The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures
Abstract
:1. Introduction
2. Clinical Presentation and Common Biomarkers
3. Etiology
4. ERCP-Based Techniques
4.1. Cholangiogram Appearance, Brush Cytology and Transpapillary Biopsy
4.2. Probe-Based Confocal Laser Endomicroscopy
5. Cholangioscopy
5.1. Different Types of Peroral Cholangioscopy
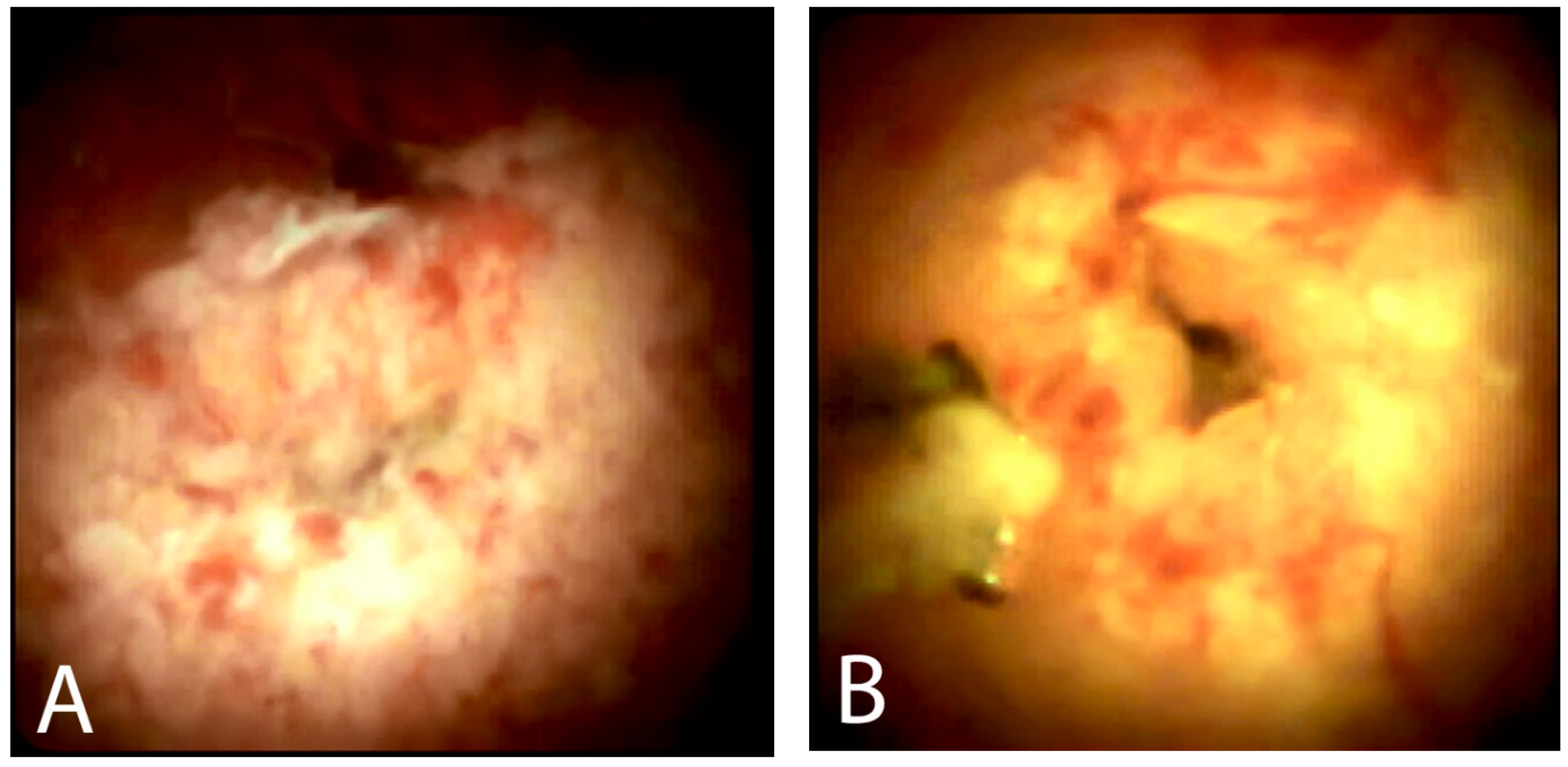
5.2. Visual Impression
5.3. Cholangioscopy-Guided Biopsy
6. Endoscopic Ultrasound
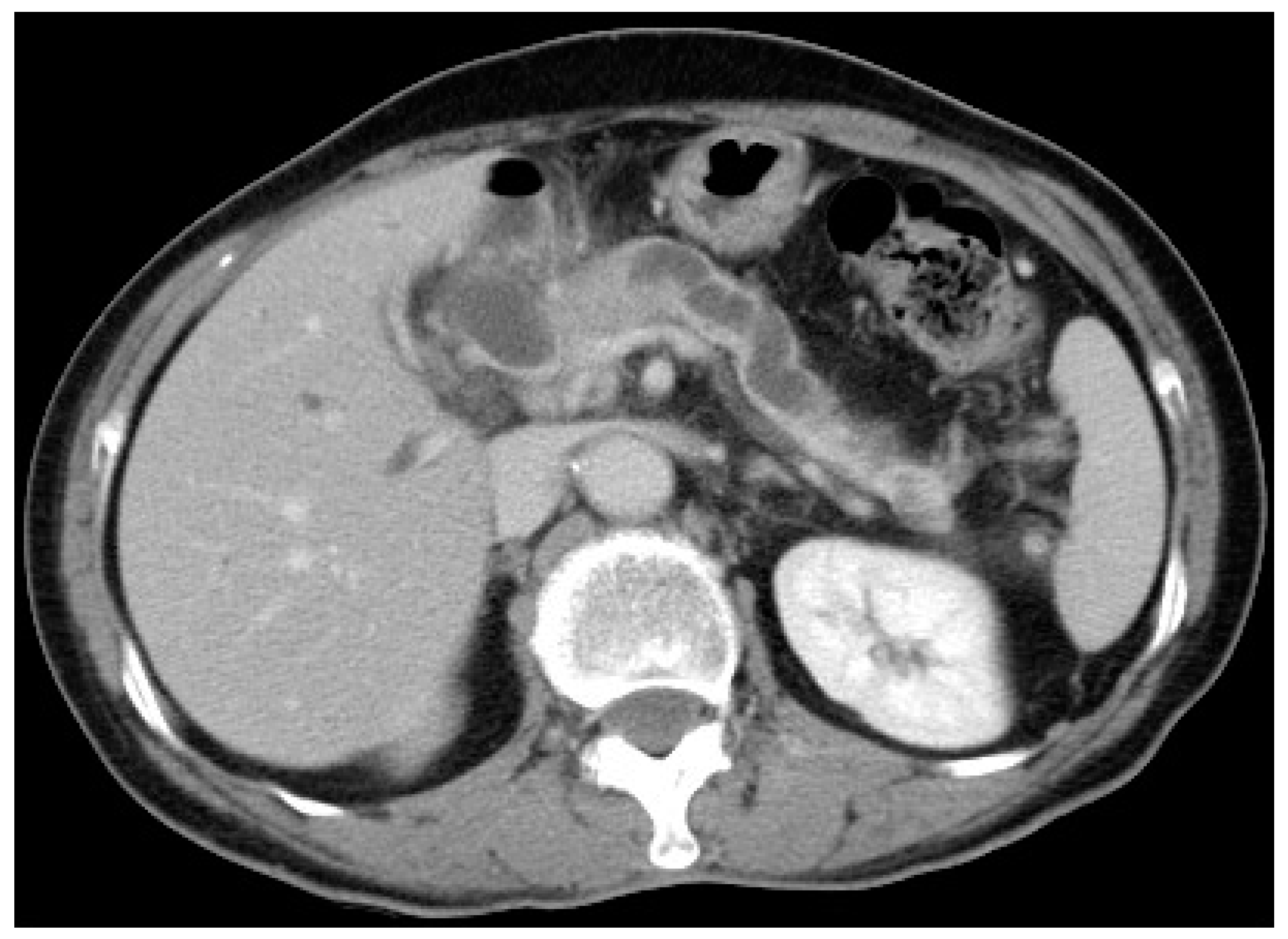
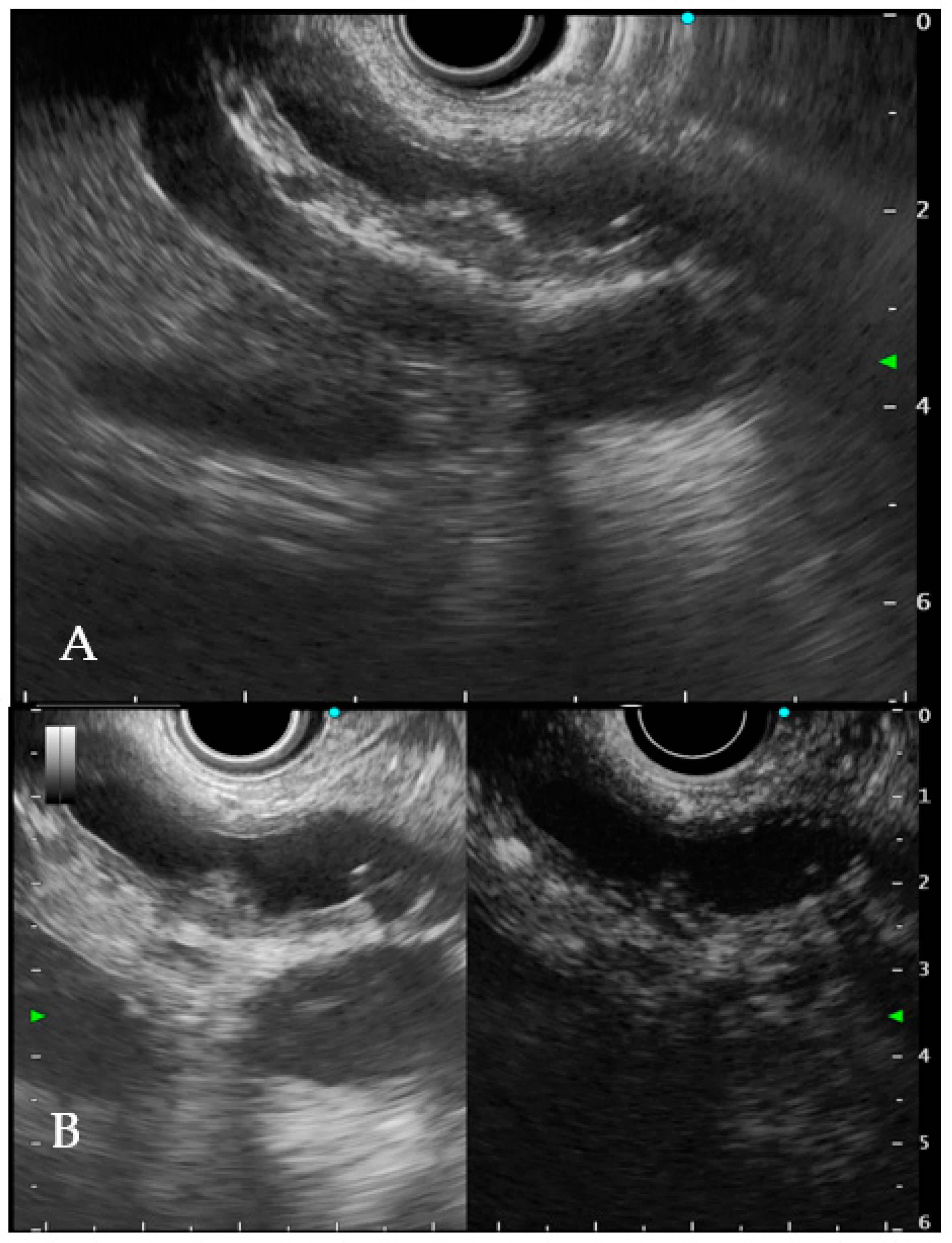
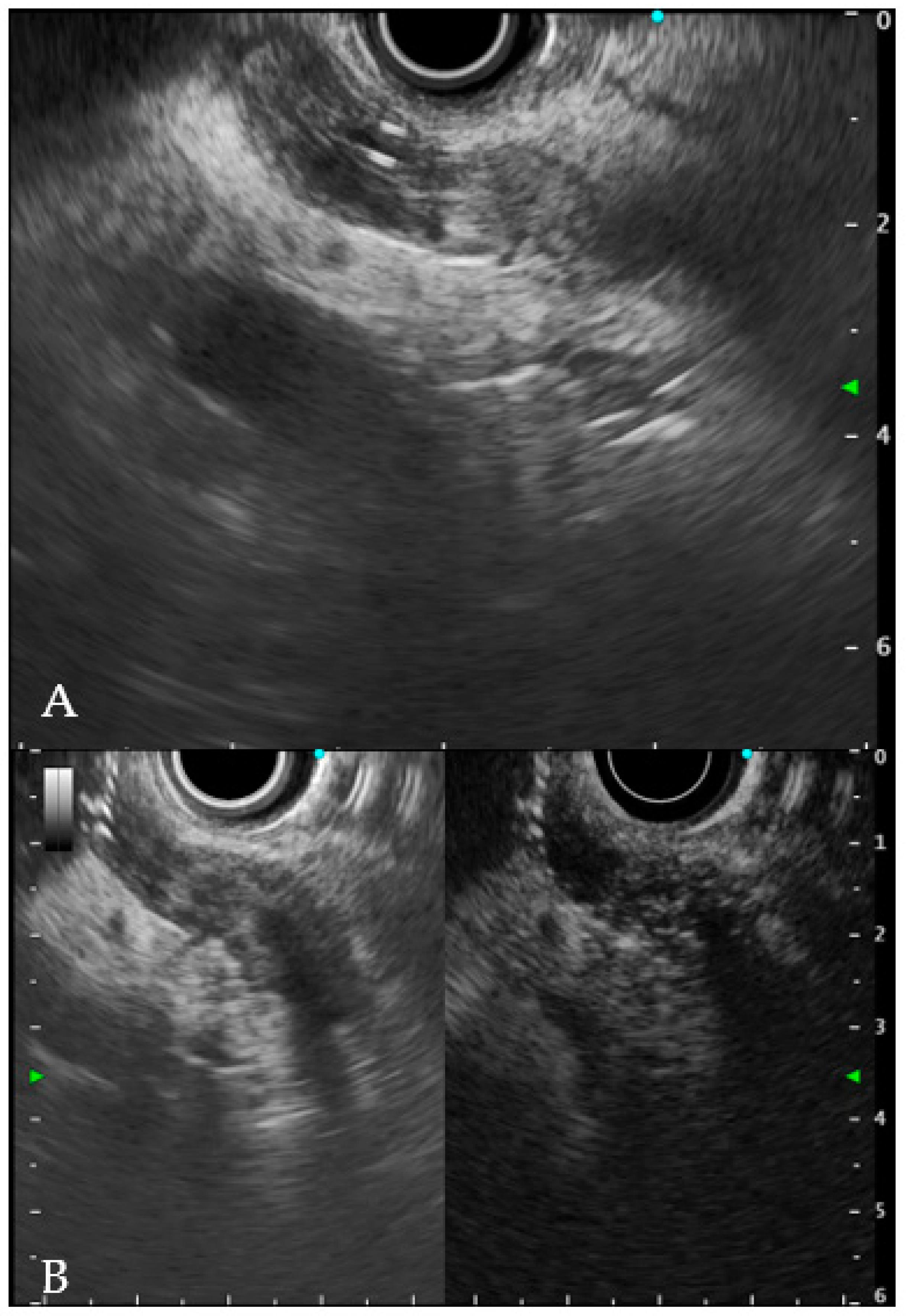
6.1. Intraductal Ultrasonography
6.2. Endoscopic Ultrasound
6.3. Tissue Is the Real Issue
7. Same-Session EUS and ERCP
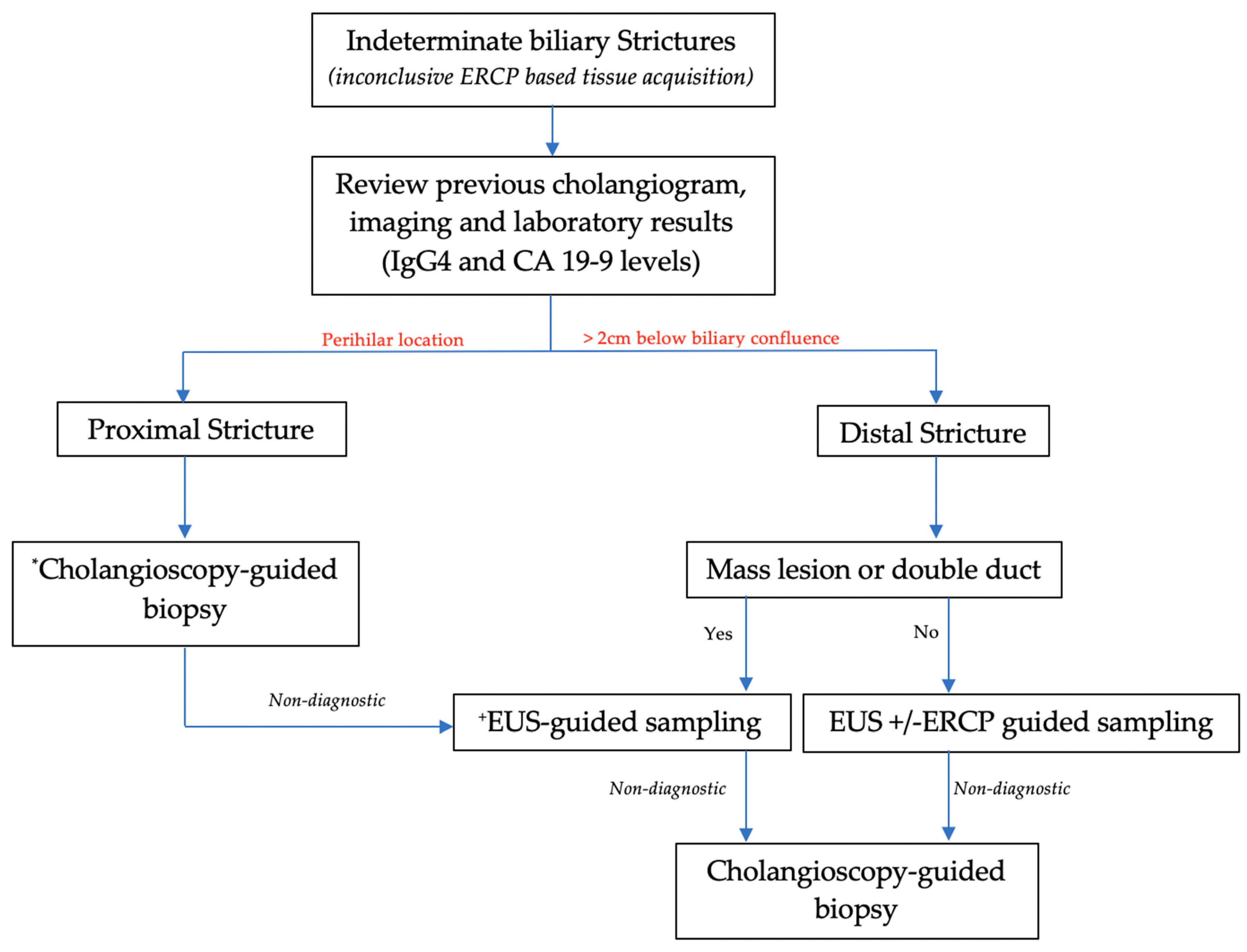
8. Biliary-Stricture-Location-Based Approach
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ERCP | endoscopic retrograde cholangiopancreatography |
| IDBS | indeterminate biliary stricture |
| EUS | endoscopic ultrasound |
| EUS-FNA | endoscopic-ultrasound-guided fine-needle aspiration |
| TPB | trans-papillary biopsy |
| MBS | malignant biliary stricture |
| CA19-9 | carbohydrate antigen 19-9 |
| CCA | cholangiocarcinoma |
| CEA | carcinoembryonic antigen |
| CT | computed tomography |
| PSC | primary sclerosing cholangitis |
| IgG4-SC | IgG4-related sclerosing cholangitis |
| IPNB | intraductal papillary neoplasm of the bile duct |
| FISH | fluorescence in situ hybridization |
| SOC | single-operator cholangioscopy |
| pCLE | probe-based confocal laser endomicroscopy |
| NBI | narrow band imaging |
| DC | direct cholangioscopy |
| D-SOC | digital-single-operator cholangioscopy |
| F-SOC | fibreoptic-single-operator cholangioscopy |
| ROSE | rapid on-site evaluation |
| IDUS | intraductal ultrasonography |
| CBD | common bile duct |
| CE-EUS | contrast-enhanced endoscopic ultrasound |
| RLN | regional lymph nodes |
| FNB | fine-needle biopsy |
References
- Martinez, N.S.; Trindade, A.J.; Sejpal, D.V. Determining the Indeterminate Biliary Stricture: Cholangioscopy and Beyond. Curr. Gastroenterol. Rep. 2020, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Njei, B.; Lourdusamy, V.; Konjeti, R.; Vargo, J.J.; Parsi, M.A. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2015, 81, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowlus, C.L.; Olson, K.A.; Gershwin, M.E. Evaluation of indeterminate biliary strictures. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.; Kowalski, T.E.; Loren, D.E. Practical Management of Indeterminate Biliary Strictures. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio Blanco, G.; Mossa, M.; Troncone, E.; Argirò, R.; Anderloni, A.; Repici, A.; Paoluzi, O.A.; Monteleone, G. Tips and tricks for the diagnosis and management of biliary stenosis-state of the art review. World J. Gastrointest. Endosc. 2021, 13, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.H.; Myung, S.J.; Lim, B.C.; Park, E.T.; Yoo, K.S.; Seo, D.W.; Lee, S.K.; Min, Y.I. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: Analysis using a receiver operating characteristic curve. Am. J. Gastroenterol. 1999, 94, 1941–1946. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 108–122. [Google Scholar] [CrossRef] [Green Version]
- Kamisawa, T.; Nakazawa, T.; Tazuma, S.; Zen, Y.; Tanaka, A.; Ohara, H.; Muraki, T.; Inui, K.; Inoue, D.; Nishino, T.; et al. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J. Hepatobiliary Pancreat. Sci. 2019, 26, 9–42. [Google Scholar] [CrossRef] [Green Version]
- Nour, E.; Hammami, A.; Missaoui, N.; Bdioui, A.; Dahmani, W.; Ben Ameur, W.; Braham, A.; Ajmi, S.; Ben Slama, A.; Ksiaa, M.; et al. Multi-Organ Involvement of Immunoglobulin G4-Related Disease. Gastroenterol. Insights 2021, 12, 350–357. [Google Scholar] [CrossRef]
- Gupta, A.; Bowlus, C.L. Primary sclerosing cholangitis: Etiopathogenesis and clinical management. Front. Biosci. 2012, 4, 1683–1705. [Google Scholar] [CrossRef]
- Sun, B.; Moon, J.H.; Cai, Q.; Rerknimitr, R.; Ma, S.; Lakhtakia, S.; Ryozawa, S.; Kutsumi, H.; Yasuda, I.; Shiomi, H.; et al. Review article: Asia-Pacific consensus recommendations on endoscopic tissue acquisition for biliary strictures. Aliment. Pharmacol. Ther. 2018, 48, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Porner, D.; Kaczmarek, D.J.; Heling, D.; Hausen, A.; Mohr, R.; Huneburg, R.; Matthaei, H.; Glowka, T.R.; Manekeller, S.; Fischer, H.P.; et al. Transpapillary tissue sampling of biliary strictures: Balloon dilatation prior to forceps biopsy improves sensitivity and accuracy. Sci. Rep. 2020, 10, 17423. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Matsubayashi, H.; Kimura, H.; Sasaki, K.; Nagata, K.; Ohno, S.; Uesaka, K.; Mori, K.; Imai, K.; Hotta, K.; et al. Diagnosis of bile duct cancer by bile cytology: Usefulness of post-brushing biliary lavage fluid. Endosc. Int. Open 2015, 3, E323–E328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, A.; Brown, J.M.; Berger, S.H.; Lewis, M.M.; Barr Fritcher, E.G.; Gores, G.J.; Keilin, S.A.; Woods, K.E.; Cai, Q.; Willingham, F.F. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Ther. Adv. Gastroenterol. 2015, 8, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Liew, Z.H.; Loh, T.J.; Lim, T.K.H.; Lim, T.H.; Khor, C.J.L.; Mesenas, S.J.; Kong, C.S.C.; Ong, W.C.; Tan, D.M.Y. Role of fluorescence in situ hybridization in diagnosing cholangiocarcinoma in indeterminate biliary strictures. J. Gastroenterol. Hepatol. 2018, 33, 315–319. [Google Scholar] [CrossRef]
- Njei, B.; McCarty, T.R.; Varadarajulu, S.; Navaneethan, U. Cost utility of ERCP-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastrointest. Endosc. 2017, 85, 773–781.e710. [Google Scholar] [CrossRef]
- Tringali, A.; Lemmers, A.; Meves, V.; Terheggen, G.; Pohl, J.; Manfredi, G.; Häfner, M.; Costamagna, G.; Devière, J.; Neuhaus, H.; et al. Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy 2015, 47, 739–753. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lu, Y.; Sun, B.; Zhang, W.M.; Zhang, Z.Z.; He, Y.P.; Yang, X.J. Probe-based confocal laser endomicroscopy for the diagnosis of undetermined biliary stenoses: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 666–673. [Google Scholar] [CrossRef]
- Turowski, F.; Hügle, U.; Dormann, A.; Bechtler, M.; Jakobs, R.; Gottschalk, U.; Nötzel, E.; Hartmann, D.; Lorenz, A.; Kolligs, F.; et al. Diagnostic and therapeutic single-operator cholangiopancreatoscopy with SpyGlassDS™: Results of a multicenter retrospective cohort study. Surg. Endosc. 2018, 32, 3981–3988. [Google Scholar] [CrossRef]
- Farnik, H.; Weigt, J.; Malfertheiner, P.; Grutzmann, A.; Gossner, L.; Friedrich-Rust, M.; Zeuzem, S.; Sarrazin, C.; Albert, J.G. A multicenter study on the role of direct retrograde cholangioscopy in patients with inconclusive endoscopic retrograde cholangiography. Endoscopy 2014, 46, 16–21. [Google Scholar] [CrossRef]
- Lee, Y.N.; Moon, J.H.; Lee, T.H.; Choi, H.J.; Itoi, T.; Beyna, T.; Neuhaus, H. Prospective randomized trial of a new multibending versus conventional ultra-slim endoscope for peroral cholangioscopy without device or endoscope assistance (with video). Gastrointest. Endosc. 2020, 91, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Itoi, T.; Okabe, Y. Types of Peroral Cholangioscopy: How to Choose the Most Suitable Type of Cholangioscopy. Curr. Treat. Options Gastroenterol. 2016, 14, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Gerges, C.; Beyna, T.; Tang, R.S.Y.; Bahin, F.; Lau, J.Y.W.; van Geenen, E.; Neuhaus, H.; Nageshwar Reddy, D.; Ramchandani, M. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: A prospective, randomized, multicenter trial (with video). Gastrointest. Endosc. 2020, 91, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.; de Moura, D.T.H.; Ribeiro, I.B.; Bazarbashi, A.N.; Franzini, T.A.P.; Dos Santos, M.E.L.; Bernardo, W.M.; de Moura, E.G.H. Efficacy of digital single-operator cholangioscopy in the visual interpretation of indeterminate biliary strictures: A systematic review and meta-analysis. Surg. Endosc. 2020, 34, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Stassen, P.M.C.; Goodchild, G.; de Jonge, P.J.F.; Erler, N.S.; Anderloni, A.; Cennamo, V.; Church, N.I.; Fernandez-Urien Sainz, I.; Huggett, M.T.; James, M.W.; et al. Diagnostic accuracy and interobserver agreement of digital single-operator cholangioscopy for indeterminate biliary strictures. Gastrointest. Endosc. 2021, 94, 1059–1068. [Google Scholar] [CrossRef]
- Almadi, M.A.; Itoi, T.; Moon, J.H.; Goenka, M.K.; Seo, D.W.; Rerknimitr, R.; Lau, J.Y.; Maydeo, A.P.; Lee, J.K.; Nguyen, N.Q.; et al. Using single-operator cholangioscopy for endoscopic evaluation of indeterminate biliary strictures: Results from a large multinational registry. Endoscopy 2020, 52, 574–582. [Google Scholar] [CrossRef]
- de Vries, A.B.; van der Heide, F.; Ter Steege, R.W.F.; Koornstra, J.J.; Buddingh, K.T.; Gouw, A.S.H.; Weersma, R.K. Limited diagnostic accuracy and clinical impact of single-operator peroral cholangioscopy for indeterminate biliary strictures. Endoscopy 2020, 52, 107–114. [Google Scholar] [CrossRef]
- Kahaleh, M.; Gaidhane, M.; Shahid, H.M.; Tyberg, A.; Sarkar, A.; Ardengh, J.C.; Kedia, P.; Andalib, I.; Gress, F.; Sethi, A.; et al. Digital single-operator cholangioscopy interobserver study using a new classification: The Mendoza Classification (with video). Gastrointest. Endosc. 2022, 95, 319–326. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.K.; Lourdusamy, V.; Njei, B.; Varadarajulu, S.; Hawes, R.H. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: A systematic review. Gastrointest. Endosc. 2015, 82, 608–614.e602. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.J.; Chen, J.H.; Xu, H.J.; Yu, Q.; Liu, K. Efficacy and Safety of Digital Single-Operator Cholangioscopy in the Diagnosis of Indeterminate Biliary Strictures by Targeted Biopsies: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 666. [Google Scholar] [CrossRef]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Sutton, B.; Hawes, R.; Varadarajulu, S. Optimizing Outcomes of Single-Operator Cholangioscopy-Guided Biopsies Based on a Randomized Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 441–448.e441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navaneethan, U.; Hasan, M.K.; Kommaraju, K.; Zhu, X.; Hebert-Magee, S.; Hawes, R.H.; Vargo, J.J.; Varadarajulu, S.; Parsi, M.A. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: A multicenter clinical experience (with video). Gastrointest. Endosc. 2016, 84, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Meves, V.; Ell, C.; Pohl, J. Efficacy and safety of direct transnasal cholangioscopy with standard ultraslim endoscopes: Results of a large cohort study. Gastrointest. Endosc. 2014, 79, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Deprez, P.H.; Garces Duran, R.; Moreels, T.; Furneri, G.; Demma, F.; Verbeke, L.; Van der Merwe, S.W.; Laleman, W. The economic impact of using single-operator cholangioscopy for the treatment of difficult bile duct stones and diagnosis of indeterminate bile duct strictures. Endoscopy 2018, 50, 109–118. [Google Scholar] [CrossRef]
- Sun, B.; Hu, B. The role of intraductal ultrasonography in pancreatobiliary diseases. Endosc. Ultrasound 2016, 5, 291–299. [Google Scholar] [CrossRef]
- Meister, T.; Heinzow, H.S.; Woestmeyer, C.; Lenz, P.; Menzel, J.; Kucharzik, T.; Domschke, W.; Domagk, D. Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J. Gastroenterol. 2013, 19, 874–881. [Google Scholar] [CrossRef]
- Heinzow, H.S.; Kammerer, S.; Rammes, C.; Wessling, J.; Domagk, D.; Meister, T. Comparative analysis of ERCP, IDUS, EUS and CT in predicting malignant bile duct strictures. World J. Gastroenterol. 2014, 20, 10495–10503. [Google Scholar] [CrossRef]
- Heinzow, H.S.; Lenz, P.; Lallier, S.; Lenze, F.; Domagk, D.; Domschke, W.; Meister, T. Ampulla of Vater tumors: Impact of intraductal ultrasound and transpapillary endoscopic biopsies on diagnostic accuracy and therapy. Acta Gastroenterol. Belg. 2011, 74, 509–515. [Google Scholar]
- Cha, S.-W. Recent advances of diagnostic approaches for indeterminate biliary tract obstruction. Int. J. Gastrointest. Interv. 2021, 10, 114–119. [Google Scholar] [CrossRef]
- Lee, J.H.; Salem, R.; Aslanian, H.; Chacho, M.; Topazian, M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am. J. Gastroenterol. 2004, 99, 1069–1073. [Google Scholar] [CrossRef]
- Garrow, D.; Miller, S.; Sinha, D.; Conway, J.; Hoffman, B.J.; Hawes, R.H.; Romagnuolo, J. Endoscopic ultrasound: A meta-analysis of test performance in suspected biliary obstruction. Clin. Gastroenterol. Hepatol. 2007, 5, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Malikowski, T.; Levy, M.J.; Gleeson, F.C.; Storm, A.C.; Vargas, E.J.; Topazian, M.D.; Abu Dayyeh, B.K.; Iyer, P.G.; Rajan, E.; Gores, G.J.; et al. Endoscopic Ultrasound/Fine Needle Aspiration Is Effective for Lymph Node Staging in Patients With Cholangiocarcinoma. Hepatology 2020, 72, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.T.; Chew, M.C.H.; Tang, R.S.Y. Troubleshooting Difficult Bile Duct Access: Advanced ERCP Cannulation Techniques, Percutaneous Biliary Drainage, or EUS-Guided Rendezvous Technique? Gastroenterol. Insights 2021, 12, 405–422. [Google Scholar] [CrossRef]
- De Moura, D.T.H.; Moura, E.G.H.; Bernardo, W.M.; De Moura, E.T.H.; Baraca, F.I.; Kondo, A.; Matuguma, S.E.; Almeida Artifon, E.L. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc. Ultrasound 2018, 7, 10–19. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mohamadnejad, M.; Islami, F.; Keshtkar, A.; Biglari, M.; Malekzadeh, R.; Eloubeidi, M.A. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: A systematic review and meta-analysis. Gastrointest. Endosc. 2016, 83, 290–298.e291. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef]
- Kovacevic, B.; Vilmann, P. EUS tissue acquisition: From A to B. Endosc. Ultrasound 2020, 9, 225–231. [Google Scholar] [CrossRef]
- van Riet, P.A.; Larghi, A.; Attili, F.; Rindi, G.; Nguyen, N.Q.; Ruszkiewicz, A.; Kitano, M.; Chikugo, T.; Aslanian, H.; Farrell, J.; et al. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest. Endosc. 2019, 89, 329–339. [Google Scholar] [CrossRef]
- van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2021, 53, 411–423. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Chafic, A.H.; Dewitt, J.; Leblanc, J.K.; El Hajj, I.I.; Cote, G.; House, M.G.; Sherman, S.; McHenry, L.; Pitt, H.A.; Johnson, C.; et al. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy 2013, 45, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, A.; Anwar, M.M.; Donohoe, C.; O’Keeffe, S.; Mushtaq, H.; Kelleher, B.; Clarke, E.; Kirca, M.; McKiernan, S.; Mahmud, N.; et al. The diagnostic accuracy of endoscopic ultrasound in suspected biliary obstruction and its impact on endoscopic retrograde cholangiopancreatography burden in real clinical practice: A consecutive analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Cho, C.M.; Jun, J.H.; Chung, M.J.; Kim, T.H.; Seo, D.W.; Kim, J.; Park, D.H. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: A multicenter experience. J. Gastroenterol. Hepatol. 2019, 34, 799–805. [Google Scholar] [CrossRef] [PubMed]
- de Moura, D.T.H.; Ryou, M.; de Moura, E.G.H.; Ribeiro, I.B.; Bernardo, W.M.; Thompson, C.C. Endoscopic Ultrasound-Guided Fine Needle Aspiration and Endoscopic Retrograde Cholangiopancreatography-Based Tissue Sampling in Suspected Malignant Biliary Strictures: A Meta-Analysis of Same-Session Procedures. Clin. Endosc. 2020, 53, 417–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.N.; Moon, J.H.; Choi, H.J.; Kim, H.K.; Lee, H.W.; Lee, T.H.; Choi, M.H.; Cha, S.W.; Cho, Y.D.; Park, S.H. Tissue acquisition for diagnosis of biliary strictures using peroral cholangioscopy or endoscopic ultrasound-guided fine-needle aspiration. Endoscopy 2019, 51, 50–59. [Google Scholar] [CrossRef] [Green Version]


| Benign | Malignant |
|---|---|
| Iatrogenic bile duct injury Post-endoscopic sphincterotomy, cholecystectomy, liver transplantation | Cholangiocarcinoma Sporadic, PSC-associated |
| Autoinflammatory Primary sclerosing cholangitis (PSC), IgG4-SC, chronic pancreatitis | Pancreatic and ampullary adenocarcinoma |
| Vascular Ischemic cholangiopathy, vasculitis, portal hypertensive biliopathy | Hepatocellular and gallbladder carcinoma |
| Infectious Recurrent pyogenic cholangitis, tuberculosis, human immunodeficiency virus cholangiopathy | Intrahepatic metastasis Lymphoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siu, W.; Tang, R.S.Y. The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures. Gastroenterol. Insights 2022, 13, 192-205. https://doi.org/10.3390/gastroent13020020
Siu W, Tang RSY. The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures. Gastroenterology Insights. 2022; 13(2):192-205. https://doi.org/10.3390/gastroent13020020
Chicago/Turabian StyleSiu, Wilson, and Raymond S. Y. Tang. 2022. "The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures" Gastroenterology Insights 13, no. 2: 192-205. https://doi.org/10.3390/gastroent13020020
APA StyleSiu, W., & Tang, R. S. Y. (2022). The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures. Gastroenterology Insights, 13(2), 192-205. https://doi.org/10.3390/gastroent13020020







