Chronic Calcifying Pancreatitis Associated with Secondary Diabetes Mellitus and Hepatosplenic Abscesses in a Young Male Patient: A Case Report
Abstract
:1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CP | chronic pancreatitis |
| PTH | parathyroid hormone |
| DWI | diffusion-weighted MRI |
| ADC | apparent diffusion coefficient MRI |
| CFTR | cystic fibrosis transmembrane conductance regulator gene |
| PNETs | pancreatic neuroendocrine tumors |
| IPMN | intraductal papillary mucinous neoplasia |
| CT | computerized tomography |
| MRI | magnetic resonance imaging |
| MRCP | magnetic resonance cholangiopancreatography |
| PEI | pancreatic exocrine insufficiency |
| FE1 | fecal elastase 1 |
| BMI | body mass index |
References
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Clain, J.E.; Pearson, R.K. Diagnosis of chronic pancreatitis. Is a gold standard necessary? Surg. Clin. N. Am. 1999, 79, 829–845. [Google Scholar] [CrossRef]
- De la Iglesia-Garcia, D.; Vallejo-Senra, N.; Iglesias-Garcia, J.; López-López, A.; Nieto, L.; Domínguez-Muñoz, J.E. Increased risk of mortality associated with pancreatic exocrine insufficiency in patients with chronic pancreatitis. J. Clin. Gastroenterol. 2018, 52, e63–e72. [Google Scholar] [CrossRef]
- Struyvenberg, M.R.; Martin, C.R.; Freedman, S.D. Practical guide to exocrine pancreatic insufficiency—Breaking the myths. BMC Med. 2017, 15, 29. [Google Scholar] [CrossRef]
- Majumder, S.; Chari, S.T. Chronic pancreatitis. Lancet 2016, 387, 1957–1966. [Google Scholar] [CrossRef]
- Chatti, N.; Chaieb, L.; Jemni, L.; Letaief, R.; Tlili-Graies, K.; Hochlef, S.; Zinelabidine, Y.; Allegue, M.; Hamida, R.B.H. Juvenile idiopathic chronic calcifying pancreatitis: Report of 10 cases from central Tunisia. Pancreas 1990, 5, 354–357. [Google Scholar] [CrossRef]
- Layer, P.; DiMagno, E.P. Early and late onset in idiopathic and alcoholic chronic pancreatitis. Different clinical courses. Surg. Clin. N. Am. 1999, 79, 847–860. [Google Scholar] [CrossRef]
- Aslam, M.; Jagtap, N.; Karyampudi, A.; Talukdar, R.; Reddy, D.N. Risk factors for development of endocrine insufficiency in chronic pancreatitis. Pancreatology 2021, 21, 15–20. [Google Scholar] [CrossRef]
- Fiore, M.; Cascella, M.; Bimonte, S.; Maraolo, A.E.; Gentile, I.; Schiavone, V.; Pace, M.C. Liver fungal infections: An overview of the etiology and epidemiology in patients affected or not affected by oncohematologic malignancies. Infect. Drug Resist. 2018, 11, 177–186. [Google Scholar] [CrossRef]
- Lipsett, P.; Huang, C.J.; Lillemoe, K.; Cameron, J.L.; Pitt, H.A. Fungal hepatic abscesses: Characterization and management. J. Gastrointest. Surg. 1997, 1, 78–84. [Google Scholar] [CrossRef]
- Javadi, S.; Menias, C.O.; Korivi, B.R.; Shaaban, A.M.; Patnana, M.; Alhalabi, K.; Elsayes, K.M. Pancreatic Calcifications and Calcified Pancreatic Masses: Pattern Recognition Approach on CT. AJR Am. J. Roentgenol. 2017, 209, 77–87. [Google Scholar] [CrossRef]
- Misgar, R.A.; Bhat, M.H.; Rather, T.A.; Masoodi, S.R.; Wani, A.I.; Bashir, M.I.; Wani, M.A.; Malik, A.A. Primary hyperparathyroidism and pancreatitis. J. Endocrinol. Investig. 2020, 43, 1493–1498. [Google Scholar] [CrossRef]
- Radlović, N. Cystic fibrosis. SRP Arh. Celok. Lek. 2012, 140, 244–249. [Google Scholar] [CrossRef]
- Iannaccone, G.; Antonelli, M. Calcification of the pancreas in cystic fibrosis. Pediatr. Radiol. 1980, 9, 85–89. [Google Scholar] [CrossRef]
- Amin, S.; Kim, M.K. Islet Cell Tumors of the Pancreas. Gastroenterol. Clin. N. Am. 2016, 45, 83–100. [Google Scholar] [CrossRef]
- Buetow, P.C.; Parrino, T.V.; Buck, J.L.; Pantongrag-Brown, L.; Ros, P.R.; Dachman, A.H.; Cruess, D.F. Islet cell tumors of the pancreas: Pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am. J. Roentgenol. 1995, 165, 1175–1179. [Google Scholar] [CrossRef]
- Eelkema, E.A.; Stephens, D.H.; Ward, E.M.; Sheedy, P.F. CT features of nonfunctioning islet cell carcinoma. AJR Am. J. Roentgenol. 1984, 143, 943–948. [Google Scholar] [CrossRef]
- Imhof, H.; Frank, P. Pancreatic calcifications in malignant islet cell tumors. Radiology 1977, 122, 333–337. [Google Scholar] [CrossRef]
- Kendig, T.A.; Johnson, R.M.; Shackford, B.C. Calcification in pancreatic carcinoma. Ann. Intern. Med. 1966, 65, 122–124. [Google Scholar] [CrossRef]
- Campisi, A.; Brancatelli, G.; Vullierme, M.-P.; Levy, P.; Ruzniewski, P.; Vilgrain, V. Are pancreatic calcifications specific for the diagnosis of chronic pancreatitis? A multidetector-row CT analysis. Clin. Radiol. 2009, 64, 903–911. [Google Scholar] [CrossRef]
- Tanaka, M. Intraductal papillary mucinous neoplasm of the pancreas: Diagnosis and treatment. Pancreas 2004, 28, 282–288. [Google Scholar] [CrossRef]
- Perez-Johnston, R.; Narin, O.; Mino-Kenudson, M.; Ingkakul, T.; Warshaw, A.; Castillo, C.F.-D.; Sahani, V. Frequency and significance of calcification in IPMN. Pancreatology 2013, 13, 43–47. [Google Scholar] [CrossRef]
- Procacci, C.; Graziani, R.; Bicego, E.; A Bergamo-Andreis, I.; Mainardi, P.; Zamboni, G.; Pederzoli, P.; Cavallini, G.; Valdo, M.; Pistolesi, G.F. Intraductal mucin-producing tumors of the pancreas: Imaging findings. Radiology 1996, 198, 249–257. [Google Scholar] [CrossRef]
- Willnow, U.; Willberg, B.; Schwamborn, D.; Körholz, D.; Göbel, U. Pancreatoblastoma in children. Case report and review of the literature. Eur. J. Pediatr. Surg. 1996, 6, 369–372. [Google Scholar] [CrossRef]
- Montemarano, H.; Lonergan, G.J.; Bulas, D.I.; Selby, D.M. Pancreatoblastoma: Imaging findings in 10 patients and review of the literature. Radiology 2000, 214, 476–482. [Google Scholar] [CrossRef]
- Klein, K.A.; Stephens, D.H.; Welch, T.J. CT characteristics of metastatic disease of the pancreas. Radiographics 1998, 18, 369–378. [Google Scholar] [CrossRef]
- Bilgin, M.; Balci, N.C.; Momtahen, A.J.; Bilgin, Y.; Klör, H.-U.; Rau, W.S. MRI and MRCP findings of the pancreas in patients with diabetes mellitus: Compared analysis with pancreatic exocrine function determined by fecal elastase 1. J. Clin. Gastroenterol. 2009, 43, 165–170. [Google Scholar] [CrossRef]
- Shetty, R.; Kumbhar, G.; Thomas, A.; Pearlin, B.; Chowdhury, S.D.; Chandramohan, A. How Are Imaging Findings Associated with Exocrine Insufficiency in Idiopathic Chronic Pancreatitis? Indian J. Radiol. Imaging 2022, 32, 182–190. [Google Scholar] [CrossRef]
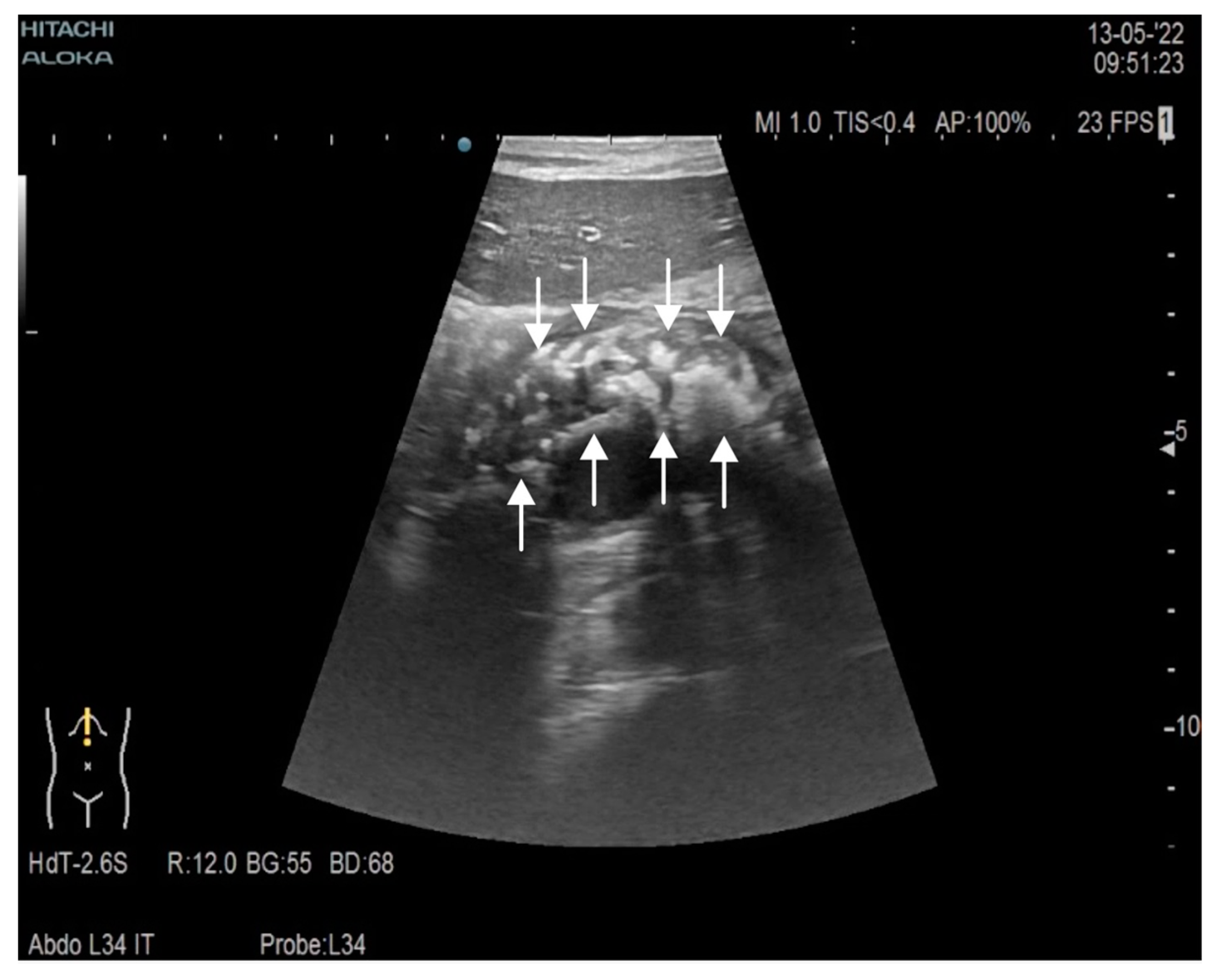
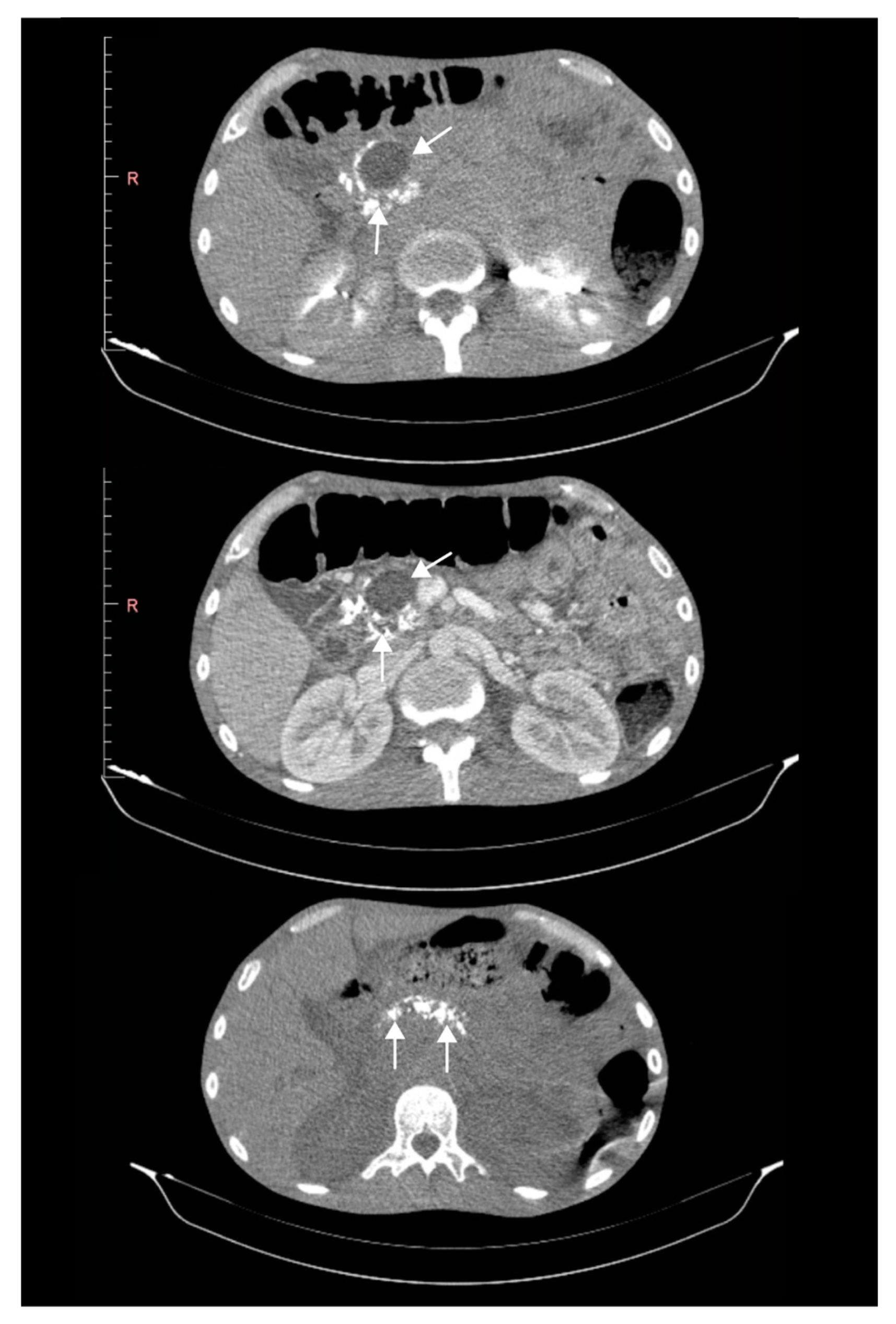
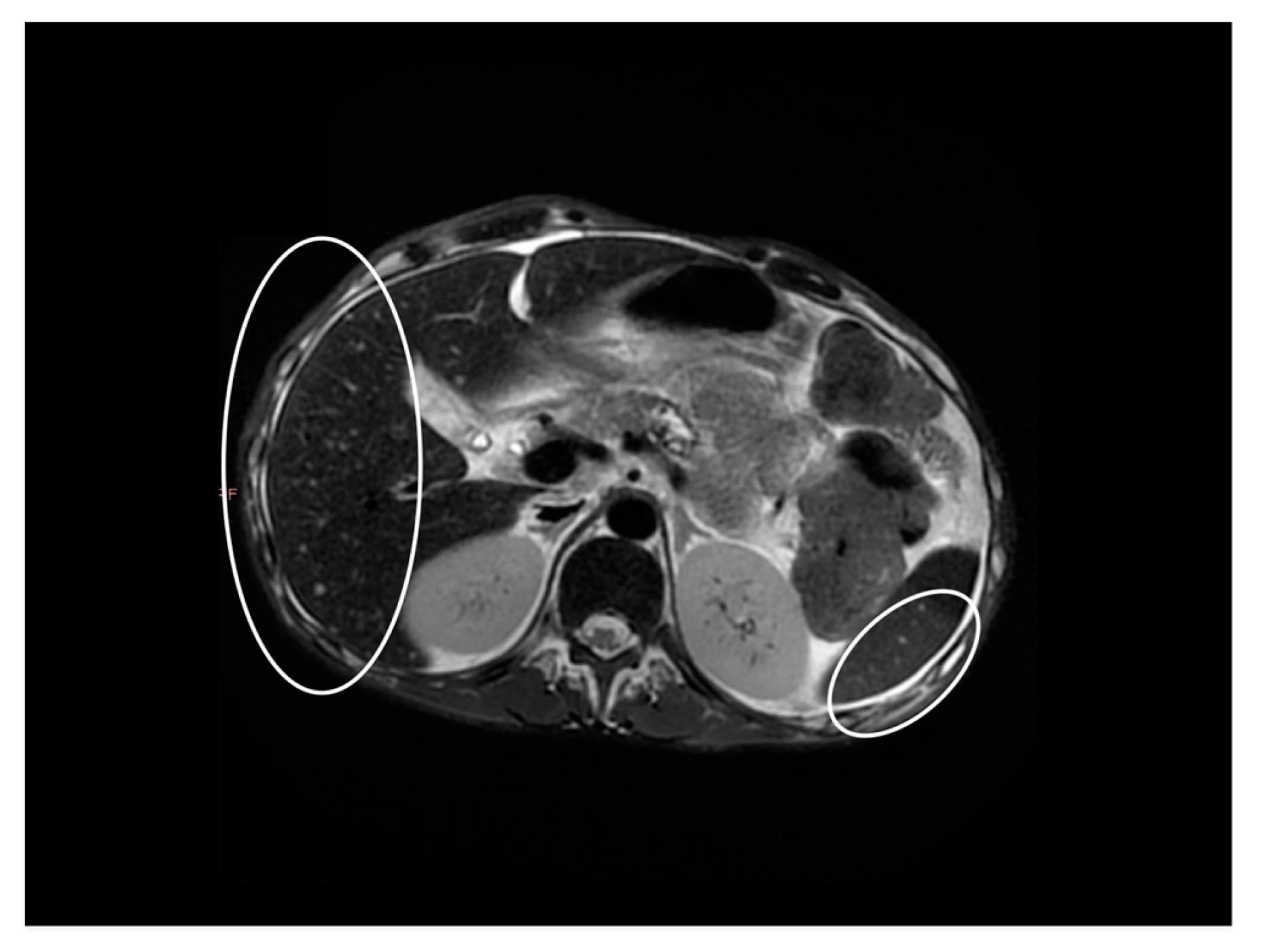
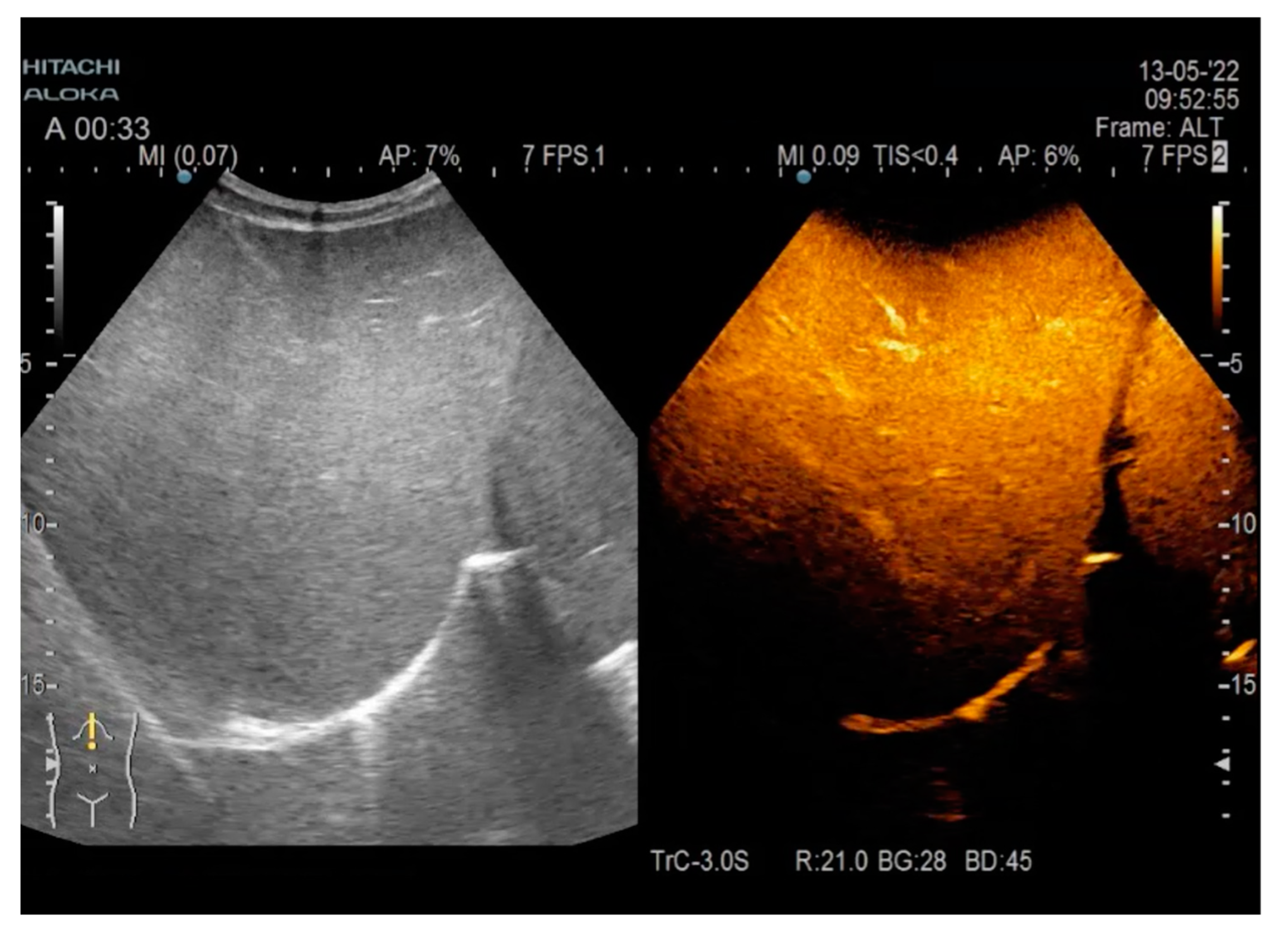
| Parameter | Admission | During Hospitalization | Discharge |
|---|---|---|---|
| Leukocyte count (×103/μL) | 2.60 | 4.54 | 6.86 |
| Neutrophil count (×103/μL) | 1.50 | 2.84 | 3.86 |
| Lymphocyte count (×103/μL) | 0.80 | 1.37 | 2.42 |
| Platalet count (×103/μL) | 198.00 | 148.80 | 200.20 |
| Erythrocyte count (×106/μL) | 2.88 | 3.26 | 3.07 |
| Hemoglobin (g/dL) | 10.00 | 10.79 | 10.51 |
| Chloride (mmol/L) | 91.00 | 98.00 | 98.00 |
| Potassium (mmol/L) | 2.80 | 4.00 | 3.50 |
| Sodium (mmol/L) | 129.00 | 134.00 | 136.00 |
| Calcium (mmol/L) | - | 6.80 | - |
| Glucose (mg/dL) | 281.59 | 126.00 | 137.42 |
| Total serum protein (g/dL) | 5.60 | 5.60 | 6.00 |
| Serum albumin (g/dL) | 2.4 | 2.8 | 2.8 |
| ALT (U/L) | 63.30 | 424.00 | 166.00 |
| AST (U/L) | 23.20 | 472.00 | 35.00 |
| GGT (U/L) | 314.00 | 452.00 | 381.00 |
| BUN (mg/dL) | 35.00 | 20.00 | - |
| Creatinine (mg/dL) | 0.54 | 0.57 | - |
| CRP (mg/dL) | - | 24.00 | - |
| ESR (mm/h) | - | 23.00 | - |
| Amylase (U/L) | 44.00 | 56.00 | - |
| C-peptide (ng/mL) | - | 0.23 | - |
| PTH (pg/mL) | - | 28.46 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marginean, C.M.; Popescu, M.; Vasile, C.M.; Stanciu, M.; Popescu, I.A.; Biciusca, V.; Ciobanu, D.; Dobrescu, A.; Sandulescu, L.D.; Bondari, S.; et al. Chronic Calcifying Pancreatitis Associated with Secondary Diabetes Mellitus and Hepatosplenic Abscesses in a Young Male Patient: A Case Report. Gastroenterol. Insights 2022, 13, 305-312. https://doi.org/10.3390/gastroent13030031
Marginean CM, Popescu M, Vasile CM, Stanciu M, Popescu IA, Biciusca V, Ciobanu D, Dobrescu A, Sandulescu LD, Bondari S, et al. Chronic Calcifying Pancreatitis Associated with Secondary Diabetes Mellitus and Hepatosplenic Abscesses in a Young Male Patient: A Case Report. Gastroenterology Insights. 2022; 13(3):305-312. https://doi.org/10.3390/gastroent13030031
Chicago/Turabian StyleMarginean, Cristina Maria, Mihaela Popescu, Corina Maria Vasile, Mihaela Stanciu, Iulian Alin Popescu, Viorel Biciusca, Daniela Ciobanu, Amelia Dobrescu, Larisa Daniela Sandulescu, Simona Bondari, and et al. 2022. "Chronic Calcifying Pancreatitis Associated with Secondary Diabetes Mellitus and Hepatosplenic Abscesses in a Young Male Patient: A Case Report" Gastroenterology Insights 13, no. 3: 305-312. https://doi.org/10.3390/gastroent13030031
APA StyleMarginean, C. M., Popescu, M., Vasile, C. M., Stanciu, M., Popescu, I. A., Biciusca, V., Ciobanu, D., Dobrescu, A., Sandulescu, L. D., Bondari, S., Popescu, M. S., & Mitrut, P. (2022). Chronic Calcifying Pancreatitis Associated with Secondary Diabetes Mellitus and Hepatosplenic Abscesses in a Young Male Patient: A Case Report. Gastroenterology Insights, 13(3), 305-312. https://doi.org/10.3390/gastroent13030031








