An Insight on Pharmacological and Mechanical Preventive Measures of Post-ERCP PANCREATITIS (PEP)—A Review
Abstract
1. Introduction
2. Risk Factors
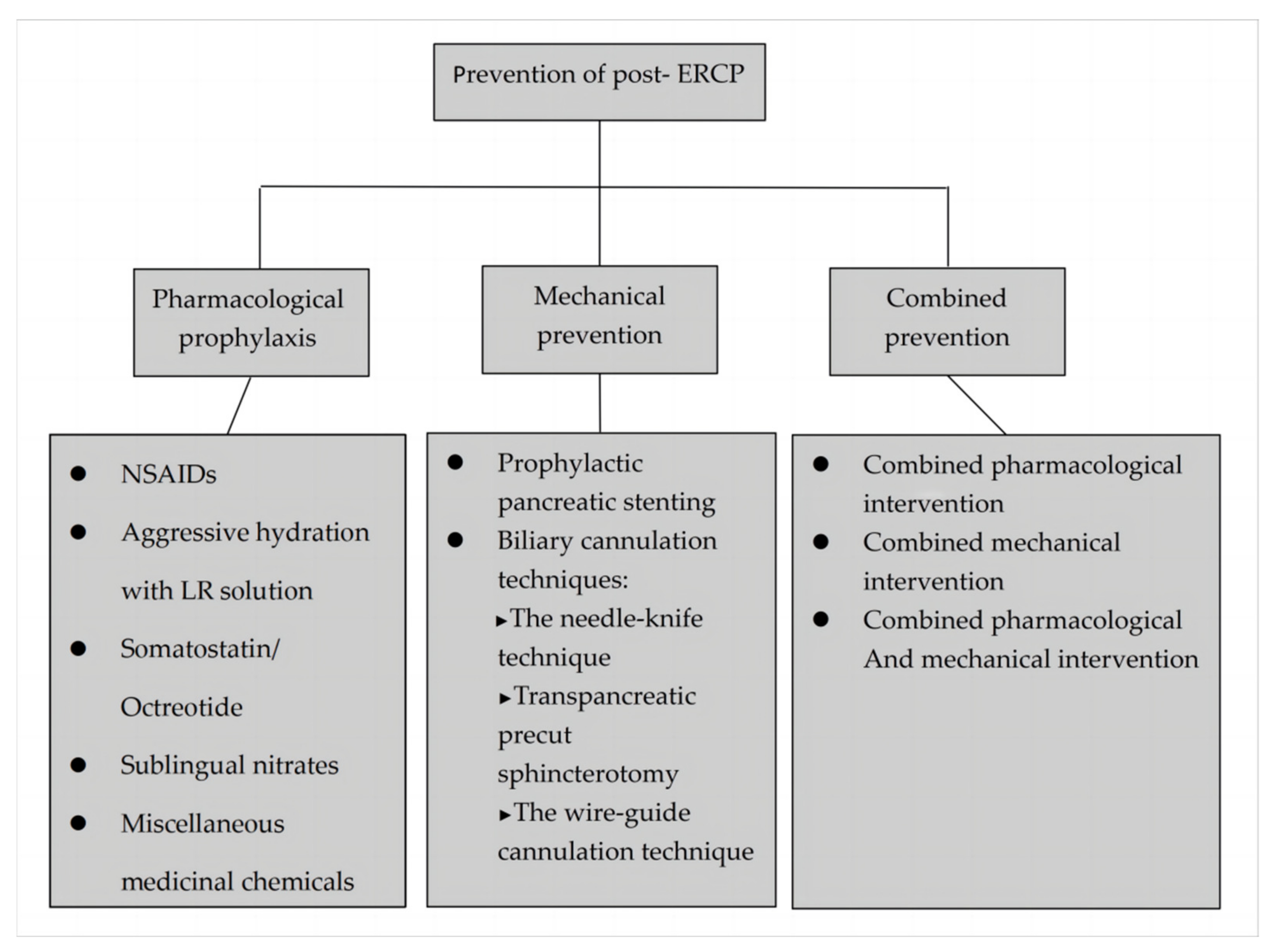
3. Prevention
3.1. Pharmacological Prophylaxis
3.1.1. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
3.1.2. Somatostatin/Octreotide
3.1.3. Sublingual Nitrates
3.1.4. Aggressive Hydration with Lactated Ringer’s Solution
3.1.5. Miscellaneous Medicinal Chemicals
3.2. Mechanical Prevention
3.2.1. Prophylactic Pancreatic Stenting
3.2.2. Biliary Cannulation
3.2.3. Needle-Knife Precut Sphincterotomy
3.2.4. Transpancreatic Precut Sphincterotomy (TPS)
3.2.5. The Wire-Guide Cannulation (WGC) Technique
4. Combined Prevention
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andriulli, A.; Loperfido, S.; Napolitano, G.; Niro, G.; Valvano, M.R.; Spirito, F.; Pilotto, A.; Forlano, R. Incidence rates of post-ERCP complications: A systematic survey of prospective studies. Am. J. Gastroenterol. 2007, 102, 1781–1788. [Google Scholar] [CrossRef]
- Kochar, B.; Akshintala, V.S.; Afghani, E.; Elmunzer, B.J.; Kim, K.J.; Lennon, A.M.; Khashab, M.A.; Kalloo, A.N.; Singh, V.K. Incidence, severity, and mortality of post-ERCP pancreatitis: A systematic review by using randomized, controlled trials. Gastrointest. Endosc. 2015, 81, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Lehman, G.; Vennes, J.; Geenen, J.E.; Russell, R.C.; Meyers, W.C.; Liguory, C.; Nickl, N. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest. Endosc. 1991, 37, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Romboli, E.; Campana, D.; Corinaldesi, R. Mechanisms involved in the onset of post-ERCP pancreatitis. JOP 2002, 3, 162–168. [Google Scholar]
- Cheng, C.L.; Sherman, S.; Watkins, J.L.; Barnett, J.; Freeman, M.; Geenen, J.; Ryan, M.; Parker, H.; Frakes, J.T.; Fogel, E.L.; et al. Risk factors for post-ERCP pancreatitis: A prospective multicenter study. Am. J. Gastroenterol. 2006, 101, 139–147. [Google Scholar] [CrossRef]
- Cotton, P.B.; Garrow, D.A.; Gallagher, J.; Romagnuolo, J. Risk factors for complications after ERCP: A multivariate analysis of 11,497 procedures over 12 years. Gastrointest. Endosc. 2009, 70, 80–88. [Google Scholar] [CrossRef]
- Christoforidis, E.; Goulimaris, I.; Kanellos, I.; Tsalis, K.; Demetriades, C.; Betsis, D. Post-ERCP pancreatitis and hyperamylasemia: Patient-related and operative risk factors. Endoscopy 2002, 34, 286–292. [Google Scholar] [CrossRef]
- Cheon, Y.K.; Cho, K.B.; Watkins, J.L.; McHenry, L.; Fogel, E.L.; Sherman, S.; Lehman, G.A. Frequency and severity of post-ERCP pancreatitis correlated with extent of pancreatic ductal opacification. Gastrointest. Endosc. 2007, 65, 385–393. [Google Scholar] [CrossRef]
- Chen, J.-J.; Wang, X.-M.; Liu, X.-Q.; Li, W.; Dong, M.; Suo, Z.-W.; Ding, P.; Li, Y. Risk factors for post-ERCP pancreatitis: A systematic review of clinical trials with a large sample size in the past 10 years. Eur. J. Med. Res. 2014, 19, 26. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, F.; Wang, Y. Risk factors for post-ERCP pancreatitis: A systematic review and meta-analysis. Surgeon 2015, 13, 218–229. [Google Scholar] [CrossRef]
- Dumonceau, J.-M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef]
- Fujisawa, T.; Kagawa, K.; Hisatomi, K.; Kubota, K.; Sato, H.; Nakajima, A.; Matsuhashi, N. Obesity with abundant subcutaneous adipose tissue increases the risk of post-ERCP pancreatitis. J. Gastroenterol. 2016, 51, 931–938. [Google Scholar] [CrossRef]
- Perney, P.; Berthier, E.; Pageaux, G.P.; Hillaire-Buys, D.; Roques, V.; Fabbro-Peray, P.; Melki, M.; Hanslik, B.; Bauret, P.; Larrey, D.; et al. Are drugs a risk factor of post-ERCP pancreatitis? Gastrointest. Endosc. 2003, 58, 696–700. [Google Scholar] [CrossRef]
- Day, L.W.; Lin, L.; Somsouk, M. Adverse events in older patients undergoing ERCP: A systematic review and meta-analysis. Endosc. Int. Open 2014, 2, E28–E36. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Tal, A.; Ajouaou, M.; Filmann, N.; Zeuzem, S.; Waidmann, O.; Albert, J. ERCP in elderly patients: Increased risk of sedation adverse events but low frequency of post-ERCP pancreatitis. Gastrointest. Endosc. 2015, 82, 1051–1059. [Google Scholar] [CrossRef]
- Lukens, F.J.; Howell, D.A.; Upender, S.; Sheth, S.G.; Jafri, S.-M.R. ERCP in the Very Elderly: Outcomes Among Patients Older than Eighty. Am. J. Dig. Dis. 2009, 55, 847–851. [Google Scholar] [CrossRef]
- Huibregtse, K. Complications of Endoscopic Sphincterotomy and Their Prevention. N. Engl. J. Med. 1996, 335, 961–963. [Google Scholar] [CrossRef]
- Freeman, M.L. Adverse outcomes of ERCP. Gastrointest. Endosc. 2002, 56, S273–S282. [Google Scholar] [CrossRef]
- Mäkelä, A.; Kuusi, T.; Schröder, T. Inhibition of serum phospholipase-A2 in acute pancreatitis by pharmacological agents in vitro. Scand J Clin Lab Invest. 1997, 57, 401–407. [Google Scholar] [CrossRef]
- Davies, N.M.; Anderson, K.E. Clinical phamacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin. Pharamakokinet. 1997, 33, 184–213. [Google Scholar] [CrossRef] [PubMed]
- Sotoudehmanesh, R.; Khatibian, M.; Kolahdoozan, S.; Ainechi, S.; Malboosbaf, R.; Nouraie, M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am. J. Gastroenterol. 2007, 102, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.F.; Wang, X.W.; Zhao, K. Role of nonsteroidal anti-inflammatory drugs in the prevention of post-ERCP pancreatitis: A meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 11–16. [Google Scholar] [PubMed]
- Elmunzer, B.J.; Waljee, A.K.; Elta, G.H.; Taylor, J.R.; Fehmi, S.M.A.; Higgins, P.D.R. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut 2008, 57, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, M.; Huang, S.; Zhang, S.; Zou, X. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: A meta-analysis. Gastrointest. Endosc. 2012, 76, 1152–1159. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Li, W.; Zhu, S.; Yang, H.; Zhang, Y.; Liu, X.; Peng, N.; Fan, P.; Jin, X. Rectal nonsteroidal anti-inflammatory drugs administration is effective for the prevention of post-ERCP pancreatitis: An updated meta-analysis of randomized controlled trials. Pancreatology 2017, 17, 681–688. [Google Scholar] [CrossRef]
- Li, X.; Tao, L.P.; Wang, C.H. Effectiveness of nonsteroidal anti-inflammatory drugs in prevention of post-ERCP pancreatitis: A meta-analysis. World J. Gastroenterol. 2014, 20, 12322–12329. [Google Scholar] [CrossRef]
- Yoshihara, T.; Horimoto, M.; Kitamura, T.; Osugi, N.; Ikezoe, T.; Kotani, K.; Sanada, T.; Higashi, C.; Yamaguchi, D.; Ota, M.; et al. 25 mg versus 50 mg dose of rectal diclofenac for prevention of post-ERCP pancreatitis in Japanese patients: A retrospective study. BMJ Open 2015, 5, e006950. [Google Scholar] [CrossRef]
- Fogel, E.L.; Lehman, G.A.; Tarnasky, P.; Cote, G.; Schmidt, S.E.; Waljee, A.K.; Higgins, P.D.R.; Watkins, J.L.; Sherman, S.; Kwon, R.S.Y.; et al. US Cooperative for Outcomes Research in Endoscopy (USCORE). Rectal indometacin dose escalation for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography in high-risk patients: A double-blind, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2020, 5, 132–141. [Google Scholar] [CrossRef]
- Raptis, S.; Schlegel, W.; Lehmann, E.; Dollinger, H.C.; Zoupas, C. Effects of somatostatin on the exocrine pancreas and the release of duodenal hormones. Metabolism 1978, 27, 1321–1328. [Google Scholar] [CrossRef]
- Dollinger, H.C.; Raptis, S.; Pfeiffer, E.F. Effects of somatostatin on exocrine and endocrine pancreatic function stimulated by intestinal hormones in man. Horm. Metab. Res. 1976, 8, 74–78. [Google Scholar] [CrossRef]
- Schlegel, W.; Raptis, S.; Harvey, R.F.; Oliver, J.M.; Pfeiffer, E.F. Inhibition of cholecystokinin-pancreozymin release by somatostatin. Lancet 1977, 2, 166–168. [Google Scholar] [CrossRef]
- Foster, E.; Leung, J. Pharmacotherapy for the prevention of post-ERCP pancreatitis. Am. J. Gastroenterol. 2007, 102, 52–55. [Google Scholar] [CrossRef]
- Andriulli, A.; Leandro, G.; Federici, T.; Ippolito, A.; Forlano, R.; Iacobellis, A.; Annese, V. Prophylactic administration of somatostatin or gabexate does not prevent pancreatitis after ERCP: An updated meta-analysis. Gastrointest. Endosc. 2007, 65, 624–632. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, J.; Zou, D.W.; Li, Z.S. Prophylactic octreotide administration does not prevent post-endoscopic retrograde cholangiopancreatography pancreatitis: A meta-analysis of randomized controlled trials. Pancreas 2008, 37, 241–256. [Google Scholar] [CrossRef]
- Rudin, D.; Kiss, A.; Wetz, R.V.; Sottile, V.M. Somatostatin and gabexate for post-endoscopic retrograde cholangiopancreatography pancreatitis prevention: Meta-analysis of randomized placebo-controlled trials. J. Gastroenterol. Hepatol. 2007, 22, 977–983. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.B.; Gao, Z.Y.; Xie, W.F. Meta-analysis: Octreotide prevents post-ERCP pancreatitis, but only at sufficient doses. Aliment. Pharmacol. Ther. 2009, 29, 1155–1164. [Google Scholar] [CrossRef]
- Omata, F.; Deshpande, G.; Tokuda, Y.; Takahashi, O.; Ohde, S.; Carr-Locke, D.L.; Jacobs, J.L.; Mine, T.; Fukui, T. Meta-analysis: Somatostatin or its long-acting analogue, octreotide, for prophylaxis against post-ERCP pancreatitis. J. Gastroenterol. 2010, 45, 885–895. [Google Scholar] [CrossRef]
- Qin, X.; Lei, W.S.; Xing, Z.X.; Shi, F. Prophylactic effect of somatostatin in preventing Post-ERCP pancreatitis: An updated meta-analysis. Saudi J. Gastroenterol. 2015, 21, 372–378. [Google Scholar]
- Hu, J.; Li, P.L.; Zhang, T.; Chen, J.P.; Hu, Y.J.; Yu, Z.; Wang, J.P.; Zhu, D.; Tong, X.F. Role of Somatostatin in Preventing Post-endoscopic Retrograde Cholangiopancreatography (ERCP) Pancreatitis: An Update Meta-analysis. Front. Pharmacol. 2016, 7, 489. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, G.; Xu, L.; Qiu, P.; Li, T.; Wang, X.; Wen, P.; Wen, J.; Xiao, X. Effect of somatostatin on prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis and hyperamylasemia: A systematic review and meta-analysis. Pancreatology 2018, 18, 370–378. [Google Scholar] [CrossRef]
- Luman, W.; Pryde, A.; Heading, R.C.; Palmer, K.R. Topical glyceryltrinitrate relaxes the sphincter of Oddi. Gut 1997, 40, 541–543. [Google Scholar] [CrossRef]
- Shao, L.M.; Chen, Q.Y.; Chen, M.Y.; Cai, J.T. Nitroglycerin in the prevention of post-ERCP pancreatitis: A meta-analysis. Dig. Dis. Sci.. 2010, 55, 1–7. [Google Scholar] [CrossRef]
- Chen, B.; Fan, T.; Wang, C.-H. A meta-analysis for the effect of prophylactic GTN on the incidence of post-ERCP pancreatitis and on the successful rate of cannulation of bile ducts. BMC Gastroenterol. 2010, 10, 85. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, C.; Yang, X.; Gao, J.; Zou, D.-W.; Li, Z.-S. Glyceryl trinitrate for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography: A meta-analysis of randomized, double-blind, placebo-controlled trials. Endoscopy 2009, 41, 690–695. [Google Scholar] [CrossRef]
- Ding, J.; Jin, X.; Pan, Y.; Liu, S.; Li, Y. Glyceryl trinitrate for prevention of post-ERCP pancreatitis and improve the rate of cannulation: A meta-analysis of prospective, randomized, controlled trials. PLoS ONE 2013, 8, e75645. [Google Scholar] [CrossRef]
- Wall, I.; Badalov, N.; Baradarian, R.; Iswara, K.; Li, J.J.; Tenner, S. Decreased mortality in acute pancreatitis related to early aggressive hydration. Pancreas 2011, 40, 547–550. [Google Scholar] [CrossRef]
- Wu, B.U.; Hwang, J.Q.; Gardner, T.H.; Repas, K.; Delee, R.; Yu, S.; Smith, B.; Banks, P.A.; Conwell, D.L. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 710–717. [Google Scholar] [CrossRef]
- Wu, D.; Wan, J.; Xia, L.; Chen, J.; Zhu, Y.; Lu, N. The Efficiency of Aggressive Hydration With Lactated Ringer Solution for the Prevention of Post-ERCP Pancreatitis: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2017, 51, e68–e76. [Google Scholar] [CrossRef]
- Radadiya, D.; Devani, K.; Arora, S.; Charilaou, P.; Brahmbhatt, B.; Young, M.; Reddy, C. Peri-Procedural Aggressive Hydration for Post Endoscopic Retrograde Cholangiopancreatography (ERCP) Pancreatitis Prophylaxsis: Meta-analysis of Randomized Controlled Trials. Pancreatology 2019, 19, 819–827. [Google Scholar] [CrossRef]
- Grunwald, D.; Wadhwa, V.; Sawhney, M.S. Hemodynamic Variation and Intravenous Fluids Administered During ERCP and the Association With Post-ERCP Pancreatitis. Pancreas 2016, 45, 293–297. [Google Scholar] [CrossRef] [PubMed]
- de-Madaria, E.; Buxbaum, J.L.; Maisonneuve, P.; García García de Paredes, A.; Zapater, P.; Guilabert, L.; Vaillo-Rocamora, A.; Rodríguez-Gandía, M.Á.; Donate-Ortega, J.; Lozada-Hernández, E.E.; et al. Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis. N. Engl. J. Med. 2022, 387, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, W.; Tenner, S. Acute pancreatitis. N. Engl. J. Med. 1994, 330, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Lasser, E.C.; Lang, J.H.; Lyon, S.G.; Hamblin, A.E.; Howard, M. Glucocorti-coid-induced elevations of C1-esterase inhibitor: A mechanism for protection against lethal dose range contrast challenge in rabbits. Investig. Radiol. 1981, 16, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, J.R.; Grendell, J.H. Intracellular events in the pathogenesis of acute pancreatitis. Pancreas 1991, 6 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef]
- Weiner, G.R.; Geenen, J.E.; Hogan, W.J.; Catalano, M.F. Use of corticosteroids in the prevention of post-ERCP pancreatitis. Gastrointest. Endosc. 1995, 42, 579–583. [Google Scholar] [CrossRef]
- De Palma, G.D.; Catanzano, C. Use of corticosteriods in the prevention of post-ERCP pancreatitis: Results of a controlled prospective study. Am. J. Gastroenterol. 1999, 94, 982–985. [Google Scholar] [CrossRef]
- Sherman, S.; Blaut, U.; Watkins, J.L.; Barnett, J.; Freeman, M.; Geenen, J.; Ryan, M.; Parker, H.; Frakes, J.T.; Fogel, E.L.; et al. Does prophylactic administration of corticosteroid reduce the risk and severity of post-ERCP pancreatitis: A randomized, prospective, multicenter study. Gastrointest. Endosc. 2003, 58, 23–29. [Google Scholar] [CrossRef]
- Zheng, M.-H.; Bai, J.; Yuan, B.; Lin, F.; You, J.; Lu, M.; Gong, Y.; Chen, Y. Meta-analysis of prophylactic corticosteroid use in post-ERCP pancreatitis. BMC Gastroenterol. 2008, 8, 6. [Google Scholar] [CrossRef]
- Guelrud, M.; Mendoza, S.; Rossiter, G.; Ramirez, L.; Barkin, J. Effect of nifedipine on sphincter of Oddi motor activity: Studies in healthy volunteers and patients with biliary dyskinesia. Gastroenterology 1988, 95, 1050–1055. [Google Scholar] [CrossRef]
- Prat, F.; Amaris, J.; Ducot, B.; Bocquentin, M.; Fritsch, J.; Choury, A.D.; Pelletier, G.; Buffet, C. Nifedipine for prevention of post-ERCP pancreatitis: A prospective, double-blind randomized study. Gastrointest. Endosc. 2002, 56, 202–208. [Google Scholar] [CrossRef]
- Karlstrom, L.; Cassuto, J.; Jodal, M.; Lundgren, O. The importance of the enteric nervous system for the bile-salt-induced secretion in the small intestine of the rat. Scand. J. Gastroenterol. 1983, 18, 117–123. [Google Scholar] [CrossRef]
- Louie, D.S.; May, D.; Miller, P.; Owyang, C. Cholecystokinin mediates feedback regulation of pancreatic enzyme secretion in rats. Am. J. Physiol. 1986, 250, G252–G259. [Google Scholar] [CrossRef]
- Schwartz, J.J.; Lew, R.J.; Ahmad, N.A.; Shah, J.N.; Ginsberg, G.G.; Kochman, M.L.; Brensinger, C.M.; Long, W.B. The effect of lidocaine sprayed on the major duodenal papilla on the frequency of post-ERCP pancreatitis. Gastrointest. Endosc. 2004, 59, 179–184. [Google Scholar] [CrossRef]
- Salas, A.; Sans, M.; Soriano, A.; Reverter, J.C.; Anderson, D.C.; Piqué, J.M.; Panés, J. Heparin attenuates TNF-alpha induced inflammatory response through a CD11b dependent mechanism. Gut 2000, 47, 88–96. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Horne, A.P.; Holme, K.R.; Page, C.P. Heparin in inflammation: Potential therapeutic applications beyond anti-coagulation. Adv. Pharmacol. 1999, 46, 151–208. [Google Scholar]
- Barkay, O.; Niv, E.; Santo, E.; Bruck, R.; Hallak, A.; Konikoff, F.M. Low-dose heparin for the prevention of post-ERCP pancreatitis: A randomized placebo-controlled trial. Surg. Endosc. 2008, 22, 1971–1976. [Google Scholar] [CrossRef]
- Rabenstein, T.; Fischer, B.; Wießner, V.; Schmidt, H.; Radespiel-Tröger, M.; Hochberger, J.; Mühldorfer, S.; Nusko, G.; Messmann, H.; Schölmerich, J.; et al. Low–molecular-weight heparin does not prevent acute post-ERCP pancreatitis. Gastrointest. Endosc. 2004, 59, 606–613. [Google Scholar] [CrossRef]
- Sanfey, H.; Bulkley, G.; Cameron, J.L. Pathogenesis of acute pancreatitis: Role of oxygen-derived free radicals in the pathogenesis. Surg. Forum. 1983, 33, 222–224. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, J.; Zhang, W.; Zou, D.; Li, Z. Meta-analysis: Allopurinol in the prevention of postendoscopic retrograde cholangiopancreatography pancreatitis. Aliment. Pharmacol. Ther. 2008, 28, 557–564. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, Y.; Bai, J.; Xin, Y.; Pan, X.; Zhao, L. Meta-analysis of prophylactic allopurinol use in post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas 2008, 37, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.L.; Yan, W.S.; Xiang, X.H.; Chen, K.; Xia, S.H. Prevention effect of allopurinol on post-endoscopic retrograde cholangiopancreatography pancreatitis: A meta-analysis of prospective randomized controlled trials. PLoS ONE 2014, 9, e107350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Francesco, V.; Mariani, A.; Angelini, G.; Masci, E.; Frulloni, L.; Talamini, G.; Passaretti, S.; Testoni, P.; Cavallini, G. Effects of gabexate mesilate, a protease inhibitor, on human sphincter of Oddi motility. Dig. Dis. Sci. 2002, 47, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, G.; Tittobello, A.; Frulloni, L.; Masci, E.; Mariana, A.; Di Francesco, V. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. Gabexate in digestive endoscopy--Italian Group. N. Engl. J. Med. 1996, 335, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Masci, E.; Cavallini, G.; Mariani, A.; Frulloni, L.; Testoni, P.A.; Curioni, S.; Tittobello, A.; Uomo, G.; Costamagna, G.; Zambelli, S.; et al. Gabexate in Digestive Endoscopy-Italian Group II. Comparison of two dosing regimens of gabexate in the prophylaxis of post-ERCP pancreatitis. Am. J. Gastroenterol. 2003, 98, 2182–2186. [Google Scholar] [CrossRef]
- Xing, G.S.; Wu, S.M.; Zhang, X.W.; Ge, Z.Z. Clinical trial of gabexate in the prophylaxis of post-endoscopic retrograde cholangiopan-creatography pancreatitis. Braz. J. Med. Biol. Res. 2006, 39, 85–90. [Google Scholar] [CrossRef]
- Tsujino, T.; Komatsu, Y.; Isayama, H.; Hirano, K.; Sasahira, N.; Yamamoto, N.; Toda, N.; Ito, Y.; Nakai, Y.; Tada, M.; et al. Ulinastatin for pancreatitis after endoscopic retrograde cholangiopancreatography: A randomized, controlled trial. Clin. Gastroenterol. Hepatol. 2005, 3, 376–383. [Google Scholar] [CrossRef]
- Fujishiro, H.; Adachi, K.; Imaoka, T.; Hashimoto, T.; Kohge, N.; Moriyama, N.; Suetsugu, H.; Kawashima, K.; Komazawa, Y.; Ishimura, N.; et al. Ulinastatin shows preventive effect on post-endoscopic retrograde cholangiopancreatography pancreatitis in a multicenter prospective randomized study. J. Gastroenterol. Hepatol. 2006, 21, 1065–1069. [Google Scholar] [CrossRef]
- Ueki, T.; Otani, K.; Kawamoto, K.; Shimizu, A.; Fujimura, N.; Sakaguchi, S.; Matsui, T. Comparison between ulinastatin and gabexate mesylate for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: A prospective, randomized trial. J. Gastroenterol. 2007, 42, 161–167. [Google Scholar] [CrossRef]
- Zheng, M.H.; Bai, J.L.; Meng, M.B.; Chen, Y.P. Gabexate mesylate in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review and meta-analysis update. Curr. Ther. Res. Clin. Exp. 2008, 69, 288–304. [Google Scholar] [CrossRef]
- Zheng, M.-H.; Chen, Y.; Yang, X.; Li, J.; Zhang, Y.; Zeng, Q. Gabexate in the prophylaxis of post-ERCP pancreatitis: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2007, 7, 6. [Google Scholar] [CrossRef]
- Lella, F.; Bagnolo, F.; Colombo, E.; Bonassi, U. A simple way of avoiding post-ERCP pancreatitis. Gastrointest. Endosc. 2004, 59, 830–834. [Google Scholar] [CrossRef]
- Fan, J.-H.; Qian, J.-B.; Wang, Y.-M.; Shi, R.-H.; Zhao, C.-J. Updated meta-analysis of pancreatic stent placement in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis. World J. Gastroenterol. 2015, 21, 7577–7583. [Google Scholar] [CrossRef]
- Chahal, P.; Tarnasky, P.R.; Petersen, B.T.; Topazian, M.D.; Levy, M.J.; Gostout, C.J.; Baron, T.H. Short 5Fr vs long 3Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin. Gastroenterol. Hepatol. 2009, 7, 834–839. [Google Scholar] [CrossRef]
- Singh, P.; Das, A.; Isenberg, G.; Wong, R.C.; Sivak, M.V.; Agrawal, D.; Chak, A. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest. Endosc. 2004, 60, 544–550. [Google Scholar] [CrossRef]
- Mazaki, T.; Masuda, H.; Takayama, T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: A systematic review and meta-analysis. Endoscopy 2010, 42, 842–853. [Google Scholar] [CrossRef]
- Mazaki, T.; Mado, K.; Masuda, H.; Shiono, M. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: An updated meta-analysis. J. Gastroenterol. 2014, 49, 343–355. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Sato, Y.; Irie, H.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; et al. Pancreatic stents to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis: A meta-analysis. World J. Meta-Anal. 2019, 7, 249–258. [Google Scholar] [CrossRef]
- Das, A.; Singh, P.; Sivak, M.V., Jr.; Chak, A. Pancreatic-stent placement for prevention of post-ERCP pancreatitis: A cost-effectiveness analysis. Gastrointest. Endosc. 2007, 65, 960–968. [Google Scholar] [CrossRef]
- Rashdan, A.; Fogel, E.L.; McHenry, L.; Sherman, S.; Temkit, M.; Lehman, G.A. Improved stent characteristics for prophylaxis of post-ERCP pancreatitis. Clin. Gastroenterol. Hepatol. 2004, 2, 322–329. [Google Scholar] [CrossRef]
- Lee, T.H.; Moon, J.H.; Choi, H.J.; Han, S.H.; Cheon, Y.K.; Cho, Y.D.; Park, S.-H.; Kim, S.-J. Prophylactic temporary 3F pancreatic duct stent to prevent post-ERCP pancreatitis in patients with a difficult biliary cannulation: A multicenter, prospective, randomized study. Gastrointest. Endosc. 2012, 76, 578–585. [Google Scholar] [CrossRef]
- Zolotarevsky, E.; Fehmi, S.M.; Anderson, M.A.; Schoenfeld, P.S.; Elmunzer, B.J.; Kwon, R.S.; Piraka, C.R.; Wamsteker, E.J.; Scheiman, J.M.; Korsnes, S.J.; et al. Prophylactic 5-Fr pancreatic duct stents are superior to 3-Fr stents: A randomized controlled trial. Endoscopy 2011, 43, 325–330. [Google Scholar] [CrossRef]
- Afghani, E.; Akshintala, V.S.; Khashab, M.A.; Law, J.K.; Hutfless, S.M.; Kim, K.J.; Lennon, A.M.; Kalloo, A.N.; Singh, V.K. 5-Fr vs. 3-Fr pancreatic stents for the prevention of post-ERCP pancreatitis in high-risk patients: A systematic review and network meta-analysis. Endoscopy 2014, 46, 573–580. [Google Scholar] [CrossRef]
- Freeman, M.L. Adverse outcomes of endoscopic retrograde cholangiopancreatography: Avoidance and management. Gastrointest. Endosc. Clin. N. Am. 2003, 13, 775–798. [Google Scholar] [CrossRef]
- Freeman, M.L.; DiSario, J.A.; Nelson, D.B.; Fennerty, M.B.; Lee, J.G.; Bjorkman, D.J.; Overby, C.S.; Aas, J.; Ryan, M.E.; Bochna, G.S.; et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest. Endosc. 2001, 54, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Huibregste, K.K.M. Endoscopic retrograde cholangiopancreatography, endoscopic sphincterotomy and endoscopic biliary and pancreatic drainage. In Textbook of Gastroenterology; Yamada, T., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1995; pp. 2590–2617. [Google Scholar]
- Bailey, A.; Bourke, M.; Williams, S.; Walsh, P.; Murray, M.; Lee, E.; Kwan, V.; Lynch, P. A prospective randomized trial of cannulation technique in ERCP: Effects on technical success and post-ERCP pancreatitis. Endoscopy 2008, 40, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Pécsi, D.; Farkas, N.; Hegyi, P.; Balaskó, M.; Czimmer, J.; Garami, A.; Illés, A.; Mosztbacher, D.; Pár, G.; Párniczky, A.; et al. Transpancreatic sphincterotomy has a higher cannulation success rate than needle-knife precut papillotomy—A meta-analysis. Endoscopy 2017, 49, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; Guda, N.M. ERCP cannulation: A review of reported techniques. Gastrointest. Endosc. 2005, 61, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J. Precut Papillotomy: A method to improve success of ERCP and Papillotomy. Endoscopy 1980, 12, 130–133. [Google Scholar] [CrossRef]
- Saritas, U.; Ustundag, Y.; Harmandar, F. Precut sphincterotomy: A reliable salvage for difficult biliary cannulation. World J. Gastroenterol. 2013, 19, 1–7. [Google Scholar] [CrossRef]
- Masci, E.; Mariani, A.; Curioni, S.; Testoni, P.A. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: A meta-analysis. Endoscopy 2003, 35, 830–834. [Google Scholar]
- Vandervoort, J.; Soetikno, R.M.; Tham, T.C.; Wong, R.C.; Ferrari, A.P., Jr.; Montes, H.; Roston, A.D.; Slivka, A.; Lichtenstein, D.R.; Ruymann, F.W.; et al. Risk factors for complications after performance of ERCP. Gastrointest. Endosc. 2002, 56, 652–656. [Google Scholar] [CrossRef]
- Testoni, P.A.; Giussani, A.; Vailati, C.; Testoni, S.; Di Leo, M.; Mariani, A. Precut sphincterotomy, repeated cannulation and post-ERCP pancreatitis in patients with bile duct stone disease. Dig. Liver Dis. 2011, 43, 792–796. [Google Scholar] [CrossRef]
- Bailey, A.A.; Bourke, M.J.; Kaffes, A.J.; Byth, K.; Lee, E.Y.; Williams, S.J. Needle-knife sphincterotomy: Factors predicting its use and the relationship with post-ERCP pancreatitis (with video). Gastrointest. Endosc. 2010, 71, 266–271. [Google Scholar] [CrossRef]
- Testoni, P.A.; Testoni, S.; Giussani, A. Difficult biliary cannulation during ERCP: How to facilitate biliary access and minimize the risk of post-ERCP pancreatitis. Dig. Liver Dis. 2011, 43, 596–603. [Google Scholar] [CrossRef]
- Swan, M.P.; Alexander, S.; Moss, A.; Williams, S.J.; Ruppin, D.; Hope, R.; Bourke, M.J. Needle knife sphincterotomy does not increase the risk of pancreatitis in patients with difficult biliary cannulation. Clin. Gastroenterol. Hepatol. 2013, 11, 430–436. [Google Scholar] [CrossRef]
- Choudhary, A.; Winn, J.; Siddique, S.; Arif, M.; Arif, Z.; Hammoud, G.M.; Puli, S.R.; Ibdah, J.A.; Bechtold, M.L. Effect of precut sphincterotomy on post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review and meta-analysis. World J. Gastroenterol. 2014, 20, 4093–4101. [Google Scholar] [CrossRef]
- Aronson, N.; Flamm, C.R.; Bohn, R.L.; Mark, D.H.; Speroff, T. Evidence-based assessment: Patient, procedure, or operator factors associated with ERCP complications. Gastrointest. Endosc. 2002, 56, S294–S302. [Google Scholar] [CrossRef]
- Chan, T.T.; Chew, M.C.H.; Tang, R.S.Y. Troubleshooting Difficult Bile Duct Access: Advanced ERCP Cannulation Techniques, Percutaneous Biliary Drainage, or EUS-Guided Rendezvous Technique? Gastroenterol. Insights 2021, 12, 405–422. [Google Scholar] [CrossRef]
- Catalano, M.F.; Linder, J.D.; Geenen, J.E. Endoscopic transpancreatic papillary septotomy for inaccessible obstructed bile ducts: Comparison with standard pre-cut papillotomy. Gastrointest. Endosc. 2004, 60, 557–561. [Google Scholar] [CrossRef]
- Liang, K.-S.; Chen, C.-C.; Liao, W.-C.; Kuo, Y.-T.; Tseng, L.-W.; He, W.-T.; Wang, H.-P. Comparison between transpancreatic sphincterotomy and needle-knife fistulotomy in difficulty biliary access, a retrospective study in Taiwan. BMC Gastroenterol. 2020, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Artifon, E.L.; Sakai, P.; Cunha, J.E.; Halwan, B.; Ishioka, S.; Kumar, A. Guidewire Cannulation Reduces Risk of Post-ERCP Pancreatitis and Facilitates Bile Duct Cannulation. Am. J. Gastroenterol. 2007, 102, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Park, D.H.; Park, J.Y.; Kim, E.O.; Lee, Y.S.; Park, J.H.; Lee, S.H.; Chung, I.K.; Kim, H.S.; Park, S.H.; et al. Can wire-guided cannulation prevent post-ERCP pancreatitis? A prospective randomized trial. Gastrointest. Endosc. 2009, 69, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Nambu, T.; Ukita, T.; Shigoka, H.; Omuta, S.; Maetani, I. Wire-guided selective cannulation of the bile duct with a sphincterotome: A prospective randomized comparative study with the standard method. Scand. J. Gastroenterol. 2011, 46, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Isayama, H.; Tsujino, T.; Sasahira, N.; Hirano, K.; Kogure, H.; Sasaki, T.; Kawakubo, K.; Yagioka, H.; Yashima, Y.; et al. Impact of introduction of wire-guided cannulation in therapeutic biliary endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 2011, 26, 1552–1558. [Google Scholar] [CrossRef]
- Cheung, J.; Tsoi, K.K.; Quan, W.L.; Lau, J.Y.; Sung, J.J. Guidewire versus conventional contrast cannulation of the common bile duct for the prevention of post-ERCP pancreatitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2009, 70, 1211–1219. [Google Scholar] [CrossRef]
- Cennamo, V.; Fuccio, L.; Zagari, R.M.; Eusebi, L.H.; Ceroni, L.; Laterza, L.; Fabbri, C.; Bazzoli, F. Can a Wire-Guided Cannulation Technique Increase Bile Duct Cannulation Rate and Prevent Post-ERCP Pancreatitis?: A Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2009, 104, 2343–2350. [Google Scholar] [CrossRef]
- Tse, F.; Yuan, Y.; Moayyedi, P.; Leontiadis, G.I. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: A systematic review and meta-analysis. Endoscopy 2013, 45, 605–618. [Google Scholar] [CrossRef]
- Gyökeres, T.; Duhl, J.; Varsányi, M.; Schwab, R.; Burai, M.; Pap, A. Double guide wire placement for endoscopic pancreaticobiliary procedures. Endoscopy 2003, 35, 95–96. [Google Scholar] [CrossRef]
- Maeda, S.; Hayashi, H.; Hosokawa, O.; Dohden, K.; Hattori, M.; Morita, M.; Kidani, E.; Ibe, N.; Tatsumi, S. Prospective Randomized Pilot Trial of Selective Biliary Cannulation Using Pancreatic Guide-Wire Placement. Endoscopy 2003, 35, 721–724. [Google Scholar] [CrossRef]
- Gotoh, Y.; Tamada, K.; Tomiyama, T.; Wada, S.; Ohashi, A.; Satoh, Y.; Higashizawa, T.; Miyata, T.; Ido, K.; Sugano, K. A new method for deep cannulation of the bile duct by straightening the pancreatic duct. Gastrointest. Endosc. 2001, 53, 820–822. [Google Scholar] [CrossRef]
- Tse, F.; Yuan, Y.; Moayyedi, P.; Leontiadis, G.I.; Barkun, A.N. Double-guidewire technique in difficult biliary cannulation for the prevention of post-ERCP pancreatitis: A systematic review and meta-analysis. Endoscopy 2016, 49, 15–26. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Devière, J.; Cremer, M. A new method of achieving deep cannulation of the common bile duct during endoscopic retrograde cholangiopancreatography. Endoscopy 1998, 30, S80. [Google Scholar] [CrossRef]
- Eminler, A.T.; Parlak, E.; Koksal, A.S.; Toka, B.; Uslan, M.I. Wire-guided cannulation over a pancreatic stent method increases the need for needle-knife precutting ın patients with difficult biliary cannulations. Gastrointest. Endosc. 2018, 89, 301–308. [Google Scholar] [CrossRef]
- Freeman, M.L.; Guda, N.M. Prevention of post-ERCP pancreatitis: A comprehensive review. Gastrointest. Endosc. 2004, 59, 845–864. [Google Scholar] [CrossRef]
- Sotoudehmanesh, R.; Eloubeidi, M.A.; Asgari, A.A.; Farsinejad, M.; Khatibian, M. A randomized trial of rectal indomethacin and sublingual nitrates to prevent post-ERCP pancreatitis. Am. J. Gastroenterol. 2014, 109, 903–909. [Google Scholar] [CrossRef]
- Tomoda, T.; Kato, H.; Ueki, T.; Akimoto, Y.; Hata, H.; Fujii, M.; Harada, R.; Ogawa, T.; Wato, M.; Takatani, M.; et al. Combination of Diclofenac and Sublingual Nitrates Is Superior to Diclofenac Alone in Preventing Pancreatitis After Endoscopic Retrograde Cholangiopancreatography. Gastroenterology 2019, 156, 1753–1760. [Google Scholar] [CrossRef]
- Katsinelos, P.; Fasoulas, K.; Paroutoglou, G.; Chatzimavroudis, G.; Beltsis, A.; Terzoudis, S.; Dimou, E.; Zavos, C.; Kaltsa, A.; Kountouras, J. Combination of diclofenac plus somatostatin in the prevention of post-ERCP pancreatitis: A randomized, double-blind, placebo-controlled trial. Endoscopy 2011, 44, 53–59. [Google Scholar] [CrossRef]
- Hajalikhani, M.; Emami, M.H.; Khodadoostan, M.; Shavakhi, A.; Rezaei, M.; Soluki, R. Combination of diclofenac and aggressive hydration for the prevention of post-ERCP pancreatitis. Gastroenterol. Hepatol. Bed. Bench. 2018, 11, 319–324. [Google Scholar]
- Mok, S.R.S.; Ho, H.C.; Shah, P.; Patel, M.; Gaughan, J.P.; Elfant, A.B. Lactated Ringer’s solution in combination with rectal indomethacin for prevention of post-ERCP pancreatitis and readmission: A prospective randomized, double-blinded, placebo-controlled trial. Gastrointest. Endosc. 2017, 85, 1005–1013. [Google Scholar] [CrossRef]
- Oh, H.C.; Kang, H.; Park, T.Y.; Choi, G.J.; Lehman, G.A. Prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis with a combination of pharmacological agents based on rectal non-steroidal anti-inflammatory drugs: A systematic review and network meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Ghanim, M.; Sheikh, T.; Sharma, S.; Ghazaleh, S.; Fatima, R.; Khan, Z.; Lee-Smith, W.; Nawras, A. Rectal indomethacin with topical epinephrine versus indomethacin alone for preventing Post-ERCP pancreatitis—A systematic review and meta-analysis. Pancreatology 2020, 20, 356–361. [Google Scholar] [CrossRef] [PubMed]
| Types | Severity Grading | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Cotton et al. [3] | • Clinical pancreatitis • Amylase at least three times more than normal at 24 h after the procedure • Requiring admission or prolongation of planned admission to 2–3 days | • Requiring hospitalization of 4–10 days | • Hospitalization for more than 10 days OR Hemorrhagic pancreatitis, phlegmon pseudocyst, or intervention OR Need for percutaneous drainage or surgery |
| Bank et al. [4] | • No organ failure • No local or systemic complications | • Organ failure that resolves within 48 h (transient organ failure) OR Local or systemic complications without persistent organ failure | • Persistent organ failure (>48 h) (single or multiple organ failure) |
| Risk Factors | Odds Ratios |
|---|---|
| Patient-related risk factors | |
| ●Female sex [14,15] | 1.40–2.23 |
| ●Previous pancreatitis [14,15] | 2.00–2.90 |
| ●Previous PEP [14,15] | 2.90–8.50 |
| ●Suspected SOD [14,15] | 2.04–4.37 |
| ●Intraductal papillary mucinous neoplasm (IPMN) [15] | 3.01 |
| Patient-related likely risk factors | |
| ●Age [9,11,13] | 1.60–3.97 |
| ●Obesity [16] | 1.143 |
| ●Taken potent pancreatotoxic drugs [17] | 3.70 |
| Procedure-related risk factors | |
| ●Difficult cannulation [14,15] | 3.49–14.9 |
| ●Pancreatic injection [14,15] | 1.58–2.72 |
| ●Precut sphincterotomy [14,15] | 2.11–3.10 |
| ●Non-prophylactic pancreatic duct stent [10,14] | 1.84–2.10 |
| ●Difficult cannulation [14,15] | 3.49–14.9 |
| Procedure-related likely risk factors | |
| ●Trainee involvement [9] | 1.5 |
| ●Extent of pancreatogram [13] | 9.516 |
| First Author | Country | Study Design | |
|---|---|---|---|
| The Aggressive Hydration (AH) Group | The Standard Hydration (SH) Group | ||
| Buxbaum et al. [49,50] | USA | 3.0 cc/kg/h during the procedure, a bolus of 20 cc/kg immediately after ERCP, followed by a post-ERCP rate of 3.0 cc/kg/h for 8 h | 1.5 cc/kg/h during ERCP and for 8 h after ERCP without a bolus |
| Shygan-Nejad et al. [50] | Iran | 3.0 cc/kg/h during ERCP, a bolus of 20 mL/kg right after ERCP and 3.0 cc/kg/h of lactatedRinger solution for 8 h | 1.5 cc/kg/h during ERCP and the following 8 h |
| Choi et al. [49,50] | Korea | 10 cc/kg before ERCP, 3.0 cc/kg/h during and for 8 h after ERCP, and a post-ERCP bolus of 10 cc/kg | 1.5 cc/kg/h during and for 8 h after ERCP |
| Park et al. [50] | Korea | 20-mL/kg bolus and 3 cc/kg/h for 8 h after ERCP | 1.5 cc/kg/h during and for 8 h after ERCP |
| Shaygan- Nejad et al. [49,50] | Iran | 3 mL/kg/h during ERCP, 3 mL/kg/h for 8 h after the procedure to 20 mL/kg | 1.5 mL/kg/h during and for 8 h after procedure |
| Shows Consistent Benefit a | Possible Benefits/ Unclear b | No Benefit c |
|---|---|---|
| Pharmacological agents | ||
| Rectal NSAIDs | Gabexate mesilate | Corticosteroid |
| Glyceryl trinitrate | Somatostatin/ Octreotide | Nifedipine |
| Aggressive hydration with Lactated Ringer’s solution | Ulinastatin | Lidocaine |
| Allopurinol | Heparin | |
| gabexate mesylate | ||
| Mechanical measures | ||
| Prophylactic pancreatic stenting | Needle-knife precut sphincterotomy | |
| The wire-guide cannulation (WGC) technique | Transpancreatic precut sphincterotomy (TPS) | |
| Combined prevention | ||
| Rectal NSAIDs plus prophylactic pancreatic stenting | Rectal NSAIDs plus somatostatin | Rectal indomethacin plus topical epinephrine |
| Rectal NSAIDs plus sublingual nitrates | Lactated Ringer’s solution plus indomethacin | |
| The double-guidewire technique (DGT) with prophylactic pancreatic stenting | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liang, Y.; Feng, Y. An Insight on Pharmacological and Mechanical Preventive Measures of Post-ERCP PANCREATITIS (PEP)—A Review. Gastroenterol. Insights 2022, 13, 387-403. https://doi.org/10.3390/gastroent13040038
Zhang Y, Liang Y, Feng Y. An Insight on Pharmacological and Mechanical Preventive Measures of Post-ERCP PANCREATITIS (PEP)—A Review. Gastroenterology Insights. 2022; 13(4):387-403. https://doi.org/10.3390/gastroent13040038
Chicago/Turabian StyleZhang, Yinqiu, Yan Liang, and Yadong Feng. 2022. "An Insight on Pharmacological and Mechanical Preventive Measures of Post-ERCP PANCREATITIS (PEP)—A Review" Gastroenterology Insights 13, no. 4: 387-403. https://doi.org/10.3390/gastroent13040038
APA StyleZhang, Y., Liang, Y., & Feng, Y. (2022). An Insight on Pharmacological and Mechanical Preventive Measures of Post-ERCP PANCREATITIS (PEP)—A Review. Gastroenterology Insights, 13(4), 387-403. https://doi.org/10.3390/gastroent13040038






