001). The degree of intestinal distention was excellent and good in 97.1% of patients after the administration of PEG and in 94.3% of the patients after the administration of the natural beverage, without significant differences between the two products and with almost perfect (k = 0.821) and substantial (k = 0.754) inter-observer variability, respectively. No statistical differences were observed between the two expert radiologists regarding the evaluation of the imaging quality; in particular, they were considered good and excellent in 100% of patients after the administration of PEG water solution and in 97.2% of those who took the natural beverage, with substantial (k = 0.618) and almost perfect (k = 0.858) inter-observer variability, respectively. The new natural beverage demonstrated the same intestinal distension and excellent image quality compared to the synthetic standard oral contrast administered during MRE for small bowel diseases, proving to be a valid alternative with better palatability.
1. Introduction
Magnetic Resonance Enterography (MRE) is a widely recognized technique for obtaining a detailed evaluation of the small bowel, otherwise poorly detectable with conventional endoscopic examinations such as esophagogastroduodenoscopy and colonoscopy, in particular in patients with inflammatory bowel diseases (IBDs) and, specifically, Crohn’s disease (CD) [1,2].
MRE has supplanted the fluoroscopic examinations carried out with barium, such as small bowel follow-through, with the advantage of avoiding radiation, being more tolerated by the patient and offering more diagnostic information. In fact, fluoroscopic examinations are able to opacify the intestinal lumen, identifying only the indirect signs of the pathology affecting the intestinal wall [3]. Conversely, MRE directly evaluates wall thickening, segmental mural hyperenhancement, intramural edema, strictures with or without upstream dilation, ulcerations and sacculations [4,5,6]. In addition, unlike traditional imaging examinations, MRE provides a panoramic overview of the extra-intestinal abdominal compartments, such as vasculature, lymph nodes, mesentery and abdominal and pelvic main organs [4]. Currently, MRE is characterized by a high diagnostic value, not only in identifying disease involvement, activity, and signs of complicated disease, such as fistula and/or abscesses but also in monitoring the response to medical treatment or progression to a fibrotic disease [7,8,9]. This imaging technique is also preliminary to the videocapsule examination, allowing the detection of any stenosis which may preclude its feasibility [10]. Furthermore, some sequences of MRE also currently allow the evaluation of intestinal peristalsis, which could be altered in IBDs [11].
To date, thanks to the undoubted advantages obtained in the last decade owing to technical refinements, MRE also plays a pivotal role, not only in the setting of IBDs, but also in a large variety of other clinical situations involving the small bowel, such as chronic diarrhea, chronic anemia, melena, unexplained weight loss, malabsorption, intestinal polyposis, celiac disease with new onset disorders, suspected intestinal parasites, suspected intestinal metastases and, finally, indeterminate abdominal pain which has been investigated with various methods without success [1,5,12].
However, in order to obtain information regarding all the above-mentioned pathologies, it is mandatory to perform an optimal MRE examination based on a good distention of the intestinal loops, obtained by means of the administration of an oral contrast agent water solution called polyethylene glycol (PEG) electrolyte approximately 45 min before the examination [13].
Unfortunately, PEG is often not well tolerated by many patients for various reasons: its unpalatability, the large quantity that has to be consumed in a relatively short period of time and the possible adverse reactions, such as abdominal pain, diarrhea and nausea. Furthermore, this product presents multiple contraindications to its use which are reported in the package leaflet of the drug, such as hypersensitivity to the active substances or to any of the excipients, gastrointestinal perforation, severe IBDs, subocclusive or stenotic occlusive forms of the intestine, gastric stasis, dynamic ileus, paralytic ileus, abdominal pain of unknown origin, acute colitis, nausea, vomiting, marked accentuation or reduction of peristalsis, rectal bleeding and a severe state of dehydration. It is generally contraindicated in children under the age of 8 or weighing less than 20 kg as well as during pregnancy.
It should be noted that many of these contraindications are quite limiting and in the majority of cases, especially in IBD patients, they represent the same indications for performing an MRE examination. Hence the idea of designing and testing a new oral contrast agent, invented and patented by our Team, with characteristics similar to those of the PEG solution, but made up of natural components and more palatable to drink in order to overcome the above-mentioned contraindications of PEG was born.
The primary aim of the study was to test, when carrying out MRE, the effectiveness of the natural beverage in adequately distending the bowel loops as compared with the standard technique achieved after the oral administration of the PEG water solution. The secondary aim of the study was to evaluate the palatability of the natural beverage as compared to PEG, linking this parameter with the quantity of beverage consumed.
2. Materials and Methods
This was a single-center prospective study, that began in January 2018 and was carried out until June 2019 at the IRCCS Policlinico Sant’Orsola-Malpighi of Bologna, Bologna, Italy. This study was approved by the Institutional Review Board (Comitato Etico di Area Vasta Emilia Centro della Regione Emilia-Romagna) of the IRCCS Policlinico Sant’Orsola-Malpighi of Bologna with registration number 584/2019/Sper/AOUBo. Written informed consent was obtained from all patients. This study was conducted according to the Declaration of Helsinki for clinical studies.
The tested new natural beverage was an aqueous-based mixture of mucilages, polysaccharides, carbohydrates/saccharides, salts and antioxidants, all of natural origins, specifically formulated and produced to ensure the same effects and the same osmotic characteristics of PEG solution. The recipe of the tested beverage has been deposited as a Patent.
The validation of this natural beverage used for replacing PEG in MRE, which represented the first experimental phase of the above-mentioned protocol, was successfully tested in 20 healthy volunteers who underwent a standard MRE in order to optimize the final composition, in terms of concentration, quantity, palatability and the onset of common adverse reactions. After identifying the correct concentration for the adequate distension of the bowel loops and the absence of adverse events related to the natural beverage, the second phase of the present study began.
The present study population was composed of patients affected by ileocolic CD who agreed to undergo two sequential MRE examinations. In fact, each patient first underwent a standard MRE performed after the administration of PEG according to the timing of the scheduled diagnostic-therapeutic pathway and the second one after the consumption of the natural beverage, in order to compare the two different oral beverages in CD patients. Each patient first underwent the standard MRE with PEG in order to avoid any delay in the normal diagnostic-therapeutic pathway. The second MRE was carried out within 7 ± 3 days, using the same machine and the same protocol in order to reduce the potential bias related to disease progression or radiological changes resulting from the start of the therapy required.
At the end of the administration of each oral beverage, a satisfaction survey was given to the patient, with the purpose of evaluating the palatability of both beverages and comparing them. The satisfaction questionnaire expressed the degree of palatability on a 5-point scale: 1 (very good), 2 (good), 3 (moderate), 4 (not good) and 5 (unpleasant).
2.1. MRE Technique
The most widely used contrast medium orally administered to obtain optimal distension of the intestinal loops, for a correct evaluation of the small bowel wall, is a PEG water solution (1500 mL for adults, 80–1000 for pediatric patients), normally administered 45 min before the beginning of the examination [13].
In the Authors’ Institution, the standard oral contrast medium used was Isocolan® (Polifarma, Rome, Italy), and the dose provided, according to the references, was 1500 mL. The same maximum dose was adopted for the natural beverage under study.
After the administration of each beverage, the quantity of contrast medium consumed by each patient before the MRE was recorded on a dedicated database using a 5-point scale: 1 (100%), 2 (75–99%), 3 (50–74%), 4 (25–49%) and (0–25%).
The MRE was performed using a 1.5 T superconducting system (Signa; GE Medical Systems, Milwaukee, WI, USA), with a body phased-array multicoil for signal detection.
In the Authors’ Institution, the acquisition protocol and the parameters of the MRE sequences were carried out according to the literature [4,13,14].
2.2. Statistical Analysis
Two experienced radiologists (MR and AC) with more than 15 years of experience in MRE, blinded both to the amount and the type of beverage administered (Isocolan® or Bever1one) prior to every examination, evaluated the images in a blinded fashion.
A comparison between Group 1 (Isocolan® group) and Group 2 (Bever1one group) was carried out based on the evaluation of the degree of intestinal distention in the standard sequences.
The intestinal distention was graded using a 4-point scale: Grade 1 (excellent distention); Grade 2 (good but not excellent distention); Grade 3 (modest distention) and Grade 4 (inadequate-absent distention).
The radiologists also evaluated the quality of all the MREs for each patient to avoid any possible impact of the type of beverage ingested on the overall quality of the examination. The quality of the images, acquired after the consumption of Isocolan® and Bever1one, was graded using a 4-point scale: Grade 1 (excellent image quality with high homogeneous signal intensity within the intestinal lumen); Grade 2 (good but not excellent quality with a good signal intensity of the intestinal lumen); Grade 3 (modest image quality with a low signal intensity of the biliary lumen) and Grade 4 (non-diagnostic quality with no or minimal signal intensity within the intestinal lumen).
To assess the agreement between the two radiologists, Cohen’s kappa values (κ) were calculated. Kappa values less than 0 indicated no agreement, 0–0.20 indicated a slight agreement, 0.21–0.40 indicated a fair agreement, 0.41–0.60 indicated a moderate agreement, 0.61–0.80 indicated a substantial agreement and 0.81–1 indicated an almost perfect agreement [15,16].
All the tests were two-tailed and a p-value of < 0.05 was considered statistically significant. The statistical analyses were carried out using the SPSS 26.0 package (SPSS Inc., Chicago, IL, USA) and STATA v16 (StataCorp LLC, College Station, TX, USA).
3. Results
The final study population included 35 patients (mean age, 45.94 ± 16.16 years; 16 females [45.7%]). The median interval between the two different MREs, performed after the consumption of Isocolan® and Bever1one, respectively, was 6 days (IQR, 5–9 days).
Table 1 summarises the results of the satisfaction questionnaire in which every patient expressed a degree of satisfaction concerning the taste of Isocolan® and Bever1one. In particular, 34 (97.1%) patients expressed positive judgments (very good and good) regarding Bever1one as compared with only 3 (8.6%) patients who expressed good judgments on Isocolan® (no very good judgment), having statistical differences (p = 0.0001).

Table 1.
Results of the Satisfaction questionnaire in which every patient expressed a degree of satisfaction concerning the taste of Isocolan and Bever1one.
The quantity of each product, Isocolan® and Bever1one, consumed by each patient is reported in Table 2. Specifically, 34 (97.1%) patients consumed 100% or 75% of the predefined quantity of Bever1one as compared to 24 (68.5%) patients who consumed the same quantity of Isocolan®, showing statistical differences (p = 0.0001).

Table 2.
Quantity of each product, Isocolan and Bever1one, consumed by each patient.
Table 3 shows data regarding the comparison between the evaluations carried out by the two expert radiologists concerning the degree of intestinal distention in the entire study population after the consumption of Isocolan® and Bever1one. In general, the majority of the examinations, including those carried out after Isocolan® (97.1%) and those after Bever1one (94.3%), achieved excellent or good intestinal distension, without significant differences between the readers (Figure 1).

Table 3.
Comparison between two expert radiologists in evaluating the degree of intestinal distention in the entire study population after the consumption of Isocolan or Bever1one.
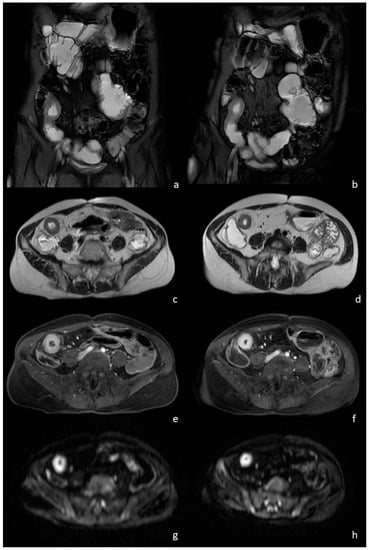
Figure 1.
A 32-year-old female patient with a known history of Crohn’s disease. The coronal T2-w with fat saturation sequence shows a narrowing of the last ileal loop caused by a severe wall thickening in both the MRE performed with the PEG water solution (a) and the study performed with the natural beverage (b). No differences regarding intestinal distension and image quality were observed also on the axial T2-w images (c,d). The same segment showed an intense mural hyperenhancement after intravenous contrast injection (arrows), regardless of the oral contrast used, the PEG water solution (e) or the natural beverage (f). Hyperintensity on the axial DW images was observed in both groups (g,h), without significant differences due to the different oral contrast administrated.
Furthermore, there were no statistical differences between the two expert radiologists in evaluating the imaging quality regarding the entire study population after the consumption of the Isocolan® and Bever1one, as reported in Table 4.

Table 4.
Comparison between two expert radiologists in evaluating the imaging quality on the entire study population after the consumption of Isocolan or Bever1one.
Agreement between Radiologists 1 and 2 concerning the degree of intestinal distension after the consumption of Bever1one and Isocolan® regarding each case is reported in Table 5, Table 6, respectively. In particular, almost perfect agreement (κ = 0.82) was found between the readers concerning the degree of intestinal distention after the consumption of Bever1one and substantial agreement (κ = 0.75) in the evaluation of the MREs performed with Isocolan®.

Table 5.
Agreement between Radiologists 1 and 2 concerning the degree of intestinal distension after the consumption of Bever1one.

Table 6.
Agreement between Radiologists 1 and 2 concerning the degree of intestinal distension after the consumption of Isocolan.
Agreement between Radiologists 1 and 2 concerning the quality of images after the consumption of Bever1one and Isocolan® regarding each case is reported in Table 7, Table 8, respectively. In particular, almost perfect agreement (κ = 0.86) was found between the readers concerning the quality of images after the consumption of Bever1one and substantial agreement (κ = 0.62) in the evaluation of the MREs performed with Isocolan®.

Table 7.
Agreement between Radiologists 1 and 2 concerning the quality of images after the consumption of Bever1one.

Table 8.
Agreement between Radiologists 1 and 2 concerning the quality of images after the consumption of Isocolan.
No patients reported abdominal discomfort, diarrhea, nausea or other gastrointestinal symptoms at the end of the examination or the day after.
4. Discussion
MRE is a standardized, widely adopted technique with elevated diagnostic accuracy for many diseases affecting the small bowel and not only. The best performance in terms of accuracy regarding MRE is strongly dependent on optimal preliminary bowel distention with PEG which, however, has many limitations and contraindications. The aim of the present study was to validate the use of a new oral agent, made up of natural components, for MRE in order to overcome the limits of PEG.
The population of the present study, even though limited in number, proved to be suitable for the study endpoints and evenly well-balanced in terms of gender and age.
The first result, which was also expected and emerged from the satisfaction survey, showed that Bever1one received positive feedback (either very good or good) from the majority of patients as opposed to PEG (Isocolan® in the present study). The difference between these results was statistically significant. The second and consequent result was that the patients consumed a greater amount of the natural beverage as compared to Isocolan® and this gap reached a difference that was statistically significant. In the era of medicine focusing on patient comfort, these results represent relevant elements as patients who appreciate the taste of the beverage will likely consume larger amounts, resulting in optimal bowel distension.
It is also crucial to point out how Bever1one and Isocolan® presented fundamentally similar results in terms of bowel distension and resulting image quality. This was demonstrated by showing two different experienced radiologists, in a blinded fashion and as statistically required, the images obtained after the administration of both oral agents. There was no statistically significant difference in the evaluation of bowel distention by the two readers it was considered to be excellent or good in more than 90% of patients for both agents. This should especially be taken into consideration as good bowel distension plays a pivotal role in evaluating bowel walls, and therefore, in the detection and grading of many pathologies, such as CD [11]. In fact, there was no statistically significant difference in image quality between Isocolan® and Bever1one in the entire study population, thus confirming that the use of the natural beverage did not affect the quality of imaging and, therefore, did not alter the final diagnosis.
Finally, the inter-reader agreement for each patient in terms of bowel distension and image quality was very high for both Isocolan® and Bever1one, although it was slightly better for the latter (almost perfect agreement for Bever1one as compared to the substantial agreement for Isocolan®). The reason for this discrepancy could probably be explained by the different amount of contrast agent consumed by the patients which, as already stated, was significantly less for Isocolan® due to its unpleasant taste; this may have affected the assessment of the images by the radiologists.
It has been recognized that the diseases which require MRE to be evaluated are greatly increasing. Therefore, for the purpose of obtaining the technical optimization of MRE, it will be important to improve its quality and accuracy. The results of this study, if externally validated, would improve this technique, especially in the era of patient-centered medicine. Furthermore, the issue of patient acceptance of PEG as a contrast agent is extremely relevant, in particular, in the pediatric population that often needs to undergo invasive procedures, such as nasogastric tube positioning, to administer an adequate amount of contrast agent, as reported in the literature [17,18]. The authors believe that their idea could be the starting point for future studies regarding this vulnerable population.
The innovative combination of ingredients (Patent deposited) of this new oral contrast medium explains its excellent organoleptic and sensory characteristics. The mucilages contained in the formulation have a soothing and gently relaxing action and stimulate intestinal peristalsis. Similarly, the polysaccharides, which have a slightly gelling action, act both as light osmotic laxatives and intestinal peristalsis stimulants. The presence of carbohydrates/saccharides further contributes to the osmotic laxative proprieties of the beverage and additionally promotes intestinal peristalsis. Similarly, salts have the effect of mild osmotic purgatives. Lastly, the adjunction of antioxidants ensures the final stabilization of the beverage. When suitable combined, these elements guarantee the same effects and the same osmotic characteristics as Isocolan®. Furthermore, due to their natural origin, these ingredients are more pleasant to the taste and have fewer contraindications compared to the synthetic counterpart PEG.
The present study had some limitations. The first is the relatively limited number of patients enrolled even though it was sufficient to reach statistical significance. However, the Authors believe that further research with larger cohorts and involving different centers is necessary to validate the present results and assess the long-term safety and efficacy of this new natural beverage before it can be widely adopted in clinical practice. Another limitation was that the beverage under study had not been tested on a pediatric population; however, this is the next step necessary after the validation of the present results.
5. Conclusions
In conclusion, this paper reports the first experience with the use of a new oral agent for MRE, composed of natural ingredients. This new beverage proved to be an excellent substitute for its synthetic counterpart PEG, overcoming its numerous contraindications. Thanks to its better palatability, Bever1one is much more appreciated by the patients with respect to PEG, leading to large amounts of beverage being consumed, consequently achieving perfect intestinal distention and allowing a diagnostic performance comparable to standard MRE.
Author Contributions
Conceptualization, M.R. and A.C. (Alberta Cappelli); methodology, M.R., M.B. and F.A.G.; software, M.B. and A.C. (Alberta Cappelli); validation, M.R. and A.C. (Alberta Cappelli); formal analysis, M.B., F.A.G. and M.S.; investigation, M.R., M.A.C., A.C. (Arrigo Cattabriga) and A.C. (Alberta Cappelli); resources, M.B., F.A.G., M.S., P.G., G.P. and S.L.; data curation, N.B.; writing—original draft preparation, M.R. and M.A.C.; writing—review and editing, M.R., M.A.C. and N.B.; visualization, M.R. and A.C. (Alberta Cappelli); supervision, M.R. and A.C. (Alberta Cappelli); project administration, R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of IRCCS Policlinico Sant’Orsola-Malpighi of Bologna (Comitato Etico di Area Vasta Emilia Centro della Regione Emilia-Romagna) with registration number 584/2019/Sper/AOUBo.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gatti, M.; Allois, L.; Carisio, A.; Dianzani, C.; Garcia Martinez, M.; Ruggirello, I.; Varello, S.; Darvizeh, F.; Faletti, R. Magnetic resonance enterography. Minerva Gastroenterol. Dietol. 2019, 65, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Bufman, H.; Eliakim, R.; Tau, N.; Amitai, M.M. Magnetic resonance enterography in Crohn’s disease patients: Current state of the art and future perspectives. Expert Rev. Med. Devices 2021, 18, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Dambha, F.; Tanner, J.; Carroll, N. Diagnostic imaging in Crohn’s disease: What is the new gold standard? Best Pract. Res. Clin. Gastroenterol. 2014, 28, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Verma, R.; Verma, S.; Rajesh, A. MR enterography of Crohn disease: Part 1, rationale, technique, and pitfalls. Am. J. Roentgenol. 2011, 197, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, F.F.; Anupindi, S.A.; Fletcher, J.G.; Al-Hawary, M.M.; Dillman, J.R.; Grand, D.J.; Bruining, D.H.; Chatterji, M.; Darge, K.; Fidler, J.L.; et al. Small Bowel Crohn Disease at CT and MR Enterography: Imaging Atlas and Glossary of Terms. Radiographics 2020, 40, 354–375. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, Y.; Park, S.H.; Kang, B.K.; Seo, N.; Yang, S.K.; Ye, B.D.; Park, S.H.; Kim, S.Y.; Baek, S.; et al. Diffusion-weighted MR enterography for evaluating Crohn’s disease: How does it add diagnostically to conventional MR enterography? Inflamm. Bowel Dis. 2015, 21, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H. DWI at MR Enterography for Evaluating Bowel Inflammation in Crohn Disease. Am. J. Roentgenol. 2016, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.F.; Lin, Q.Y.; Yin, H.L.; Li, Z.Q. Magnetic Resonance Imaging for Evaluation of Bowel Inflammation and Disease Activity in Crohn’s Disease: A Systematic Review and Meta-Analysis. Gastroenterol. Hepatol. 2022, 46, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Fidler, J.L.; Guimaraes, L.; Einstein, D.M. MR imaging of the small bowel. MR imaging of the small bowel. Radiographics 2009, 29, 1811–1825. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Planell, N.; Rodríguez, S.; Delgado, S.; Ordás, I.; Ramírez-Morros, A.; Ayuso, C.; Aceituno, M.; Ricart, E.; Jauregui-Amezaga, A.; et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am. J. Gastroenterol. 2015, 110, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Grajo, J.R.; Huang, C.; Dillman, J.R.; Gee, M.S.; Jaffe, T.A.; Soto, J.A.; Baker, M.E. MR Enterography of Complicated Crohn Disease: Stricturing and Penetrating Disease. Top Magn. Reason. Imaging 2021, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chatterji, M.; Fidler, J.L.; Taylor, S.A.; Anupindi, S.A.; Yeh, B.M.; Guglielmo, F.F. State of the Art MR Enterography Technique. Top Magn. Reason. Imaging 2021, 30, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Ascenti, G.; Bottari, A.; Catanzariti, F.; Blandino, A.; Mazziotti, S. MR enterography: What is next after Crohn’s disease? Jpn. J. Radiol. 2019, 37, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Vasuri, F.; Golfieri, R.; Fiorentino, M.; Capizzi, E.; Renzulli, M.; Pinna, A.D.; Grigioni, W.F.; D’Errico-Grigioni, A. OATP 1B1/1B3 expression in hepatocellular carcinomas treated with orthotopic liver transplantation. Virchows Arch. 2011, 459, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Pecorelli, A.; Brandi, N.; Brocchi, S.; Tovoli, F.; Granito, A.; Carrafiello, G.; Ierardi, A.M.; Golfieri, R. The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool? J. Clin. Med. 2022, 11, 4399. [Google Scholar] [CrossRef] [PubMed]
- Frush, D.P. Oral Contrast Agents for Pediatric CT and MR Enterography: It’s a Matter of Good Taste. Radiology 2018, 288, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.R.; Towbin, A.J.; Imbus, R.; Young, J.; Gates, E.; Trout, A.T. Comparison of Two Neutral Oral Contrast Agents in Pediatric Patients: A Prospective Randomized Study. Radiology 2018, 288, 245–251. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).