Value of Acute Kidney Injury in Predicting Mortality in Vietnamese Patients with Decompensated Cirrhosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Study Population
2.2. Data Collection and Definitions
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of Patients
| General Characteristics | Frequency | Percentage (%) |
|---|---|---|
| Average age | 61.1 ± 12.9 years | |
| Gender | ||
| Male | 167 | 66.8 |
| Female | 83 | 33.2 |
| Causes of cirrhosis | ||
| Hepatitis B virus | 94 | 37.6 |
| Hepatitis C virus | 42 | 16.8 |
| Alcohol | 50 | 20 |
| Hepatitis B virus + alcohol | 11 | 4.4 |
| Hepatitis C virus + alcohol | 1 | 0.4 |
| Hepatitis B virus + hepatitis C virus | 4 | 1.6 |
| Other causes (primary biliary cholangitis, primary sclerosing cholangitis, alpha-1-antitrypsin deficiency, and Wilson’s disease) | 48 | 19.2 |
| Child–Pugh Score | ||
| Child–Pugh B | 181 | 72.4 |
| Child–Pugh C | 69 | 27.6 |
| Acute kidney injury | ||
| Yes | 64 | 25.6 |
| No | 186 | 74.4 |
| Acute kidney injury stages | ||
| AKI 1 | 20 | 31.3 |
| AKI 2 | 31 | 48.4 |
| AKI 3 | 13 | 20.3 |
3.2. Treatment Results for Acute Kidney Injury
| Results of Treatment | Frequency | Percentage (%) |
|---|---|---|
| Overall mortality | 35 | 14 |
| Mortality rate of acute kidney injury | 24 | 37.5 |
| Mortality rate of hepatorenal syndrome | 16 | 84.2 |
| Mortality rate by stage of acute kidney injury | ||
| AKI 1 | 4 | 20 |
| AKI 2 | 13 | 41.9 |
| AKI 3 | 7 | 51.8 |
| Average survival time | ||
| AKI | 10.6 ± 0.7 days | |
| Non-AKI | 24 ± 0.5 days | |
| Mean MELD score (±SD) | 21 ± 8.5 scores | |
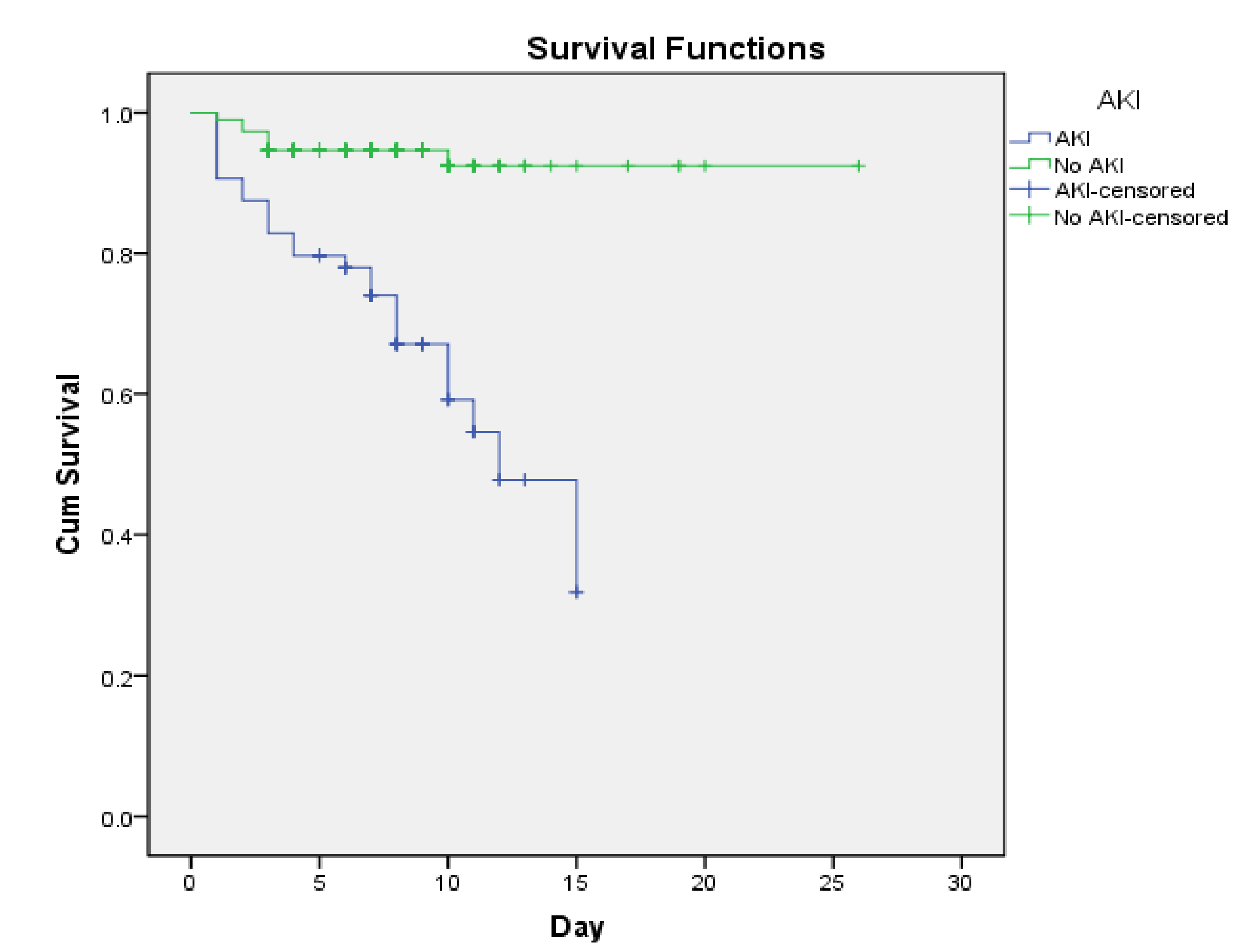
3.3. Factors Related to Mortality Prognosis in Patients with Decompensated Cirrhosis
| Factors | Dead | Survival | Univariable | Multivariable | ||
|---|---|---|---|---|---|---|
| n (%) | n (%) | OR | p | OR | p | |
| (95% Confidence Interval) | (95% Confidence Interval) | |||||
| Child–Pugh | ||||||
| Child C | 17 (25) | 51 (75) | 3 (1.5–6.3) | 0.02 | 0.8 (0.3–2.4) | 0.7 |
| Child B | 18 (9.9) | 164 (90.1) | ||||
| Acute kidney injury | ||||||
| Yes | 24 (37.5) | 40 (62.5) | 9.5 (4.3–21.1) | <0.0001 | 7.7 (2.9–20.1) | < 0.0001 |
| No | 11 (5.9) | 175 (94.1) | ||||
| Hepatic encephalopathy | ||||||
| Yes | 16 (30.8) | 36 (69.2) | 4.2 (1.9–8.9) | <0.0001 | 4 (1.5–10.4) | 0.005 |
| No | 19 (9.6) | 179 (90.4) | ||||
| Gastrointestinal bleeding | ||||||
| Yes | 14 (22.6) | 48 (77.4) | 2.3 (1.1–4.9) | 0.03 | 5 (1.8–13.5) | 0.002 |
| No | 21 (11.2) | 167 (88.8) | ||||
| Ascites | ||||||
| Yes | 33 (15) | 187 (85) | 2.5 (0.6–10.9) | 0.2 | - | - |
| No | 2 (6.7) | 28 (93.3) | ||||
| Infections | ||||||
| Yes | 18 (30) | 42 (70) | 4.4 (2.1–9.2) | <0.0001 | 2.4 (0.9–6.0) | 0.07 |
| No | 17 (8.9) | 173 (91.1) | ||||
| Hyponatremia | ||||||
| Yes | 20 (21.1) | 75 (78.9) | 2.5 (1.2–5.1) | 0.01 | 2.3 (0.9–5.9) | 0.09 |
| No | 15 (9.7) | 140 (90.3) | ||||
| Increased total bilirubin | ||||||
| Yes | 22 (19.1) | 93 (80.9) | 2.2 (1.1–4.6) | 0.03 | 1.2 (0.4–3.2) | 0.7 |
| No | 13 (9.6) | 122 (90.4) | ||||
| Prothrombin < 70% | ||||||
| Yes | 34 (16) | 179 (84) | 6.8 (1–51.6) | 0.03 | 0.9 (0.9–3.3) | 0.06 |
| No | 1 (2.7) | 36 (97.3) | ||||
| Thrombocytopenia | ||||||
| Yes | 25 (13.7) | 157 (86.3) | 0.8 (0.4–2.0) | 0.9 | - | - |
| No | 10 (14.7) | 58 (85.3) | ||||
| Serum albumin | ||||||
| <30 g/L | 28 (14.4) | 167 (85.6) | 1.2 (0.5–2.7) | 0.8 | - | - |
| <30 g/L | 7 (12.7) | 48 (87.3) | ||||
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Law, M.G.; Dore, G.J. An enormous hepatitis B virus-related liver disease burden projected in Vietnam by 2025. Liver Int. 2008, 28, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.; Eriksson, B.; Strömberg, U.; Buchebner, D.; Midlöv, P. Incidence, aetiology and related comorbidities of cirrhosis: A Swedish population-based cohort study. BMC Gastroenterol. 2020, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Chancharoenthana, W.; Leelahavanichkul, A. Acute kidney injury spectrum in patients with chronic liver disease: Where do we stand? World J. Gastroenterol. 2019, 25, 3684–3703. [Google Scholar] [CrossRef] [PubMed]
- Khatua, C.R.; Sahu, S.K.; Meher, D. Acute kidney injury in hospitalized cirrhotic patients: Risk factors, type of kidney injury, and survival. JGH Open 2021, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gessolo Lins, P.R.; Carvalho Padilha, W.S.; Magalhaes Giradin Pimentel, C.F. Risk factors, mortality and acute kidney injury outcomes in cirrhotic patients in the emergency department. BMC Nephrol. 2018, 19, 277. [Google Scholar] [CrossRef] [PubMed]
- Karagozian, R.; Bhardwaj, G.; Wakefield, D.B. Acute kidney injury is associated with higher mortality and healthcare costs in hospitalized patients with cirrhosis. Ann. Hepatol. 2019, 18, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Angeli, P.; Gines, P.; Wong, F.; Bernardi, M. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. Gut 2015, 64, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Dung, V.T.M. Rate of Acute Kidney Injury and Mortality Prognosis in Patients with Decompensated Cirrhosis; Ho Chi Minh City University of Medicine and Pharmacy: Ho Chi Minh City, Vietnam; Cho Ray Hospital: Ho Chi Minh City, Vietnam, 2015. [Google Scholar]
- Rosi, S.; Piano, S.; Frigo, A.C. New ICA criteria for the diagnosis of acute kidney injury in cirrhotic patients: Can we use an imputed value of serum creatinine? Liver Int. 2015, 35, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.; Jang, I. Analysis of mortality prognostic factors using model for end-stage liver disease with incorporation of serum-sodium classification for liver cirrhosis complications: A retrospective cohort study. Medicine 2019, 98, e17862. [Google Scholar] [CrossRef] [PubMed]
- Bansho, E.T.O.; Silva, P.E.S.; Colombo, B.S. Prognostic Significance of The New Criteria for Acute Kidney Injury in Cirrhosis. Ann. Hepatol. 2018, 17, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.M.; Garcia-Tsao, G.; Sanyal, A. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology 2013, 57, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Nagaraju, S.P. Acute kidney injury in patients with cirrhosis of liver: Clinical profile and predictors of outcome. Indian J. Gastroenterol. 2018, 37, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Vicco, M.H.; Rodeles, L.; Ferini, F.; Long, A.K. In-hospital mortality risk factors in patients with ascites due to cirrhosis. Rev. Assoc. Med. Bras. 2015, 61, 35–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bjerring, P.N.; Gluud, L.L. Severe hepatic encephalopathy is an independent predictor of mortality in hospitalised patients with cirrhosis. AME Med. J. 2017, 2, 134. [Google Scholar] [CrossRef]
- Kumar, A.S.; Sibia, R.S. Predictors of in-hospital mortality among patients presenting with variceal gastrointestinal bleeding. Saudi J. Gastroenterol. 2015, 21, 43–46. [Google Scholar] [PubMed]
- Gomes, C.G.O.; de Andrade, M.V.M.; Resende Guedes, L. Clinical Aspects and Prognosis Evaluation of Cirrhotic Patients Hospitalized with Acute Kidney Injury. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6567850. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.N.; Mai, T.H.N.; Vo, N.H.; Vo, C.T.; Ngo, N.T.Y.; Vi, M.T.; Nguyen, T. Value of Acute Kidney Injury in Predicting Mortality in Vietnamese Patients with Decompensated Cirrhosis. Gastroenterol. Insights 2022, 13, 139-147. https://doi.org/10.3390/gastroent13020015
Nguyen NN, Mai THN, Vo NH, Vo CT, Ngo NTY, Vi MT, Nguyen T. Value of Acute Kidney Injury in Predicting Mortality in Vietnamese Patients with Decompensated Cirrhosis. Gastroenterology Insights. 2022; 13(2):139-147. https://doi.org/10.3390/gastroent13020015
Chicago/Turabian StyleNguyen, Nghia N., Tan H. N. Mai, Nghia H. Vo, Cuong T. Vo, Nhi T. Y. Ngo, Mai T. Vi, and Thang Nguyen. 2022. "Value of Acute Kidney Injury in Predicting Mortality in Vietnamese Patients with Decompensated Cirrhosis" Gastroenterology Insights 13, no. 2: 139-147. https://doi.org/10.3390/gastroent13020015
APA StyleNguyen, N. N., Mai, T. H. N., Vo, N. H., Vo, C. T., Ngo, N. T. Y., Vi, M. T., & Nguyen, T. (2022). Value of Acute Kidney Injury in Predicting Mortality in Vietnamese Patients with Decompensated Cirrhosis. Gastroenterology Insights, 13(2), 139-147. https://doi.org/10.3390/gastroent13020015






