Hepatosplenic T-Cell Lymphoma Mimicking Acute Onset of Cholestatic Hepatitis in a Young Immunocompetent Man: A Case Report
Abstract
:1. Introduction
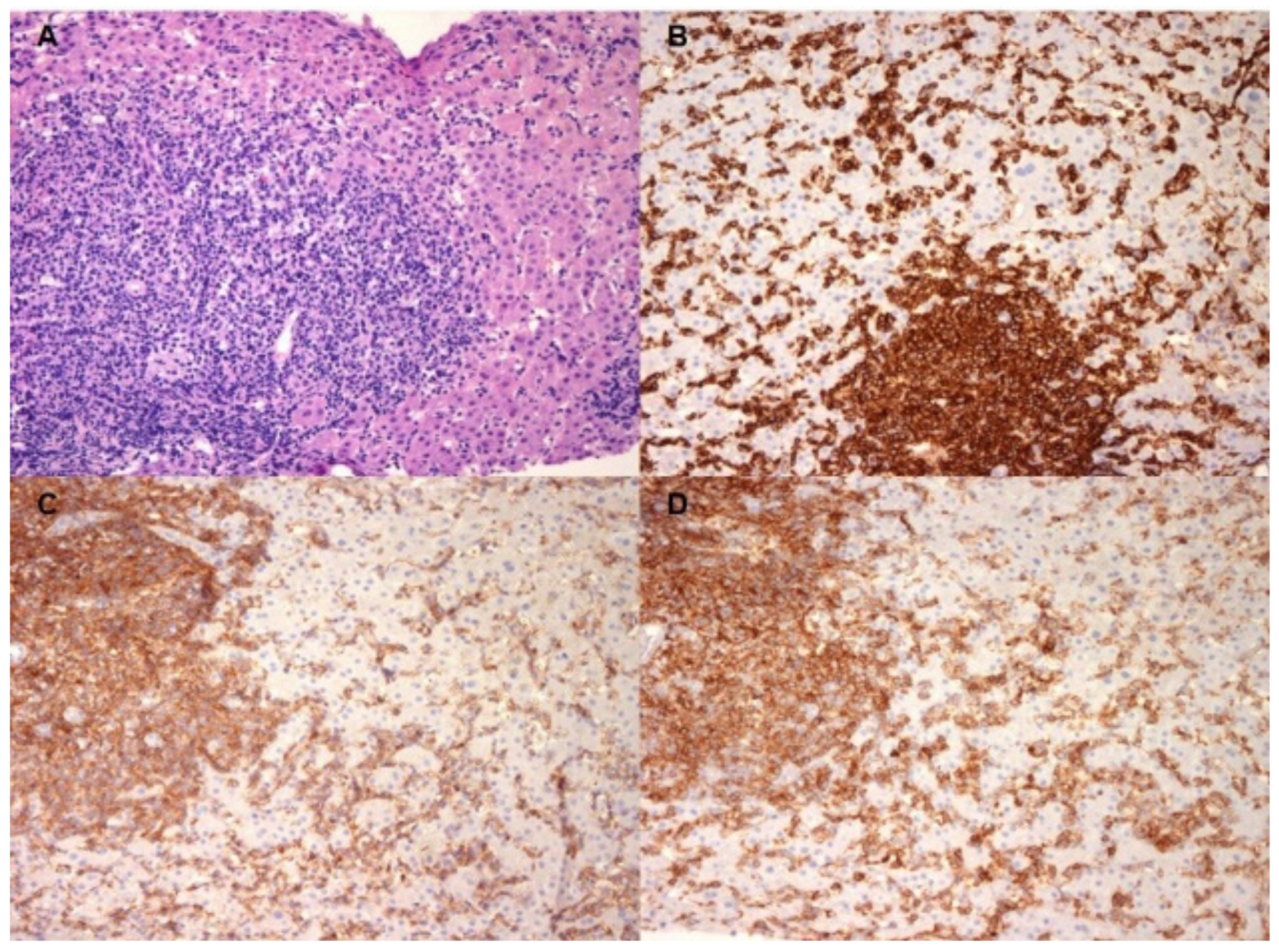
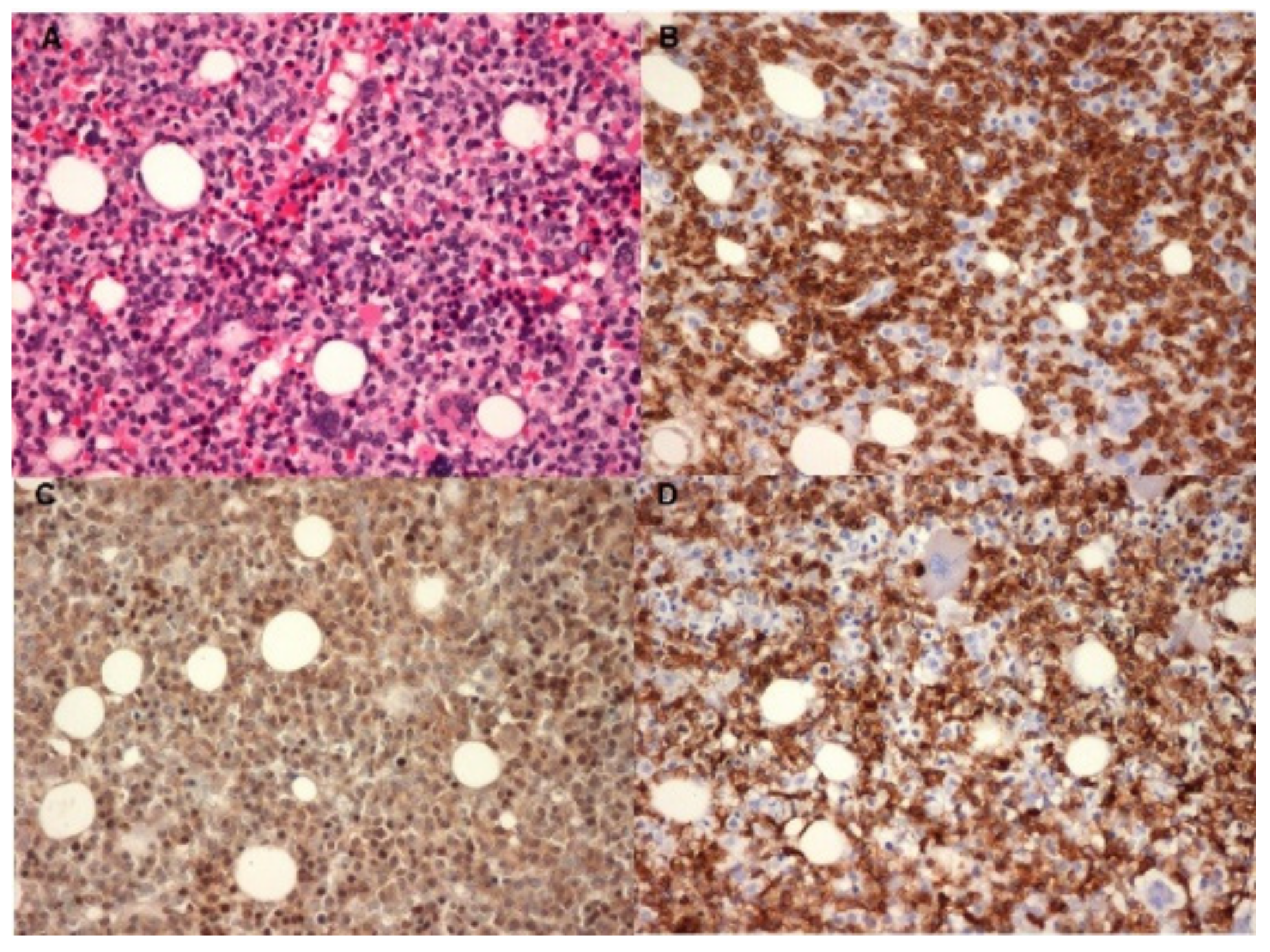
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC Press: Lyon, France, 2008; Volume 2. [Google Scholar]
- Yabe, M.; Medeiros, L.J.; Tang, G.; Wang, S.A.; Ahmed, S.; Nieto, Y.; Hu, S.; Bhagat, G.; Oki, Y.; Patel, K.P.; et al. Prognostic factors of hepatosplenic T-cell lymphoma (HSTCL): A clinicopathologic, immunophenotypic, and cytogenetic analysis of 28 patients. Am. J. Surg. Pathol. 2016, 40, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Macon, W.R.; Levy, N.B.; Kurtin, P.J.; Salhany, K.E.; Elkhalifa, M.Y.; Casey, T.T.; Craig, F.E.; Vnencak-Jones, C.L.; Gulley, M.L.; Park, J.P.; et al. Hepatosplenic alphabeta T-cell lymphomas: A report of 14 cases and comparison with hepatosplenic gammadelta T-cell lymphomas. Am. J. Surg. Pathol. 2001, 25, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Yabe, M.; Miranda, R.N.; Medeiros, L.J. Hepatosplenic T-cell Lymphoma: A review of clinicopathologic features, pathogenesis, and prognostic factors. Hum. Pathol. 2018, 74, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.; Armitage, J.; Weisenburger, D. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [PubMed]
- Shi, Y.; Wang, E. Hepatosplenic T-cell lymphoma: A clinicopathologic review with an emphasis on diagnostic differentiation from other T-cell/natural killer-cell neoplasms. Arch Pathol. Lab. Med. 2015, 139, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Travert, M.; Huang, Y.; De Leval, L.; Martin-Garcia, N.; Delfau-Larue, M.H.; Berger, F.; Bosq, J.; Brière, J.; Soulier, J.; Macintyre, E.; et al. Molecular features of hepatosplenic T-cell lymphoma unravels potential novel therapeutic targets. Blood 2012, 119, 5795–5806. [Google Scholar] [CrossRef] [PubMed]
- Chazouillères, O.; Housset, C. Intrahepatic cholestasis. In Textbook of Hepatology: From Basic Science to Clinical Practice; Blackwell: Oxford, UK, 2007; pp. 1481–1500. [Google Scholar]
- Amin, S.; Findeis, S.K.; Whiteley, A.; Krause, J.R. An unusual presentation of an uncommon lymphoma, hepatosplenic T-cell lymphoma. In Baylor University Medical Center Proceedings; Taylor & Francis: Dallas, TX, USA, 2019; Volume 32, pp. 129–130. [Google Scholar]
- Li, P.; Zhang, D.; Zhou, J.; Li, P.; Shen, Y.; Pan, Z.; Evans, A.G.; Liao, X. Hepatic involvement by T-cell neoplasms: A clinicopathologic study of 40 cases. Hum. Pathol. 2020, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.; Gomes, M.M.; Carda, J.P.; Pereira de Moura, J. Hepatosplenic T-cell lymphoma in a young immunocompetent man. BMJ Case Rep. 2016, 2016, bcr2016214414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, Y.; Kato, S.; Komiya, H.; Shirota, T.; Mukai, K.; Hayashi, T. Primary omental gamma/delta T-cell lymphoma involving the central nervous system. Leuk. Lymph. 2004, 45, 1947–1950. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metelli, F.; Solimando, R.; Alemanni, L.V.; Gafà, R.; Marasco, G. Hepatosplenic T-Cell Lymphoma Mimicking Acute Onset of Cholestatic Hepatitis in a Young Immunocompetent Man: A Case Report. Gastroenterol. Insights 2022, 13, 258-263. https://doi.org/10.3390/gastroent13030026
Metelli F, Solimando R, Alemanni LV, Gafà R, Marasco G. Hepatosplenic T-Cell Lymphoma Mimicking Acute Onset of Cholestatic Hepatitis in a Young Immunocompetent Man: A Case Report. Gastroenterology Insights. 2022; 13(3):258-263. https://doi.org/10.3390/gastroent13030026
Chicago/Turabian StyleMetelli, Flavio, Riccardo Solimando, Luigina Vanessa Alemanni, Roberta Gafà, and Giovanni Marasco. 2022. "Hepatosplenic T-Cell Lymphoma Mimicking Acute Onset of Cholestatic Hepatitis in a Young Immunocompetent Man: A Case Report" Gastroenterology Insights 13, no. 3: 258-263. https://doi.org/10.3390/gastroent13030026
APA StyleMetelli, F., Solimando, R., Alemanni, L. V., Gafà, R., & Marasco, G. (2022). Hepatosplenic T-Cell Lymphoma Mimicking Acute Onset of Cholestatic Hepatitis in a Young Immunocompetent Man: A Case Report. Gastroenterology Insights, 13(3), 258-263. https://doi.org/10.3390/gastroent13030026






