Should the Endoscopic Restrictions during COVID-19 Pandemic Remain Unchanged?
Abstract
:1. Introduction
2. Materials and Methods
- Mandatory use of a mask when entering the center;
- Limitation on number of companions (only one per patient);
- Closure of the space above the reception desk, with isolation from the administrative staff;
- Modifications in the distribution of waiting room furniture to maintain a distance of 1.5 m between users;
- Epidemiological surveys (see COVID-19 Survey, in Supplementary Materials) and temperature checks conducted for all patients;
- Endoscopy and ward cleaning teams were provided PPE and instructed regarding use (see “Protocol of PPE” below);
- Changes to endoscopic ward disinfection protocol, including disinfection of all surfaces with chlorinated substances 10 min after the end of the procedure;
- Change to the internal circulation of patients and companions (encouraging them to leave their room through the emergency side door);
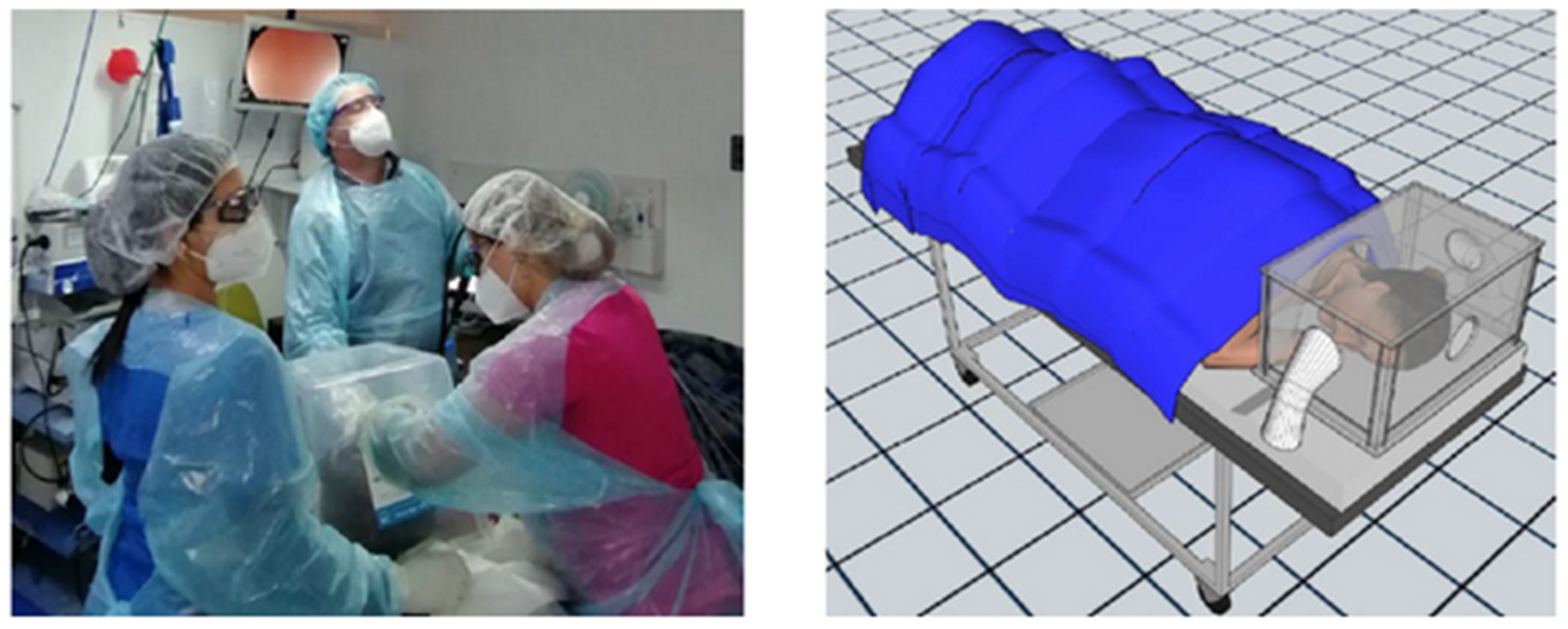
- Manufacturing of air isolation boxes to prevent air circulation around a patient’s head during high endoscopies. This box was made of plastic, is transparent, and has three holes—a small one wrapped in a plastic sleeve to enter the endoscope, another to adjust the patient’s neck, and one for the arm of the endoscopy assistant (Figure 2);
- Upon entering the endoscopy ward, the patient maintained the use of a mask. In the case of upper digestive endoscopies, the mask was removed prior to the placement of the “air isolation box”, and as soon as it is removed, the personal mask was reinstalled. During colonoscopies, it was worn during the entire procedure;
- Looking for potential COVID-19 infection, IgG and IgM antibodies were measured periodically against COVID-19 in the endoscopic and cleaning teams using a rapid test, with symptoms monitored daily among staff.
Protocol of PPE
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, D.H.; Zhao, S.; Lin, Q.Y.; Zhuang, Z.; Cao, P.; Wang, M.H.; Yang, L. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int. J. Infect. Dis. 2020, 94, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am. J. Infect. Control. 2007, 35, S65–S164. [Google Scholar] [CrossRef] [PubMed]
- Riou, J.; Althaus, C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance 2020, 25, 2000058. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.L. Environmental surface contamination with SARS-CoV-2—A short review. J. Hum. Virol. Retrovirol. 2020, 8, 15–19. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Lui, R.N.; Wong, S.H.; Sánchez-Luna, S.A.; Pellino, G.; Bollipo, S.; Wong, M.; Chiu, P.W.Y.; Sung, J.J.Y. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J. Gastroenterol. Hepatol. 2020, 35, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Cortés, P.; Heredia, C.; González, R.; Silva, V.; Bufadel, M.E.; Araya, R.J.; Moscoso, F.J.; Donoso, F.G.; Montenegro, C.U. Guía de recomendación ached-schge para el funcionamiento de las unidades de endoscopia durante el brote de coronavirus (COVID-19). Gastroenterol. Latinoam. 2020, 1, 9–20. [Google Scholar] [CrossRef]
- Sud, A.; Torr, B.; Jones, M.E.; Broggio, J.; Scott, S.; Loveday, C.; Garrett, A.; Gronthoud, F.; Nicol, D.L.; Jhanji, S.; et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: A modelling study. Lancet Oncol. 2020, 21, 1035–1044. [Google Scholar] [CrossRef]
- Ministerio de Salud (MINSAL). Situación Epidemiológica, COVID-19 Chile. 2020. Available online: https://www.minsal.cl/wp-content/uploads/2020/03/Informe_3_COVID_19_Chile.pdf (accessed on 12 March 2020).
- Instituto Nacional de Estadística (INE). Síntesis de Resultados CENSO 2017. 2017. Available online: https://www.censo2017.cl/descargas/home/sintesis-de-resultados-censo2017.pdf (accessed on 14 June 2020).
- World Health Organization. Uso Racional de Equipo de Protección Personal Frente a la COVID-19 y Aspecto que Considerar en Situación de Escasez Graves. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/331810/WHO-2019-nCoV-IPC_PPE_use-2020.3-spa.pdf (accessed on 7 February 2020).
- Colegio Médico de Chile. Recomendaciones uso de elementos de protección personal (EPP): Para trabajadores de la salud. Rev. Chil. Infectol. 2020, 37, 106–110. Available online: https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S0716-10182020000200106&lng=es. (accessed on 3 March 2021). [CrossRef] [PubMed]
- Sociedad Chilena de Medicina del Trabajo (SOCHMET). Pandemia por Coronavirus (COVID-19): Recomendaciones de Seguridad y Salud Ocupacional para Trabajadores de la Salud. 2020. Available online: http://www.colegiomedico.cl/wp-content/uploads/2020/03/Recomendaciones-SOCHMET-Covid-19-para-trabajadores-de-la-salud-V01.pdf (accessed on 7 February 2021).
- Subsecretaria de Redes Asistenciales (MINSAL). Protocolo de Referencia para Correcto uso de Equipos de Protección Personal en Pacientes Sospechosos o Confirmados de COVID-19. 2020, pp. 5–10. Available online: http://www.colegiomedico.cl/wp-content/uploads/2020/03/PROTOCOLO-DE-USO-DE-EQUIPOS-DE-PROTECCI%C3%93N-PERSONAL-EN-LA-PREVENCI%C3%93N-DE-TRANSMISI%C3%93N-COVID19.pdf (accessed on 7 February 2021).
- Castro, E.; Castro, R.; Fernandes, F.; Pereira, G.; Perazzo, H. Gastrointestinal endoscopy during the COVID-19 pandemic: An updated review of guidelines and statements from international and national societies. Gastrointest. Endosc. 2020, 92, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.W.Y.; Ng, S.C.; Inoue, H.; Reddy, D.N.; Hu, E.L.; Cho, J.Y.; Ho, L.K.Y.; Hewett, D.G.; Chiu, H.-M.; Rerknimitr, R.; et al. Practice of endoscopy during COVID-19 pandemic: Position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements). Gut 2020, 69, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Hassan, C.; Beilenhoff, U.; Antonelli, G.; Ebigbo, A.; Pellisè, M.; Arvanitakis, M.; Bhandari, P.; Bisschops, R.; Van Hooft, J.E.; et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy 2020, 52, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bresky, E.; Bresky, G.; Lancellotti, D.; Madariaga, J.; Licuime, S.; Palma, P.; Saez, F.; Rojas, M.J.; Seijas, L. Should the Endoscopic Restrictions during COVID-19 Pandemic Remain Unchanged? Gastroenterol. Insights 2021, 12, 358-365. https://doi.org/10.3390/gastroent12030034
Bresky E, Bresky G, Lancellotti D, Madariaga J, Licuime S, Palma P, Saez F, Rojas MJ, Seijas L. Should the Endoscopic Restrictions during COVID-19 Pandemic Remain Unchanged? Gastroenterology Insights. 2021; 12(3):358-365. https://doi.org/10.3390/gastroent12030034
Chicago/Turabian StyleBresky, Emilio, Gustavo Bresky, Domingo Lancellotti, Juan Madariaga, Sebastian Licuime, Paulette Palma, Fabian Saez, Maria Jose Rojas, and Luis Seijas. 2021. "Should the Endoscopic Restrictions during COVID-19 Pandemic Remain Unchanged?" Gastroenterology Insights 12, no. 3: 358-365. https://doi.org/10.3390/gastroent12030034
APA StyleBresky, E., Bresky, G., Lancellotti, D., Madariaga, J., Licuime, S., Palma, P., Saez, F., Rojas, M. J., & Seijas, L. (2021). Should the Endoscopic Restrictions during COVID-19 Pandemic Remain Unchanged? Gastroenterology Insights, 12(3), 358-365. https://doi.org/10.3390/gastroent12030034








