The Outcomes of Nutritional Support Techniques in Patients with Gastrointestinal Cancers
Abstract
1. Introduction
- A body mass index (BMI) < 18.5 kg/m2,
- Unintentional weight loss, associated either with low BMI or with a low fat free mass index [6].
- Obstruction of the digestive tract, with dysphagia and recurrent vomiting,
- Side effects of medical and surgical treatments that hinder adequate nutritional intake (nausea, anorexia, swallowing dysfunction, mucositis, etc.),
- A regular assessment of the nutritional intake, BMI and changes that occurred in body weight since the diagnosis of cancer [18].
- In patients who present abnormalities in the screening evaluation, it is recommended to proceed with the evaluation of some parameters such as a quantitative evaluation of nutritional intake, an objective evaluation of muscle mass, physical performance, and symptoms secondary to nutritional disorders, and the degree of systemic inflammation [18].
- Protein intake required for cancer patients: 1–1.5 g/kg/day [18].
- In cancer patients whose nutritional status does not improve despite oral nutritional interventions, enteral nutrition is recommended. If enteral nutrition is insufficient or not feasible, parenteral nutrition (PN) is recommended [18].
2. Nutritional Support Techniques for Digestive Cancer Patients
- Tube feeding.
- Endoscopic stents.
- Gastrojejunostomy [15].
2.1. Nasogastric and Nasojejunal Tubes
2.2. Percutaneous Endoscopic Gastrostomy (PEG)
2.3. Percutaneous Endoscopic Gastrostomy with Jejunal Tube Extension (PEG-J) and Direct Percutaneous Endoscopic Jejunostomy (DPEJ)
2.4. Non-Endoscopic Methods for Gastrostomy/Jejunostomy
2.5. Esophageal Stents
2.6. Enteral Stents
2.7. Surgical Gastrojejunostomy
2.8. Endoscopic Ultrasound-Guided (EUS-Guided) Gastroenterostomy
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Auth Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf (accessed on 31 May 2022).
- International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/populations/642-romania-fact-sheets.pdf (accessed on 31 May 2022).
- Badicu, G.; Sani, S.H.Z.; Fathirezaie, Z. Predicting tobacco and alcohol consumption based on physical activity level and demographic characteristics in Romania students. Children 2020, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Nitenberg, G.; Raynard, B. Nutritional support of the cancer patient: Issues and dilemmas. Crit. Rev. Oncol. Hematol. 2000, 34, 137–168. [Google Scholar] [CrossRef]
- Lees, J. Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. Eur. J. Cancer Care 1999, 8, 133–136. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Nugent, B.; Lewis, S.; O’Sullivan, J.M. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev. 2013, 1, CD007904. [Google Scholar] [CrossRef]
- Chen, M.J.; Wu, I.C.; Chen, Y.J.; Wang, T.E.; Chang, Y.F.; Yang, C.L.; Huang, W.C.; Chang, W.K.; Sheu, B.S.; Wu, M.S.; et al. Nutrition therapy in esophageal cancer-Consensus statement of the Gastroenterological Society of Taiwan. Dis. Esophagus 2018, 31, doy016. [Google Scholar] [CrossRef]
- Tendler, D.A. Malignant gastric outlet obstruction: Bridging another divide. Am. J. Gastroenterol. 2002, 97, 4–6. [Google Scholar] [CrossRef]
- Adler, D.G.; Baron, T.H. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: Experience in 36 patients. Am. J. Gastroenterol. 2002, 97, 72–78. [Google Scholar] [CrossRef]
- Hebuterne, X.; Lemarie, E.; Michallet, M.; Beauvillain de Montreuil, C.; Schneider, S.M.; Goldwasser, F. Prevalence of malnutrition and current use od nutrition support in patients with cancer. JPEN J. Parenter. Enteral. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Nunes, G.; Fonseca, J.; Barata, A.T.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Nutritional Suport of Cancer Patients without Oral Feeding: Hoe to Select the Most Effective Technique? GE Port. J. Gastroenterol. 2020, 27, 172–184. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 28–42. [Google Scholar] [CrossRef]
- Dijksterhuis, W.; Latenstein, A.; van Kleef, J.J.; Verhoeven, R.; de Vries, J.; Slingerland, M.; Steenhagen, E.; Heisterkamp, J.; Timmermans, L.M.; de van der Schueren, M.; et al. Cachexia and dietetic inter-ventions in patients with esophagogastric cancer: A multicenter cohort study. J. Natl. Compr. Cancer Netw. 2021, 19, 144–152. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Berts, H.; Bozzetti, F.; Hutterer, E. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Cao, D.X.; Wu, G.H.; Zhang, B.; Quan, Y.J.; Wei, J.; Jin, H.; Jiang, Y.; Yang, Z.A. Resting energy expenditure and body composition in patients with newly detected cancer. Clin. Nutr. 2010, 29, 72–77. [Google Scholar] [CrossRef]
- Wang, L.; Sesso, H.D.; Glynn, R.J.; Christen, W.G.; Bubes, V.; Manson, J.E.; Buring, J.E.; Gaziano, J.M. Vitamin E and C supplementation and risk of cancer in men: Posttrial follow-up in the Physicians’ Health Study II randomized trial. Am. J. Clin. Nutr. 2014, 100, 915–923. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The selenium and vitamin E cancer prevention trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Korber, J.; Pricelius, S.; Heidrich, M.; Müller, M.J. Increased lipid utilization in weight losing and weight stable cancer patients with normal body weight. Eur. J. Clin. Nutr. 1999, 53, 740–745. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J.; et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: A two-year randomized controlled trial. PLoS ONE 2014, 9, e108687. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.Y.; Ho, J.W.; Hui, B.P.; Lee, A.M.; Macfarlane, D.J.; Leung, S.S.; Lam, S.H.; Taylor, A.J. Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. Br. Med. J. Int. Ed. 2012, 344, e70. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Schutt, A.J.; Reitemeier, R.J.; Hahn, R.G. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer 1974, 33, 1607–1609. [Google Scholar] [CrossRef]
- Ruiz Garcia, V.; Lopez-Briz, E.; Carbonell Sanchis, R.; Gonzalvez Perales, J.L.; Bort-Marti, S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst. Rev. 2013, 2013, CD004310. [Google Scholar] [CrossRef]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef]
- Kaegi-Braun, N.; Schuetz, P.; Mueller, B.; Kutz, A. Association of Nutritional Support with Clinical Outcomes in Malnourished Cancer Patients: A Population-Based Matched Cohort Study. Front. Nutr. 2021, 10, 603370. [Google Scholar] [CrossRef]
- Yolsuriyanwong, K.; Chand, B. Update on endoscopic enteral access. Tech. Gastrointest. Endosc. 2018, 20, 172–181. [Google Scholar] [CrossRef]
- Braunschweig, C.L.; Levy, P.; Sheean, P.M.; Wang, X. Enteral compared with parenteral nutrition: A meta-analysis. Am. J. Clin. Nutr. 2001, 74, 534–542. [Google Scholar] [CrossRef]
- Pritchard, C.; Duffy, S.; Edington, J.; Pang, F. Enteral nutrition and oral nutrition supplements: A review of the economics literature. JPEN J. Parenter. Enteral. Nutr. 2006, 30, 52–59. [Google Scholar] [CrossRef]
- MacFie, J. Bacterial Translocation, Gut Barrier Function and Nutritional Support. Surgery 2002, 20, 1–2. [Google Scholar] [CrossRef]
- Szefel, J.; Kruszewski, W.J.; Buczek, T. Enteral feeding and its impact on the gut immune system and intestinal mucosal barrier. Prz. Gastroenterol. 2015, 10, 71–77. [Google Scholar] [CrossRef]
- Amano, K.; Maeda, I.; Ishiki, H.; Miura, T.; Hatano, Y.; Tsukuura, H.; Taniyama, T.; Matsumoto, Y.; Matsuda, Y.; Kohara, H.; et al. Effects of enteral nutrition and parenteral nutrition on survival in patients with advanced cancer cachexia: Analysus of a multicenter prospective cohort study. Clin. Nutr. 2021, 40, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; DeLegge, M.H.; Fang, J.C.; McClave, S.A.; Kundu, S.; d’Othee, B.J.; Martinez–Salazar, G.M.; Sacks, D.; Swan, T.L.; Towbin, R.B.; et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology 2011, 141, 742–765. [Google Scholar]
- Ho, C.S.; Yee, A.C.; McPherson, R. Complications of surgical and percutaneous non endoscopic gastrostomy: Review of 233 patients. Gastroenterology 1988, 95, 1206–1210. [Google Scholar] [CrossRef]
- Nagaraja, V.; Cox, M.R.; Eslick, G.D. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2014, 5, 119–126. [Google Scholar]
- Best, C. How to set up and administer an enteral feed via a nasogastric tube. Nurs. Stand. 2017, 31, 42–47. [Google Scholar] [CrossRef]
- Kwon, R.S.; Banerjee, S.; Desilets, D.; Diehl, D.L.; Farraye, F.A.; Kaul, V.; Mamula, P.; Pedrosa, M.C.; Rodriguez, S.A.; Varadarajulu, S.; et al. ASGE Technology Committee Enteral nutrition access devices. Gastrointest. Endosc. 2010, 72, 236–248. [Google Scholar] [CrossRef]
- Löser, C.; Aschl, G.; Hébuterne, X.; Mathus-Vliegen, E.M.; Muscaritoli, M.; Niv, Y.; Rollins, H.; Singer, P.; Skelly, R.H. ESPEN guidelines on artificial enteral nutrition—Percutaneous endoscopic gastrostomy (PEG). Clin. Nutr. 2005, 24, 848–861. [Google Scholar]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schutema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN guideline on home enteral nutrition. Clin. Nutr. 2020, 39, 5–22. [Google Scholar] [CrossRef]
- Shastri, Y.M.; Shirodkar, M.; Mallath, M.K. Endoscopic feeding tube placement in patients with cancer: A prospective clinical audit of 2055 procedures in 1866 patients. Aliment. Pharmacol. Ther. 2008, 27, 649–658. [Google Scholar] [CrossRef]
- Westaby, D.; Young, A.; O’Toole, P.; Smith, G.; Sanders, D.S. The provision of a percutaneously placed enteral tube feeding service. Gut 2010, 59, 1592–1605. [Google Scholar] [CrossRef]
- Mobily, M.; Patel, J.A. Palliative percutaneous endoscopic gastrostomy placement for gastrointestinal cancer: Roles, goals, and complications. World J. Gastrointest. Endosc. 2015, 7, 364–369. [Google Scholar] [CrossRef]
- McClave, S.A.; Ritchie, C.S. The role of endoscopically placed feeding or decompression tubes. Gastroenterol. Clin. North Am. 2006, 35, 83–100. [Google Scholar] [CrossRef]
- Villalba, C.M.; Rodriguez, J.A.V.; Sanchez, F.G. Percutaneous endoscopic gastrostomy. Indication, care and complications. Med. Clin. 2019, 152, 229–236. [Google Scholar] [CrossRef]
- Lochs, H.; Valentini, L.; Schutz, T.; Allison, S.P.; Howard, P.; Pichard, C. ESPEN Guidelines on adult enteral nutrition. Clin. Nutr. 2006, 25, 177–360. [Google Scholar] [CrossRef]
- Leeds, J.S.; McAlindon, M.E.; Grant, J.; Robson, H.E.; Lee, F.K.; Sanders, D.S. Survival analysis after gastrostomy: A single-centre, observational study comparing radiological and endoscopic insertion. Eur. J. Gastroenterol. Hepatol. 2010, 22, 591. [Google Scholar] [CrossRef]
- Johnston, S.D.; Tham, T.C.; Mason, M. Death after PEG: Results of the National Confidential Enquiry into Patient Outcome and Death. Gastrointest. Endosc. 2008, 68, 223. [Google Scholar] [CrossRef]
- Meisel, K.; Arnold, R.M.; Stijacic, C.I.; Boscardin, J.; Smith, A.K. Survival, Functional Status, and Eating Ability After Percutaneous Endoscopic Gastrostomy Tube Placement for Acute Stroke. J. Am. Geriatr. Soc. 2017, 65, 1848. [Google Scholar] [CrossRef]
- Arora, G.; Rockey, D.; Gupta, S. High in-hospital mortality after percutaneous endoscopic gastrostomy: Results of a nationwide population-based study. Clin. Gastroenterol. Hepatol. 2013, 11, 1437–1444.e3. [Google Scholar] [CrossRef]
- Blomberg, J.; Lagergren, J.; Martin, L.; Mattsson, F.; Lagergren, P. Complications after percutaneous endoscopic gastrostomy in a prospective study. Scand. J. Gastroenterol. 2012, 47, 737–742. [Google Scholar] [CrossRef]
- Keung, E.Z.; Liu, X.; Nuzhad, A.; Rabinowits, G.; Patel, V. In-hospital and long-term outcomes after percutaneous endoscopic gastrostomy in patients with malignancy. J. Am. Coll. Surg. 2012, 215, 777–786. [Google Scholar] [CrossRef]
- Raha, S.K.; Woodhouse, K. The use of percutaneous endoscopic gastrostomy (PEG) in 161 consecutive elderly patients. Age Ageing 1994, 23, 162. [Google Scholar] [CrossRef]
- Laranjo, A.; Brito, M.; Nunes, G.; Santos, C.A.; Fonseca, J. Feasibility, safety and outcome of endoscopic gastrostomy in patients with esophageal cancer. Nutr. Hosp. 2020, 37, 660–666. [Google Scholar] [CrossRef]
- Amrendra, M.; Varun, K.; Zorisadday, G.; Neel, R.; Rajan, K.; Praveen, K. Prophylactic antibiotics for PEG tube placement: An update meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 2019, 14, S326. [Google Scholar]
- Schneider, A.S.; Schettler, A.; Markowski, A.; Luettig, B.; Kaufmann, B.; Klamt, S.; Lenzen, H.; Momma, M.; Seipt, C.; Lankisch, T.; et al. Complication and mortality rate after percutaneous endoscopic gastrostomy are low and indication-dependent. Scand. J. Gastroenterol. 2014, 49, 891–898. [Google Scholar] [CrossRef]
- Hucl, T.; Spicak, J. Complications of percutaneous endoscopic gastrostomy. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 769. [Google Scholar] [CrossRef]
- Macedo, C.; Almeida, N.; Alves, A.R.; Ferreira, A.M.; Figueiredo, P. Persistent Peristomal Leakage from Percutaneous Endoscopic Gastrostomy Successfully Treated with Argon Plasma Coagulation. GE Port. J. Gastroenterol. 2021, 28, 210. [Google Scholar] [CrossRef]
- Enestvedt, B.K.; Jorgensen, J.; Sedlack, R.E.; Coyle, W.J.; Obstein, K.L.; Al-Haddad, M.A.; Christie, J.A.; Davila, R.E.; Mullady, D.K.; Kubiliun, N.; et al. ASGE Training Committee 2013–2014. Endoscopic approaches to enteral feeding and nutrition core curriculum. Gastrointest. Endosc. 2014, 80, 34–41. [Google Scholar] [CrossRef]
- Jain, R.; Maple, J.T.; Anderson, M.A.; Appalaneni, V.; Ben-Menachem, T.; Decker, G.A.; Fanelli, R.D.; Fisher, L.; Fukami, N.; Ikenberry, S.O.; et al. ASGE Standards of Practice Committee. The role of endoscopy in enteral feeding. Gastrointest. Endosc. 2011, 74, 7–12. [Google Scholar] [CrossRef]
- Finucane, T.E.; Bynum, J.P. Use of tube feeding to prevent aspiration pneumonia. Lancet 1996, 348, 1421–1424. [Google Scholar] [CrossRef]
- Allum, W.H.; Blazeby, J.M.; Griffin, S.M.; Cunningham, D.; Jankowski, J.A.; Wong, R. Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology Guidelines for the management of oesophageal and gastric cancer. Gut 2011, 60, 1449–1472. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Torrinhas, R.S. Enteral feeding. Encycl. Food Health 2016, 2, 258–263. [Google Scholar]
- Sofue, K.; Takeuchi, Y.; Tsurusaki, M.; Shibamoto, K.; Sakamoto, N.; Kitajima, K.; Sone, M.; Sugimura, K.; Arai, Y. Value of Percutaneous Radiologic Gastrostomy for Patients with Advanced Esophageal Cancer. Ann. Surg. Oncol. 2016, 23, 3623–3631. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, S.H.; Lee, C.; Seo, J.Y.; Kang, H.Y.; Yang, J.I.; Chung, S.J.; Kim, J.S. Thirty-day mortality after percutaneous gastrostomy by endoscopic versus radiologic placement: A systematic review and meta-analysis. Intest. Res. 2016, 14, 333–342. [Google Scholar] [CrossRef]
- Oliveira, G.P.; Santos, C.A.; Fonseca, J. The role of surgical gastrostomy in the age of endoscopic gastrostomy: A 13 years and 543 patients retrospective study. Rev. Esp. Enferm. Dig. 2016, 108, 776–779. [Google Scholar] [CrossRef][Green Version]
- Shin, J.H.; Park, A.W. Updates on percutaneous radiologic gastrostomy/gastrojejunostomy and jejunostomy. Gut Liver 2010, 4 (Suppl. 1), S25–S31. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Y.; Xie, T.; Hu, Y. Percutaneous endoscopic gastrostomy versus percutaneous radiological gastrostomy for swallowing distrubances. Cochrane Database Syst Rev 2016, 2, CD009198. [Google Scholar]
- Spaander, M.C.; Baron, T.H.; Siersema, P.D.; Fuccio, L.; Schumacher, B.; Escorsell, A.; Garcia-Pagán, J.C.; Dumonceau, J.M.; Conio, M.; De Ceglie, A.; et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016, 48, 939–948. [Google Scholar] [CrossRef]
- Spaander, M.C.W.; van der Bogt, R.D.; Baron, T.H.; Albers, D.; Blero, D.; De Ceglie, A.; Conio, M.; Czakó, L.; Everett, S.; Garcia-Pagán, J.C.; et al. Esophageal stenting for benign and malignant disease:European Society of Gastrointestinal Endoscopy (ESGE) Guideline–Update 2021. Endoscopy 2021, 53, 751–762. [Google Scholar]
- Sharma, B.; Raina, S.; Sharma, R.; Bodh, V.; Raina, S.K.; Sharma, N. Self-Expanding Metallic Stents (SEMS) in Inoperable Esophageal Cancer: A Prospective Analysis of Morbidity and Survival Outcomes. Indian J. Palliat. Care 2019, 25, 398–402. [Google Scholar]
- Aoki, T.; Osaka, Y.; Takagi, Y.; Okada, R.; Shinohara, M.; Tsuchida, A.; Sato, S.; Koyanagi, Y. Comparative study of self-expandable metallic stent and bypass surgery for inoperable esophageal cancer. Dis. Esophagus 2001, 14, 208–211. [Google Scholar] [CrossRef]
- Dallal, H.J.; Smith, G.D.; Grieve, D.C.; Ghosh, S.; Penman, I.D.; Palmer, K.R. A randomized trial of thermal ablative therapy versus expandable metal stents in the palliative treatment of patients with esophageal carcinoma. Gastrointest. Endosc. 2001, 54, 549–557. [Google Scholar] [CrossRef]
- Dai, Y.; Li, C.; Xie, Y.; Liu, X.; Zhang, J.; Zhou, J.; Pan, X.; Yang, S. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst. Rev. 2014, 2014, CD005048. [Google Scholar]
- Homs, M.Y.V.; Steyerberg, E.W.; Eijkenboom, W.M.H.; Tilanus, H.W.; Stalpers, L.J.A.; Bartelsman, J.F.; van Lanschot, J.J.; Wijrdeman, H.K.; Mulder, C.J.; Reinders, J.G.; et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: Multicentre randomised trial. Lancet 2004, 364, 1497–1504. [Google Scholar] [CrossRef]
- Tinusz, B.; Soos, A.; Hegyi, P.; Sarlos, P.; Szapary, L.; Eros, A.; Feczak, D.; Szakacs, Z.; Marta, K.; Venglovecz, V.; et al. Efficacy and safety of stenting and additional oncological treatment versus stenting alone in unresectable esophageal cancer: A meta-analysis and systematic review. Radiother. Oncol. 2020, 147, 169–177. [Google Scholar] [CrossRef]
- Vakil, N.; Morris, A.I.; Marcon, N.; Segalin, A.; Peracchia, A.; Bethge, N.; Zuccaro, G.; Bosco, J.J.; Jones, W.F. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am. J. Gastroenterol. 2001, 96, 1791–1796. [Google Scholar] [CrossRef]
- Conio, M.; Repici, A.; Battaglia, G.; de Pretis, G.; Ghezzo, L.; Bittinger, M.; Messmann, H.; Demarquay, J.F.; Blanchi, S.; Togni, M.; et al. A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am. J. Gastroenterol. 2007, 102, 2667–2677. [Google Scholar] [CrossRef]
- Vermeulen, B.D.; Siersema, P.D. Esophageal stenting in clinical practice: An Overview. Curr. Treat. Options Gastroenterol. 2018, 16, 260–273. [Google Scholar] [CrossRef]
- Doosti-Irani, A.; Mansournia, M.A.; Rahimi-Foroushani, A.; Haddad, P.; Holakouie-Naieni, K. Complications of stent placement in patients with esophageal cancer: A systematic review and network meta-analysis. PLoS ONE 2017, 12, e0184784. [Google Scholar] [CrossRef]
- Espinel, J.; Sanz, O.; Vivas, S.; Jorquera, F.; Munoz, F.; Olcoz, J.L.; Pinedo, E. Malignant gastrointestinal obstruction: Endoscopic stenting versus surgical palliation. Surg. Endosc. 2006, 20, 1083–1087. [Google Scholar] [CrossRef]
- Chen, Y.I.; Khashab, M.A. Endoscopic approach to gastrointestinal bypass in malignant gastric outlet obstruction. Curr. Opin. Gastroenterol. 2016, 32, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.; Alawad, A.S.; Shin, E.J.; Kim, K.; Bourdel, N.; Sing, V.K.; Lennon, A.M.; Hutfless, S.; Sharaiha, R.Z.; Amateau, S.; et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg. Endosc. 2013, 27, 2068–2075. [Google Scholar] [CrossRef]
- Balaceanu, A.; Diaconu, C.; Mateescu, D.; Stănica, A. Hepatocellular carcinoma with hepatic and pulmonary metastasis, inferior vena cava and left pulmonary artery thrombosis in a patient with asymptomatic hepatitis C. Case report. Med. Ultrason. 2010, 12, 345–348. [Google Scholar] [PubMed]
- Orr, J.; Lockwood, R.; Gamboa, A.; Slaughter, J.C.; Obstein, K.L.; Yachimski, P. Enteral Stents for Malignant Gastric Outlet Obstruction: Low Reintervention Rates for Obstruction due to Pancreatic Adenocarcinoma Versus Other Etiologies. J. Gastrointest. Surg. 2021, 25, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Jeurnink, S.M.; van Eijck, C.H.J.; Steyerberg, E.W.; Kuipers, E.J.; Siersema, P.D. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: A systematic review. BMC Gastroenterol. 2007, 7, 18. [Google Scholar] [CrossRef]
- Dawod, Q.; Issa, D.; Shah, S.L.; Daod, S.; Sharaiha, R.Z. EUS-gided stent placement for afferent limb and gastrojejunal obstruction in patient with pancreatic cancer. VideoGIE 2021, 6, 257–259. [Google Scholar] [CrossRef]
- Rimbas, M.; Larghi, A.; Costamagna, G. Endoscopic ultrasound-guided gastroenterostomy: Are we ready for prime time? Endosc. Ultrasound 2017, 6, 235–240. [Google Scholar]
- Nageswaran, H.; Belgaumkar, A.; Kumar, R. Acute afferent loop syndrome in the early postoperative period following pancreaticoduodenectomy. Ann. R. Coll. Surg. Engl. 2015, 97, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Hitawala, A.A.; Mousa, O.Y. Percutaneous Gastrostomy and Jenunostomy. Stat Pearls. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559215/ (accessed on 15 May 2022).
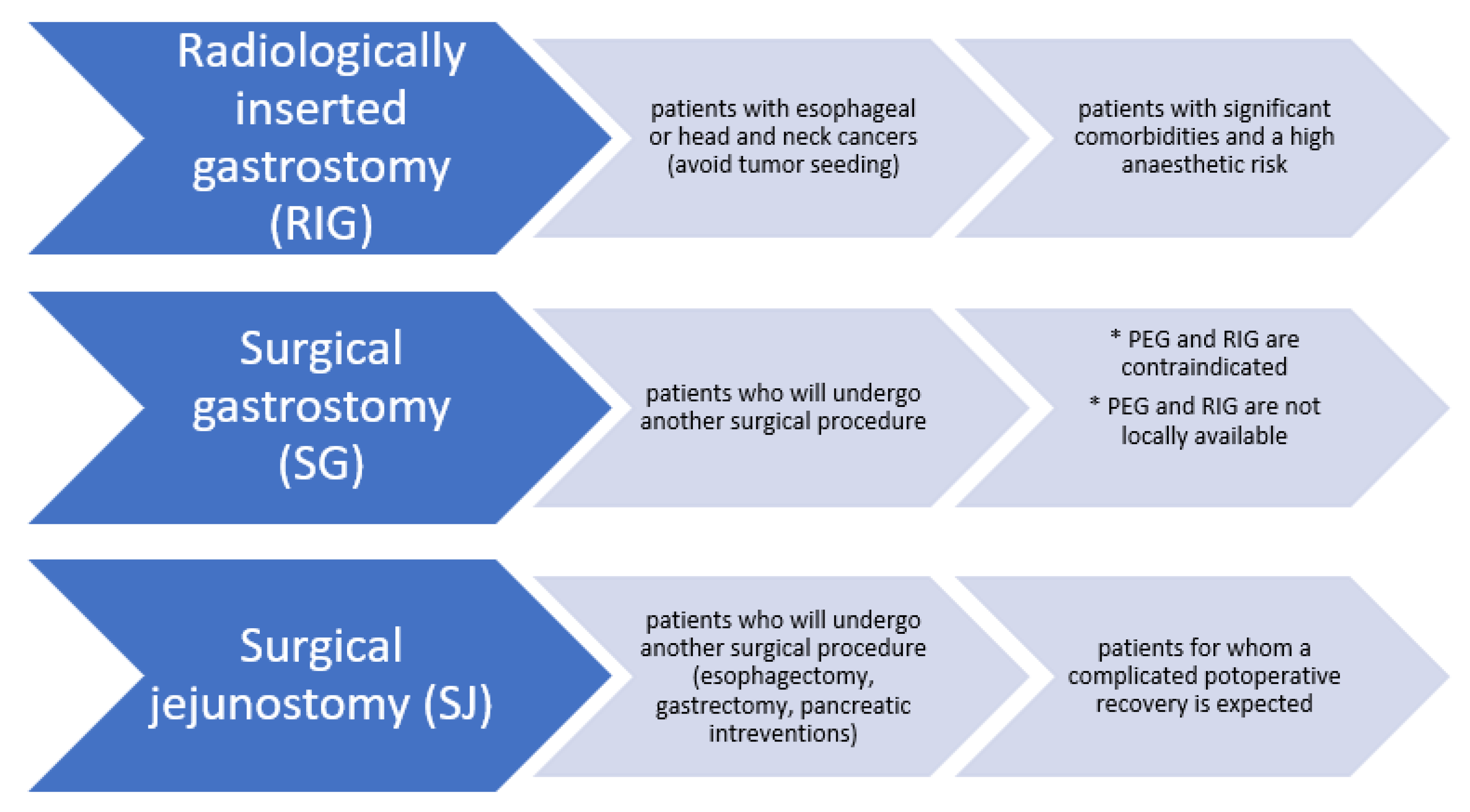

| Endoscopic Techniques for Enteral Access |
|---|
|
| Complications That May Occur at Any Time | Early Complications of PEG Placement | Late Complications of PEG Placement |
|---|---|---|
| Tube dysfunction Infection Wound infection Necrotizing fasciitis (rare complication) Bleeding Peristomal leakage Ulcerations Gastric outlet obstruction Inadvertent gastrostomy tube removal | Pneumoperitoneum Ileus Esophageal and gastric perforation Other early complications: small bowel wall hematoma with small bowel obstruction; sigmoid volvulus; transhepatic placement of a gastrostomy tube; damage of other abdominal organs | Deterioration of the gastrostomy site Buried bumper syndrome Colocutaneous fistula Persistent gastric fistula following gastrostomy tube removal PEG tract tumor seeding Other late complications: gastric herniation; persistent abdominal wall pain. |
| Major Complications | Minor Complications |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, V.-A.; Gheorghe, G.; Oprita, R.; Stan-Ilie, M.; Dascalu, R.-I.; Zaharia, O.; Jinga, V.; Diaconu, C.C.; Constantinescu, G. The Outcomes of Nutritional Support Techniques in Patients with Gastrointestinal Cancers. Gastroenterol. Insights 2022, 13, 245-257. https://doi.org/10.3390/gastroent13030025
Ionescu V-A, Gheorghe G, Oprita R, Stan-Ilie M, Dascalu R-I, Zaharia O, Jinga V, Diaconu CC, Constantinescu G. The Outcomes of Nutritional Support Techniques in Patients with Gastrointestinal Cancers. Gastroenterology Insights. 2022; 13(3):245-257. https://doi.org/10.3390/gastroent13030025
Chicago/Turabian StyleIonescu, Vlad-Alexandru, Gina Gheorghe, Ruxandra Oprita, Madalina Stan-Ilie, Raluca-Ioana Dascalu, Ondin Zaharia, Viorel Jinga, Camelia Cristina Diaconu, and Gabriel Constantinescu. 2022. "The Outcomes of Nutritional Support Techniques in Patients with Gastrointestinal Cancers" Gastroenterology Insights 13, no. 3: 245-257. https://doi.org/10.3390/gastroent13030025
APA StyleIonescu, V.-A., Gheorghe, G., Oprita, R., Stan-Ilie, M., Dascalu, R.-I., Zaharia, O., Jinga, V., Diaconu, C. C., & Constantinescu, G. (2022). The Outcomes of Nutritional Support Techniques in Patients with Gastrointestinal Cancers. Gastroenterology Insights, 13(3), 245-257. https://doi.org/10.3390/gastroent13030025








