Ventilatory Effect of Midazolam in Propofol Deep Sedation for Hepatic Tumor Patients Undergoing Percutaneous Radiofrequency Ablation Procedure
Abstract
1. Introduction
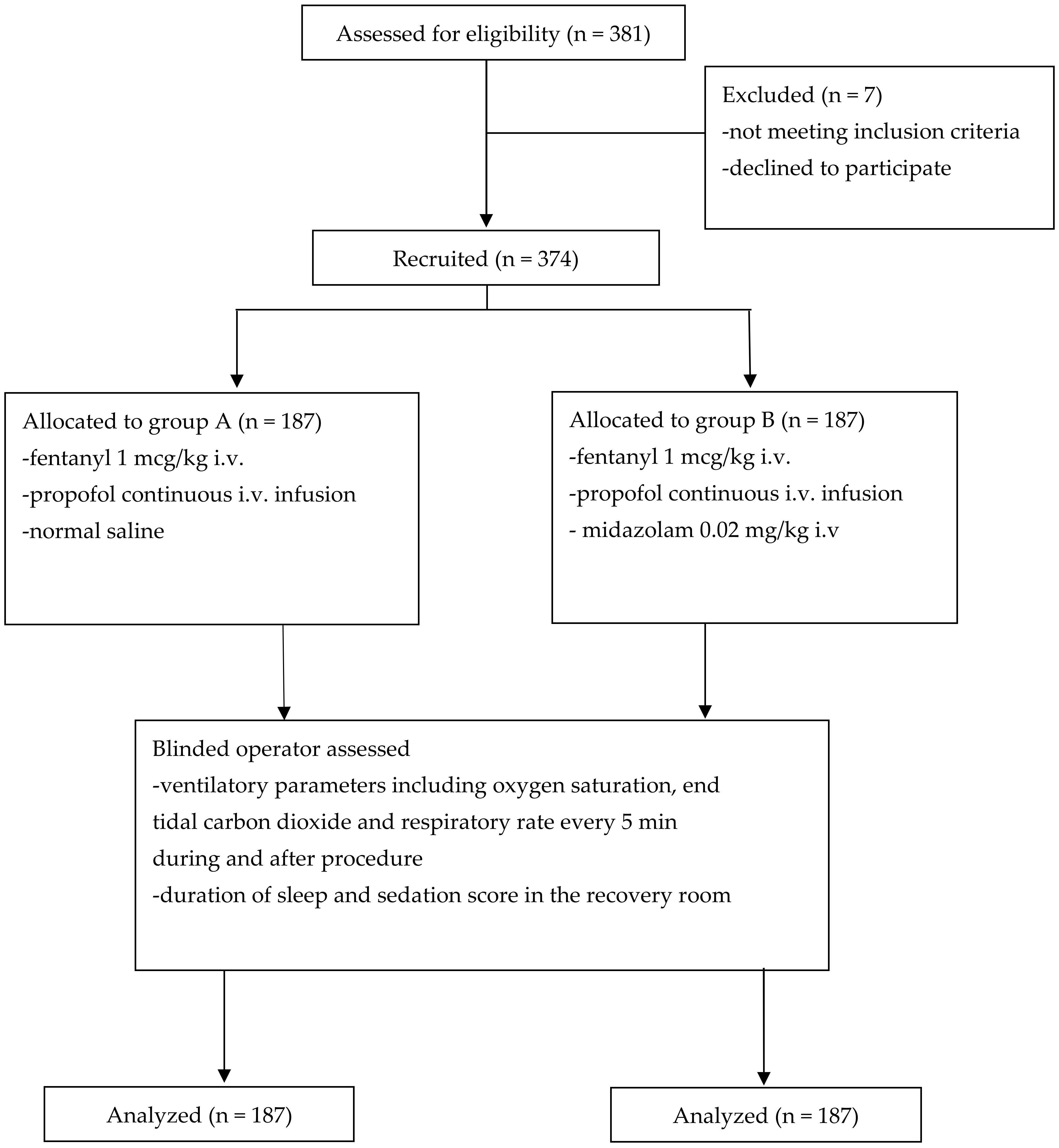
2. Materials and Methods
2.1. Patients
2.2. Study Design
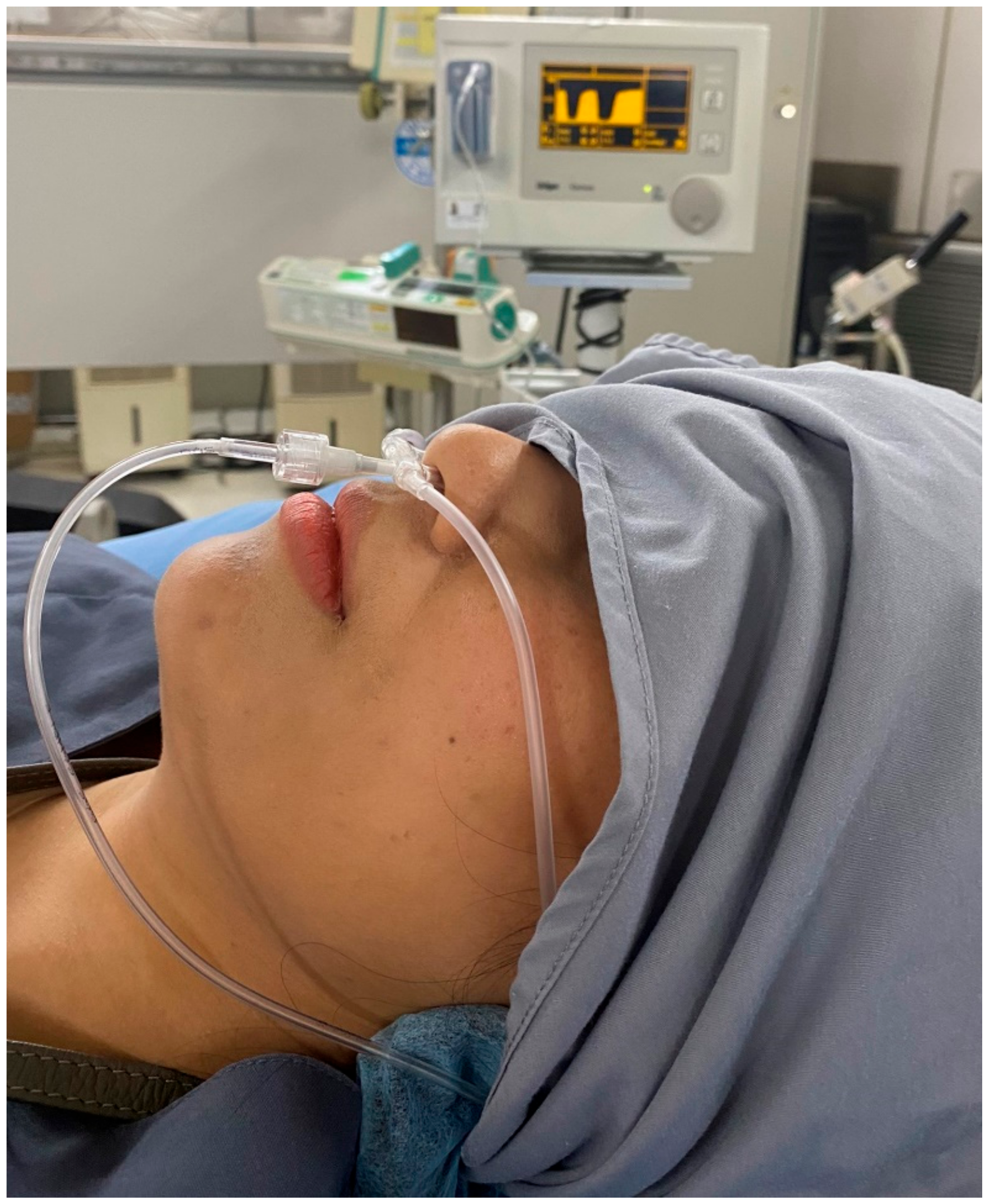
2.3. Sedation-Related Procedure
2.4. Sedation-Related Ventilatory Effects
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wells, S.A.; Hinshaw, J.L.; Lubner, M.G.; Ziemlewicz, T.J.; Brace, C.L.; Lee, F.T., Jr. Liver ablation: Best practice. Radiol. Clin. N. Am. 2015, 53, 933–971. [Google Scholar] [CrossRef]
- Facciorusso, A.; Serviddio, G.; Muscatiello, N. Local ablative treatments for hepatocellular carcinoma: An updated review. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Amornyotin, S.; Jirachaipitak, S.; Wangnatip, S. Anesthetic management for radiofrequency ablation in patients with hepatocellular carcinoma in a developing country. J. Anesth. Crit. Care. Open Access 2015, 3, 00086. [Google Scholar]
- Amornyotin, S.; Srikureja, W.; Pausawasdi, N.; Prakanrattana, U.; Kachintorn, U. Intravenous sedation for gastrointestinal endoscopy in very elderly patients of Thailand. Asian Biomed. 2011, 5, 485–491. [Google Scholar] [CrossRef]
- Reves, J.G.; Fragen, R.J.; Vinik, H.R.; Greenblatt, D.J. Midazolam: Pharmacology and uses. Anesthesiology 1985, 62, 310–324. [Google Scholar] [CrossRef]
- Amornyotin, S. Sedative and analgesic drugs for gastrointestinal endoscopic procedure. J. Gastroenterol. Hepatol. Res. 2014, 3, 1133–1144. [Google Scholar]
- American Society of Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. An update report by the American Society of Anesthesiologists Task Force on sedation and analgesia by non-anesthesiologists. Anesthesiology 2002, 96, 1004–1017. [Google Scholar]
- Cohen, L.B.; DeLegge, M.H.; Aisenberg, J.; Brill, J.V.; Inadomi, J.M.; Kochman, M.L.; Piorkowski, J.D. AGA Institute review of endoscopic sedation. Gastroenterology 2007, 133, 675–701. [Google Scholar] [CrossRef]
- Taylor, D.M.; O’Brien, D.; Ritchie, P.; Pasco, J.; Cameron, P.A. Propofol versus midazolam/fentanyl for reduction of anterior shoulder dislocation. Acad. Emerg. Med. 2005, 12, 13–19. [Google Scholar] [CrossRef]
- Amornyotin, S. Intravenous sedation techniques for gastrointestinal endoscopy. J. Gastroenterol. Hepatol. Res. 2016, 5, 2050–2057. [Google Scholar] [CrossRef][Green Version]
- Amornyotin, S. Sedation and monitoring for gastrointestinal endoscopy. World J. Gastrointest. Endosc. 2013, 5, 47–55. [Google Scholar] [CrossRef]
- Amornyotin, S.; Leelakusolvong, S.; Chalayonnawin, W.; Kongphlay, S. Age-dependent safety analysis of propofol-based deep sedation for ERCP and EUS procedures at an endoscopy training center in a developing country. Clin. Exp. Gastroenterol. 2012, 5, 123–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tobias, J.D.; Leder, M. Procedural sedation: A review of sedative agents, monitoring, and management of complications. Saudi J. Anaesth. 2011, 5, 395–410. [Google Scholar] [CrossRef]
- Nishiyama, T. Sedation during artificial ventilation by continuous intravenous infusion of midazolam: Effects of hepatocellular or renal damage. J. Intens. Care. Med. 1997, 12, 40–44. [Google Scholar] [CrossRef]
- Soleimanpour, H.; Safari, S.; Rahmani, F.; Jafari, R.A.; Alavian, S.M. Intravenous hypnotic regimens in patients with liver disease: A review article. Anesth. Pain Med. 2015, 5, e23923. [Google Scholar] [CrossRef]
- Weaver, C.S.; Hauter, W.H.; Duncan, C.E.; Brizendine, E.J.; Cordell, W.H. An assessment of the association of bispectral index with 2 clinical sedation scales for monitoring depth of procedural sedation. Am. J. Emerg. Med. 2007, 25, 918–924. [Google Scholar] [CrossRef]
- Amornyotin, S.; Chalayonnawin, W.; Kongphlay, S. Deep sedation for endoscopic retrograde cholangiopancreatography: A comparison between clinical assessment and narcotrendTM monitoring. Med. Devic. Evid. Res. 2011, 4, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Ichinohe, T.; Kaneko, Y. Is measurement of end-tidal CO2 through a nasal cannula reliable? Anesth. Prog. 1997, 44, 23–26. [Google Scholar]
- Lam, T.; Nagappa, M.; Wong, J.; Singh, M.; Wong, D.; Chung, F. Continuous pulse oximetry and capnography monitoring for postoperative respiratory depression and adverse events: A systematic review and meta-analysis. Anesth. Analg. 2017, 125, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Amornyotin, S.; Sripunjan, K. Propofol deep sedation with and without midazolam for percutaneous radiofrequency ablation in patients with hepatocellular carcinoma: A retrospective cohort study. J. Med. Assoc. Thai. 2018, 101, S109–S115. [Google Scholar]
- Gonzalez Castro, L.N.; Mehta, J.H.; Brayanov, J.B.; Mullen, G.J. Quantification of respiratory depression during pre-operative administration of midazolam using a non-invasive respiratory volume monitor. PLoS ONE 2017, 12, e0172750. [Google Scholar] [CrossRef] [PubMed]
- Holley, K.; MacNabb, C.M.; Georgiadis, P.; Minasyan, H.; Shukla, A.; Mathews, D. Monitoring minute ventilation versus respiratory rate to measure the adequacy of ventilation in patients undergoing upper endoscopic procedures. J. Clin. Monit. Comput. 2016, 30, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.H.; Williams, G.W., II; Harvey, B.C.; Grewal, N.K.; George, E.E. The relationship between minute ventilation and end tidal CO2 in intubated and spontaneously breathing patients undergoing procedural sedation. PLoS ONE 2017, 12, e0180187. [Google Scholar]
- Williams, G.W., II; George, C.A.; Harvey, B.C.; Freeman, J.E. A comparison of measurements of change in respiratory status in spontaneously breathing volunteers by the ExSpiron noninvasive respiratory volume monitor versus the capnostream capnometer. Anesth. Analg. 2017, 124, 120–126. [Google Scholar] [CrossRef] [PubMed]
| Group A | Group B | p-Value | |
|---|---|---|---|
| (n = 187) | (n = 187) | ||
| Age (year) | 63.5 (9.7) | 62.7 (9.2) | 0.638 |
| Gender: Male | 126 (67.4) | 141 (75.4) | 0.086 |
| Female | 61 (32.6) | 46 (24.6) | |
| Weight (kg) | 65.8 (12.4) | 67.6 (11.9) | 0.615 |
| Height (cm) | 163.1 (8.3) | 164.1 (7.7) | 0.372 |
| ASA physical status: | |||
| I | 0 | 0 | 0.489 |
| II | 132 (70.6) | 138 (73.8) | |
| III | 55 (29.4) | 49 (26.2) | |
| Duration of procedure (min) | 50.2 (23.6) | 58.1 (28.2) | 0.56 |
| Total dose of propofol | |||
| mg | 295.4 (139.3) | 306.9 (162.8) | 0.369 |
| mg/kg | 4.6 (2.2) | 4.6 (2.5) | 0.446 |
| mg/kg/h | 5.8 (2.3) | 5.1 (2.2) | 0.476 |
| Group A | Group B | p-Value | |
|---|---|---|---|
| (n = 187) | (n = 187) | ||
| 5 min during procedure | |||
| SpO2 (%) | 98.7 (1.8) | 98.6 (1.9) | 0.521 |
| ETCO2 (mmHg) | 35.4 (4.6) | 34.7 (4.2) | 0.349 |
| Respiratory rate (breath per minute) | 16.1 (3.6) | 16.7 (3.9) | 0.197 |
| 10 min during procedure | |||
| SpO2 (%) | 98.6 (1.9) | 98.3 (2.1) | 0.636 |
| ETCO2 (mmHg) | 35.8 (4.6) | 34.8 (4.6) | 0.285 |
| Respiratory rate (breath per minute) | 16.1 (3.5) | 16.8 (3.5) | 0.757 |
| 15 min during procedure | |||
| SpO2 (%) | 98.6 (1.9) | 98.3 (2.0) | 0.355 |
| ETCO2 (mmHg) | 35.6 (4.9) | 34.9 (4.6) | 0.585 |
| Respiratory rate (breath per minute) | 16.1 (3.4) | 17.3 (3.8) | 0.288 |
| 20 min during procedure | |||
| SpO2 (%) | 98.6 (1.9) | 98.4 (1.9) | 0.485 |
| ETCO2 (mmHg) | 35.5 (4.6) | 35.1 (4.6) | 0.217 |
| Respiratory rate (breath per minute) | 16.2 (3.4) | 17.3 (3.5) | 0.059 |
| 25 min during procedure | |||
| SpO2 (%) | 98.6 (1.8) | 98.4 (1.7) | 0.116 |
| ETCO2 (mmHg) | 35.1 (4.6) | 34.7 (4.6) | 0.755 |
| Respiratory rate (breath per minute) | 16.4 (3.5) | 17.5 (3.6) | 0.136 |
| 30 min during procedure | |||
| SpO2 (%) | 98.4 (2.0) | 98.4 (1.7) | 0.535 |
| ETCO2 (mmHg) | 34.8 (4.6) | 34.7 (4.9) | 0.327 |
| Respiratory rate (breath per minute) | 16.9 (3.5) | 17.5 (3.5) | 0.375 |
| 35 min during procedure | |||
| SpO2 (%) | 98.5 (1.8) | 98.3 (1.8) | 0.394 |
| ETCO2 (mmHg) | 34.5 (4.3) | 34.7 (4.7) | 0.236 |
| Respiratory rate (breath per minute) | 16.9 (3.4) | 17.7 (3.6) | 0.318 |
| 40 min during procedure | |||
| SpO2 (%) | 98.5 (1.7) | 98.4 (1.7) | 0.475 |
| ETCO2 (mmHg) | 34.3 (4.0) | 34.3 (4.3) | 0.304 |
| Respiratory rate (breath per minute) | 17.0 (3.8) | 17.9 (3.6) | 0.422 |
| 45 min during procedure | |||
| SpO2 (%) | 98.5 (1.8) | 98.5 (1.6) | 0.646 |
| ETCO2 (mmHg) | 34.3 (3.8) | 34.2 (4.4) | 0.691 |
| Respiratory rate (breath per minute) | 16.9 (3.2) | 18.0 (3.6) | 0.105 |
| 50 min during procedure | |||
| SpO2 (%) | 98.4 (1.8) | 98.3 (1.9) | 0.625 |
| ETCO2 (mmHg) | 34.1 (4.1) | 34.2 (5.0) | 0.238 |
| Respiratory rate (breath per minute) | 16.9 (3.7) | 18.5 (3.5) | 0.335 |
| 55 min during procedure | |||
| SpO2 (%) | 98.4 (1.8) | 98.4 (1.9) | 0.67 |
| ETCO2 (mmHg) | 33.5 (4.1) | 34.4 (4.6) | 0.451 |
| Respiratory rate (breath per minute) | 17.0 (3.3) | 18.2 (3.1) | 0.691 |
| 60 min during procedure | |||
| SpO2 (%) | 98.5 (1.7) | 98.4 (2.0) | 0.265 |
| ETCO2 (mmHg) | 33.4 (5.0) | 34.7 (4.0) | 0.654 |
| Respiratory rate (breath per minute) | 17.3 (3.3) | 18.2 (3.5) | 0.709 |
| Group A | Group B | p-Value | |
|---|---|---|---|
| (n = 187) | (n = 187) | ||
| 5 min after procedure | |||
| SpO2 (%) | 98.7 (1.7) | 98.2 (2.0) | 0.234 |
| ETCO2 (mmHg) | 32.4 (4.3) | 32.0 (4.3) | 0.079 |
| Respiratory rate (breath per minute) | 16.5 (3.3) | 17.2 (3.5) | 0.636 |
| Sedation score (0–3) | 0.8 (0.5) | 0.6 (0.6) | 0.026 * |
| 10 min after procedure | |||
| SpO2 (%) | 98.9 (1.4) | 98.5 (1.8) | 0.058 |
| ETCO2 (mmHg) | 33.0 (4.3) | 32.5 (4.4) | 0.79 |
| Respiratory rate (breath per minute) | 16.4 (3.3) | 17.1 (3.6) | 0.328 |
| Sedation score (0–3) | 1.2 (0.6) | 0.9 (0.6) | <0.001 * |
| 15 min after procedure | |||
| SpO2 (%) | 98.5 (1.7) | 98.6 (1.8) | 0.321 |
| ETCO2 (mmHg) | 33.4 (5.0) | 32.9 (4.0) | 0.685 |
| Respiratory rate (breath per minute) | 17.3 (3.3) | 17.0 (3.4) | 0.134 |
| Sedation score (0–3) | 1.5 (0.6) | 1.2 (0.6) | <0.001 * |
| 20 min after procedure | |||
| SpO2 (%) | 99.2 (1.3) | 98.9 (1.6) | 0.519 |
| ETCO2 (mmHg) | 33.7 (3.8) | 32.6 (4.1) | 0.625 |
| Respiratory rate (breath per minute) | 16.3 (3.6) | 17.1 (3.2) | 0.069 |
| Sedation score (0–3) | 1.6 (0.5) | 1.5 (0.5) | 0.203 |
| 25 min after procedure | |||
| SpO2 (%) | 99.4 (1.1) | 99.0 (1.4) | 0.186 |
| ETCO2 (mmHg) | 33.9 (3.4) | 32.0 (4.1) | 0.51 |
| Respiratory rate (breath per minute) | 16.2 (3.7) | 16.6 (3.0) | 0.075 |
| Sedation score (0–3) | 1.5 (0.6) | 1.5 (0.6) | 0.658 |
| 30 min after procedure | |||
| SpO2 (%) | 99.6 (0.8) | 99.2 (1.2) | 0.397 |
| ETCO2 (mmHg) | 33.2 (3.1) | 31.8 (3.8) | 0.658 |
| Respiratory rate (breath per minute) | 15.9 (3.0) | 16.6 (3.0) | 0.821 |
| Sedation score (0–3) | 1.6 (0.6) | 1.7 (0.5) | 0.454 |
| 35 min after procedure | |||
| SpO2 (%) | 99.6 (0.8) | 98.9 (1.5) | 0.369 |
| ETCO2 (mmHg) | 34.2 (2.5) | 32.6 (4.2) | 0.822 |
| Respiratory rate (breath per minute) | 16.8 (2.8) | 16.9 (3.0) | 0.449 |
| Sedation score (0-3) | 1.6 (0.5) | 1.7 (0.5) | 0.724 |
| 40 min after procedure | |||
| SpO2 (%) | 100.0 (0.0) | 99.3 (1.0) | 0.399 |
| ETCO2 (mmHg) | 34.0 (1.7) | 34.3 (3.3) | 0.125 |
| Respiratory rate (breath per minute) | 18.7 (1.2) | 16.6 (3.7) | 0.179 |
| Sedation score (0-3) | 2.0 (0.0) | 1.9 (0.4) | 0.49 |
| Duration of sleep after procedure (min) | 18.9 (7.4) | 22.4 (7.7) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sripunjan, K.; Sombood, P.; Vichitvejpaisal, P.; Amornyotin, S. Ventilatory Effect of Midazolam in Propofol Deep Sedation for Hepatic Tumor Patients Undergoing Percutaneous Radiofrequency Ablation Procedure. Gastroenterol. Insights 2021, 12, 89-99. https://doi.org/10.3390/gastroent12010009
Sripunjan K, Sombood P, Vichitvejpaisal P, Amornyotin S. Ventilatory Effect of Midazolam in Propofol Deep Sedation for Hepatic Tumor Patients Undergoing Percutaneous Radiofrequency Ablation Procedure. Gastroenterology Insights. 2021; 12(1):89-99. https://doi.org/10.3390/gastroent12010009
Chicago/Turabian StyleSripunjan, Krongthip, Pattharaporn Sombood, Phongtara Vichitvejpaisal, and Somchai Amornyotin. 2021. "Ventilatory Effect of Midazolam in Propofol Deep Sedation for Hepatic Tumor Patients Undergoing Percutaneous Radiofrequency Ablation Procedure" Gastroenterology Insights 12, no. 1: 89-99. https://doi.org/10.3390/gastroent12010009
APA StyleSripunjan, K., Sombood, P., Vichitvejpaisal, P., & Amornyotin, S. (2021). Ventilatory Effect of Midazolam in Propofol Deep Sedation for Hepatic Tumor Patients Undergoing Percutaneous Radiofrequency Ablation Procedure. Gastroenterology Insights, 12(1), 89-99. https://doi.org/10.3390/gastroent12010009








