Imaging in the Assessment of Musculoskeletal Manifestations Associated with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Imaging in the Assessment of Inflammatory Musculoskeletal Manifestations
2.1. Conventional Radiography
2.1.1. Sacroiliac Joints
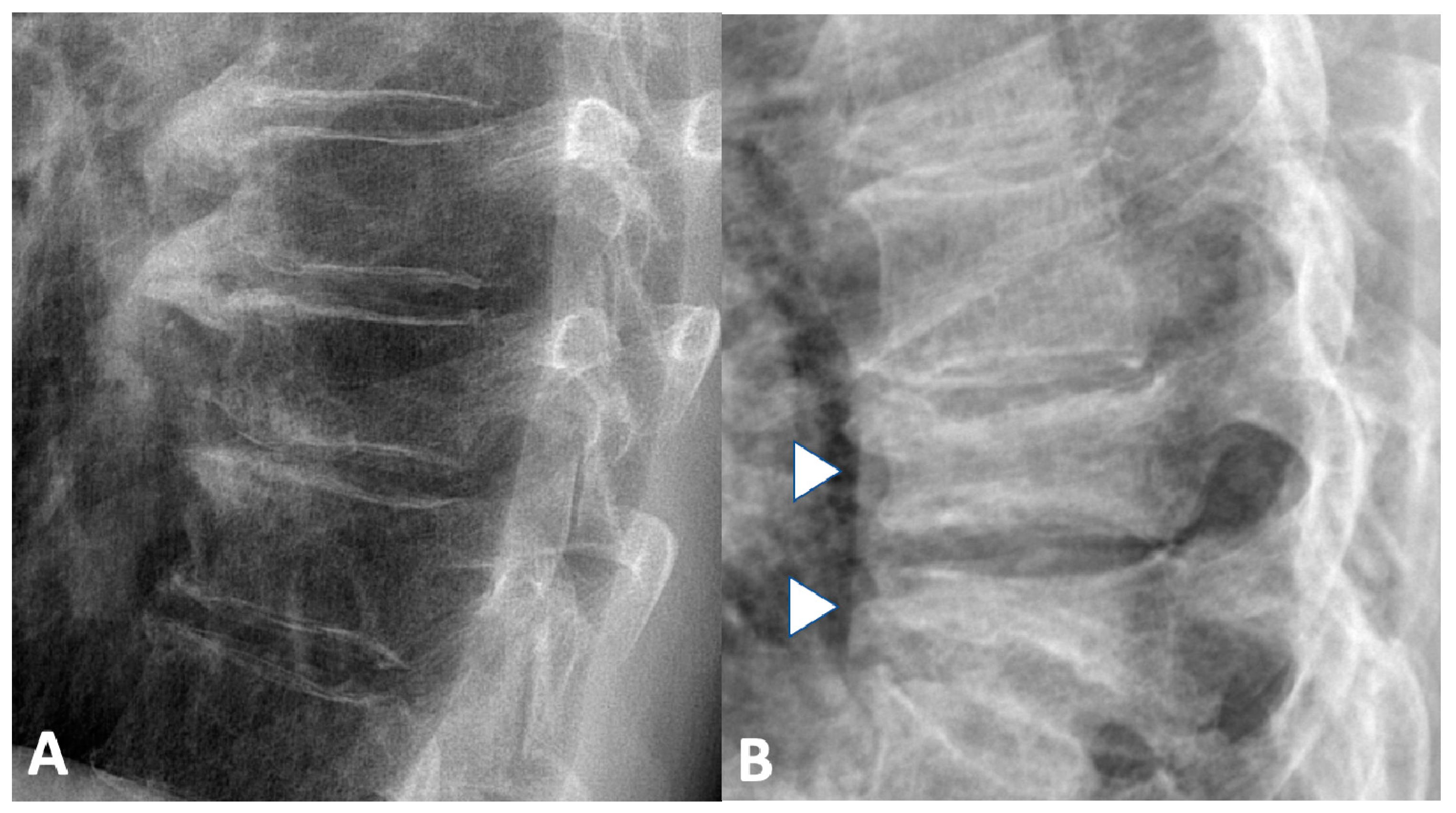
2.1.2. Spine
2.1.3. Peripheral Joints
2.2. Magnetic Resonance Imaging
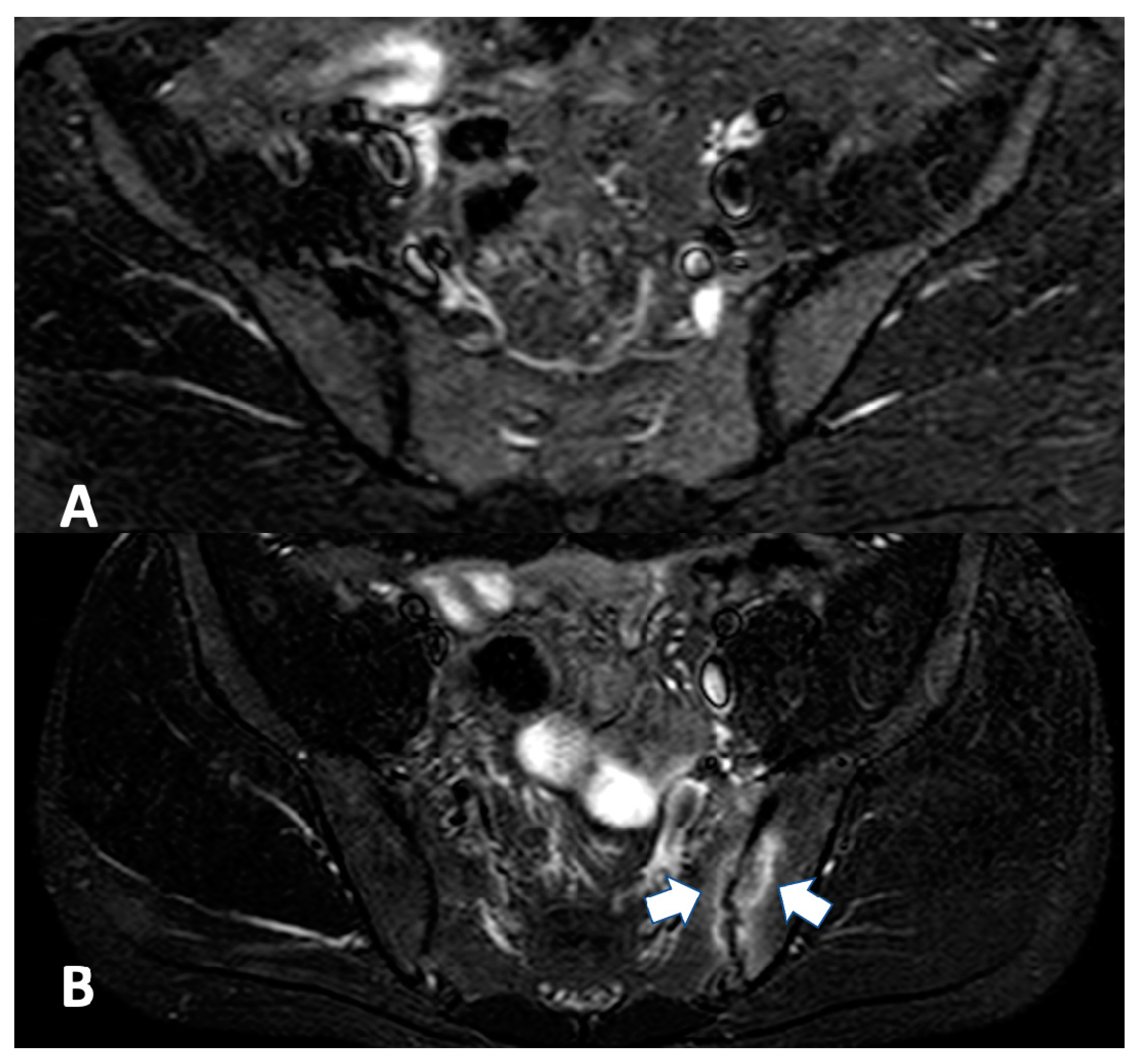
2.2.1. Sacroiliac Joint
2.2.2. Spine
2.2.3. Entheses and Tendons
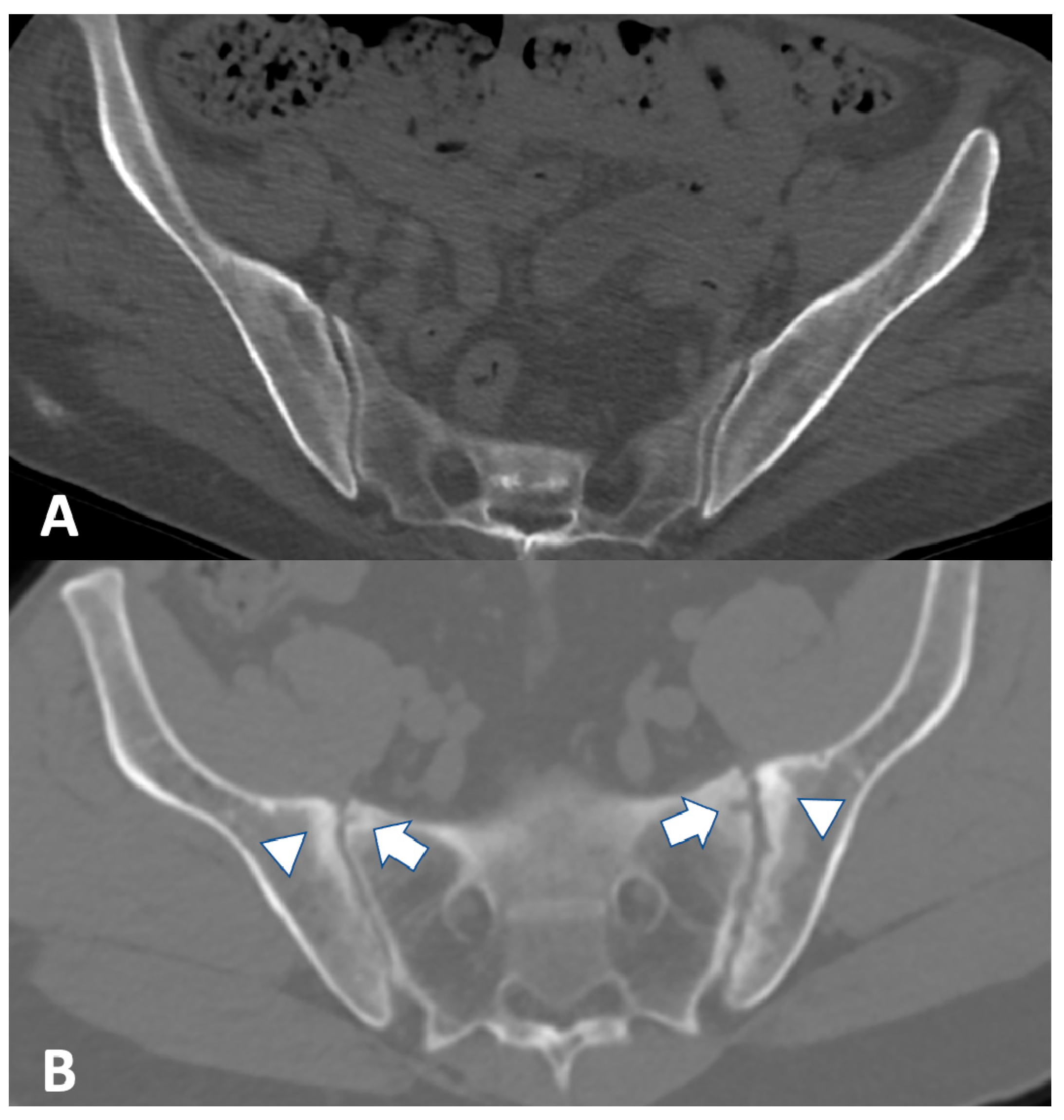
2.3. Computed Tomography
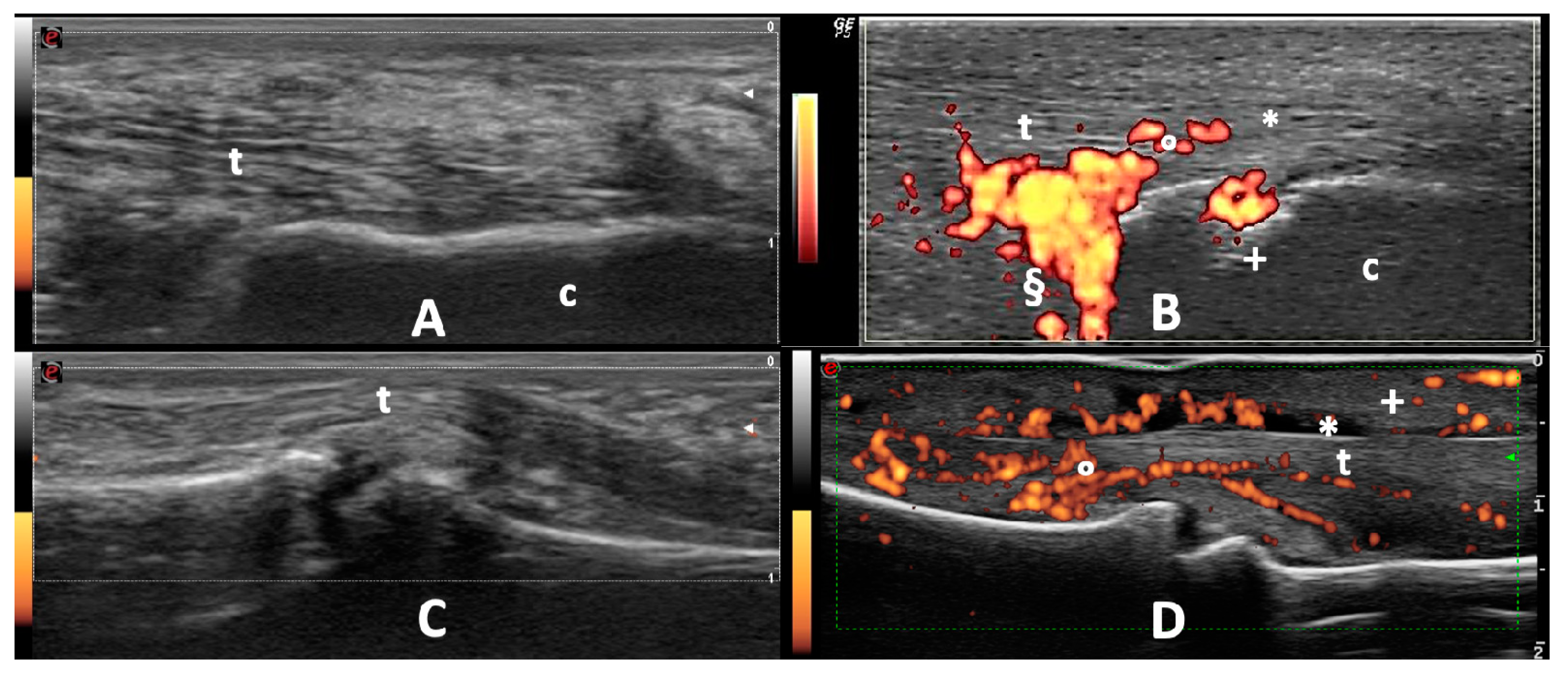
2.4. Ultrasound
2.4.1. Peripheral Joints
2.4.2. Entheses and Tendons
3. Imaging in the Assessment of Non-Inflammatory Musculoskeletal Manifestation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Pellicano, R.; Actis, G.C. The gut and the inflammatory bowel diseases inside-out: Extra-intestinal manifestations. Minerva Gastroenterol Dietol. 2019, 65, 309–318. [Google Scholar] [CrossRef]
- Marinelli, C.; Savarino, E.; Inferrera, M.; Lorenzon, G.; Rigo, A.; Ghisa, M.; Facchin, S.; D’Incà, R.; Zingone, F. Factors Influencing Disability and Quality of Life during Treatment: A Cross-Sectional Study on IBD Patients. Gastroenterol. Res. Pract. 2019, 2019, 5354320. [Google Scholar] [CrossRef] [PubMed]
- ACHESON, E.D. An Association between Ulcerative Colitis, Regional Enteritis, and Ankylosing Spondylitis. Q. J. Med. 1960, 29, 489–499. [Google Scholar] [PubMed]
- Van Tubergen, A.; Weber, U. Diagnosis and Classification in Spondyloarthritis: Identifying a Chameleon. Nat. Rev. Rheumatol. 2012, 8, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Defendenti, C.; Ditto, M.C.; Batticciotto, A.; Ventura, D.; Antivalle, M.; Ardizzone, S.; Sarzi-Puttini, P. Rheumatic Manifestations in Inflammatory Bowel Disease. Autoimmun. Rev. 2014, 13, 20–23. [Google Scholar] [CrossRef]
- Bourikas, L.A.; Papadakis, K.A. Musculoskeletal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2009, 15, 1915–1924. [Google Scholar] [CrossRef]
- Van Schaik, F.D.M.; Verhagen, M.A.M.T.; Siersema, P.D.; Oldenburg, B. High Prevalence of Low Bone Mineral Density in Patients with Inflammatory Bowel Disease in the Setting of a Peripheral Dutch Hospital. J. Crohns Colitis 2008, 2, 208–213. [Google Scholar] [CrossRef]
- Mandl, P.; Navarro-Compán, V.; Terslev, L.; Aegerter, P.; van der Heijde, D.; D’Agostino, M.A.; Baraliakos, X.; Pedersen, S.J.; Jurik, A.G.; Naredo, E.; et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 2015, 74, 1327–1339. [Google Scholar] [CrossRef]
- Bennett, P.H.; Wood, P.H.N. Population Studies of the Rheumatic Diseases. Population Studies of the Rheumatic Diseases; Excerpta Medica Foundation: Amsterdam, The Netherlands, 1968; pp. 456–457. [Google Scholar]
- Lanna, C.C.D.; de Ferrari, M.L.A.; Rocha, S.L.; Nascimento, E.; de Carvalho, M.A.P.; da Cunha, A.S. A Cross-Sectional Study of 130 Brazilian Patients with Crohn’s Disease and Ulcerative Colitis: Analysis of Articular and Ophthalmologic Manifestations. Clin. Rheumatol. 2008, 27, 503–509. [Google Scholar] [CrossRef]
- Queiro, R.; Maiz, O.; Intxausti, J.; de Dios, J.R.; Belzunegui, J.; González, C.; Figueroa, M. Subclinical Sacroiliitis in Inflammatory Bowel Disease: A Clinical and Follow-up Study. Clin. Rheumatol. 2000, 19, 445–449. [Google Scholar] [CrossRef]
- de Vlam, K.; Mielants, H.; Cuvelier, C.; De Keyser, F.; Veys, E.M.; De Vos, M. Spondyloarthropathy Is Underestimated in Inflammatory Bowel Disease: Prevalence and HLA Association. J. Rheumatol. 2000, 27, 2860–2865. [Google Scholar] [PubMed]
- Peeters, H.; Vander Cruyssen, B.; Mielants, H.; de Vlam, K.; Vermeire, S.; Louis, E.; Rutgeerts, P.; Belaiche, J.; De Vos, M. Clinical and Genetic Factors Associated with Sacroiliitis in Crohn’s Disease. J. Gastroenterol. Hepatol. 2007, 23, 132–137. [Google Scholar] [CrossRef]
- Bandinelli, F.; Terenzi, R.; Giovannini, L.; Milla, M.; Genise, S.; Bagnoli, S.; Biagini, S.; Annese, V.; Matucci-Cerinic, M. Occult Radiological Sacroiliac Abnormalities in Patients with Inflammatory Bowel Disease Who Do Not Present Signs or Symptoms of Axial Spondylitis. Clin. Exp. Rheumatol. 2014, 32, 949–952. [Google Scholar] [PubMed]
- Creemers, M.C.W. Assessment of Outcome in Ankylosing Spondylitis: An Extended Radiographic Scoring System. Ann. Rheum. Dis. 2005, 64, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Reyna, T.S.; Martínez-Reyes, C.; Yamamoto-Furusho, J.K. Rheumatic Manifestations of Inflammatory Bowel Disease. World J. Gastroenterol. 2009, 15, 5517. [Google Scholar] [CrossRef]
- Salvarani, C.; Fries, W. Clinical Features and Epidemiology of Spondyloarthritides Associated with Inflammatory Bowel Disease. World J. Gastroenterol. 2009, 15, 2449. [Google Scholar] [CrossRef]
- Fornaciari, G.; Salvarani, C.; Beltrami, M.; Macchioni, P.; Stockbrügger, R.W.; Russel, M.G. Musculoskeletal Manifestations in Inflammatory Bowel Disease. Can. J. Gastroenterol. 2001, 15, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Sudoł-Szopińska, I.; Jurik, A.; Eshed, I.; Lennart, J.; Grainger, A.; Østergaard, M.; Klauser, A.; Cotten, A.; Wick, M.; Maas, M.; et al. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin. Musculoskelet. Radiol. 2015, 19, 396–411. [Google Scholar] [CrossRef]
- Lambert, R.G.W.; Bakker, P.A.C.; van der Heijde, D.; Weber, U.; Rudwaleit, M.; Hermann, K.-G.A.; Sieper, J.; Baraliakos, X.; Bennett, A.; Braun, J.; et al. Defining Active Sacroiliitis on MRI for Classification of Axial Spondyloarthritis: Update by the ASAS MRI Working Group. Ann. Rheum. Dis. 2016, 75, 1958–1963. [Google Scholar] [CrossRef]
- Sung, S.; Kim, H.S.; Kwon, J.W. MRI Assessment of Sacroiliitis for the Diagnosis of Axial Spondyloarthropathy: Comparison of Fat-Saturated T2, STIR and Contrast-Enhanced Sequences. Br. J. Radiol. 2017, 90, 20170090. [Google Scholar] [CrossRef]
- Leclerc-Jacob, S.; Lux, G.; Rat, A.C.; Laurent, V.; Blum, A.; Chary-Valckenaere, I.; Peyrin-Biroulet, L.; Loeuille, D. The Prevalence of Inflammatory Sacroiliitis Assessed on Magnetic Resonance Imaging of Inflammatory Bowel Disease: A Retrospective Study Performed on 186 Patients. Aliment. Pharmacol. Ther. 2014, 39, 957–962. [Google Scholar] [CrossRef]
- Gotler, J.; Amitai, M.M.; Lidar, M.; Aharoni, D.; Flusser, G.; Eshed, I. Utilizing MR Enterography for Detection of Sacroiliitis in Patients with Inflammatory Bowel Disease: Sacroiliitis on MRE of IBD Patients. J. Magn. Reson. Imaging 2015, 42, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Bandyopadhyay, S.; Ghosh, P.; De, A.; Bhattacharya, A.; Dhali, G.K.; Das, K. Extraintestinal Manifestations in Inflammatory Bowel Disease: Prevalence and Predictors in Indian Patients. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2015, 34, 387–394. [Google Scholar] [CrossRef]
- Orchard, T.R.; Holt, H.; Bradbury, L.; Hammersma, J.; Mcnally, E.; Jewell, D.P.; Wordsworth, B.P. The Prevalence, Clinical Features and Association of HLA-B27 in Sacroiliitis Associated with Established Crohn’s Disease. Aliment. Pharmacol. Ther. 2009, 29, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Gentili, F.; Guerrini, S.; Di Meglio, N.; Lo Re, G.; Carotti, M.; Interlicchia, F.; Reginelli, A.; Barile, A.; Sadotti, G.; et al. Inflammatory Bowel Diseases and Coexisting Spondyloarthritis: A Neglected and Too Often Under-Reported Association by Radiologists. A Multicenter Study by Italian Research Group of Imaging in Rheumatology. Gastroenterol. Insights 2020, 11, 47–57. [Google Scholar] [CrossRef]
- Brakenhoff, L.; Stomp, W.; van Gaalen, F.; Hommes, D.; Bloem, J.; van der Heijde, D.; Fidder, H.; Reijnierse, M. Magnetic Resonance Imaging of the Hand Joints in Patients with Inflammatory Bowel Disease and Arthralgia: A Pilot Study. Scand. J. Rheumatol. 2014, 43, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Eshed, I.; Bollow, M.; McGonagle, D.G.; Tan, A.L.; Althoff, C.E.; Asbach, P.; Hermann, K.-G.A. MRI of enthesitis of the appendicular skeleton in spondyloarthritis. Ann. Rheum. Dis. 2007, 66, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Geijer, M.; Sihlbom, H.; Göthlin, J.H.; Nordborg, E. The role of CT in the diagnosis of sacro-iliitis. Acta Radiol. 1998, 39, 265–268. [Google Scholar] [CrossRef]
- McEniff, N.; Eustace, S.; McCarthy, C.; O’Malley, M.; O’Morain, C.A.; Hamilton, S. Asymptomatic Sacroiliitis in Inflammatory Bowel Disease Assessment by Computed Tomography. Clin. Imaging 1995, 19, 258–262. [Google Scholar] [CrossRef]
- Scott, W.W.; Fishman, E.K.; Kuhlman, J.E.; Caskey, C.I.; O’Brien, J.J.; Walia, G.S.; Bayless, T.M. Computed Tomography Evaluation of the Sacroiliac Joints in Crohn Disease: Radiologic/Clinical Correlation. Skelet. Radiol. 1990, 19, 207–210. [Google Scholar] [CrossRef]
- Hwangbo, Y.; Kim, H.J.; Park, J.S.; Ryu, K.N.; Kim, N.H.; Shim, J.; Jang, J.Y.; Dong, S.H.; Kim, B.H.; Chang, Y.W.; et al. Sacroiliitis Is Common in Crohn’s Disease Patients with Perianal or Upper Gastrointestinal Involvement. Gut Liver 2010, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Fauny, M.; Cohen, N.; Morizot, C.; Leclerc-Jacob, S.; Wendling, D.; Lux, G.; Laurent, V.; Blum, A.; Netter, P.; Baumann, C.; et al. Low Back Pain and Sacroiliitis on Cross-Sectional Abdominal Imaging for Axial Spondyloarthritis Diagnosis in Inflammatory Bowel Diseases. Inflamm. Intest. Dis. 2020, 5, 124–131. [Google Scholar] [CrossRef]
- Paparo, F.; Bacigalupo, L.; Garello, I.; Biscaldi, E.; Cimmino, M.A.; Marinaro, E.; Rollandi, G.A. Crohn’s Disease: Prevalence of Intestinal and Extraintestinal Manifestations Detected by Computed Tomography Enterography with Water Enema. Abdom. Imaging 2012, 37, 326–337. [Google Scholar] [CrossRef] [PubMed]
- De Kock, I.; Hindryckx, P.; De Vos, M.; Delrue, L.; Verstraete, K.; Jans, L. Prevalence of CT Features of Axial Spondyloarthritis in Patients with Crohn’s Disease. Acta Radiol. 2017, 58, 593–599. [Google Scholar] [CrossRef]
- Kelly, O.B.; Li, N.; Smith, M.; Chan, J.; Inman, R.D.; Silverberg, M.S. The Prevalence and Clinical Associations of Subclinical Sacroiliitis in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1066–1071. [Google Scholar] [CrossRef]
- Burge, A.J.; Nwawka, O.K.; Berkowitz, J.L.; Potter, H.G. Imaging of Inflammatory Arthritis in Adults. Rheum. Dis. Clin. N. Am. 2016, 42, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Kaeley, G.S.; Bakewell, C.; Deodhar, A. The Importance of Ultrasound in Identifying and Differentiating Patients with Early Inflammatory Arthritis: A Narrative Review. Arthritis Res. Ther. 2020, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Jeka, S.; Zuchowski, P.; Dura, M.; Zwierko, B.; Waszczak-Jeka, M. The Role of Ultrasonography in the Diagnostic Criteriafor Rheumatoid Arthritis and Monitoring Its Therapeutic Efficacy. Adv. Clin. Exp. Med. 2018, 27, 1303–1307. [Google Scholar] [CrossRef]
- Balint, P.V. Ultrasonography of Entheseal Insertions in the Lower Limb in Spondyloarthropathy. Ann. Rheum. Dis. 2002, 61, 905–910. [Google Scholar] [CrossRef]
- de Miguel, E.; Cobo, T.; Muñoz-Fernández, S.; Naredo, E.; Usón, J.; Acebes, J.C.; Andréu, J.L.; Martín-Mola, E. Validity of Enthesis Ultrasound Assessment in Spondyloarthropathy. Ann. Rheum. Dis. 2009, 68, 169–174. [Google Scholar] [CrossRef]
- Bandinelli, F.; Milla, M.; Genise, S.; Giovannini, L.; Bagnoli, S.; Candelieri, A.; Collaku, L.; Biagini, S.; Cerinic, M.M. Ultrasound Discloses Entheseal Involvement in Inactive and Low Active Inflammatory Bowel Disease without Clinical Signs and Symptoms of Spondyloarthropathy. Rheumatol. Oxf. Engl. 2011, 50, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Rovisco, J.; Duarte, C.; Batticcioto, A.; Sarzi-Puttini, P.; Dragresshi, A.; Portela, F.; Gutierrez, M. Hidden Musculoskeletal Involvement in Inflammatory Bowel Disease: A Multicenter Ultrasound Study. BMC Musculoskelet. Disord. 2016, 17, 84. [Google Scholar] [CrossRef]
- Bakirci Ureyen, S.; Karacaer, C.; Toka, B.; Erturk, Z.; Eminler, A.T.; Kaya, M.; Tascilar, K.; Tamer, A.; Uslan, I.; Kurum, E.; et al. Similar Subclinical Enthesitis in Celiac and Inflammatory Bowel Diseases by Ultrasound Suggests a Gut Enthesis Axis Independent of Spondyloarthropathy Spectrum. Rheumatol. Oxf. Engl. 2018, 57, 1417–1422. [Google Scholar] [CrossRef]
- Martinis, F.; Tinazzi, I.; Bertolini, E.; Citriniti, G.; Variola, A.; Geccherle, A.; Marchetta, A.; McGonagle, D.; Macchioni, P. Clinical and Sonographic Discrimination between Fibromyalgia and Spondyloarthopathy in Inflammatory Bowel Disease with Musculoskeletal Pain. Rheumatol. Oxf. Engl. 2020, 59, 2857–2863. [Google Scholar] [CrossRef]
- Bertolini, E.; Macchioni, P.; Rizzello, F.; Salice, M.; Vukatana, G.; Sandri, G.; Bertani, A.; Ciancio, G.; Govoni, M.; Zelante, A.; et al. Ultrasonographic and Clinical Assessment of Peripheral Enthesitis and Arthritis in an Italian Cohort of Inflammatory Bowel Disease Patients. Semin. Arthritis Rheum. 2020, 50, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P. Bone Loss Associated with Gastrointestinal Disease: Prevalence and Pathogenesis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 851–856. [Google Scholar] [CrossRef]
- Cooper, C.; Atkinson, E.J.; O’Fallon, W.M.; Melton, J.L. Incidence of Clinically Diagnosed Vertebral Fractures: A Population-Based Study in Rochester, Minnesota, 1985–1989. J. Bone Miner. Res. 2009, 7, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lisk, R.; Yeong, K. Reducing Mortality from Hip Fractures: A Systematic Quality Improvement Programme. BMJ Qual. Improv. Rep. 2014, 3, u205006.w2103. [Google Scholar] [CrossRef] [PubMed]
- Bartram, S.A. Mutifactorial Analysis of Risk Factors for Reduced Bone Mineral Density in Patients with Crohn’s Disease. World J. Gastroenterol. 2006, 12, 5680. [Google Scholar] [CrossRef]
- Frei, P.; Fried, M.; Hungerbühler, V.; Rammert, C.; Rousson, V.; Kullak-Ublick, G.A. Analysis of Risk Factors for Low Bone Mineral Density in Inflammatory Bowel Disease. Digestion 2006, 73, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, Y.; Hamdy, K. The Frequency of Low Bone Mineral Density and Its Associated Risk Factors in Patients with Inflammatory Bowel Diseases. Int. J. Rheum. Dis. 2010, 13, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Bundela, R.P.S.; Ashdhir, P.; Narayan, K.S.; Jain, M.; Pokharna, R.K.; Nijhawan, S. Prevalence and Risk Factors for Low Bone Mineral Density in Ulcerative Colitis. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2017, 36, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Reffitt, D.M.; Meenan, J.; Sanderson, J.D.; Jugdaohsingh, R.; Powell, J.J.; Thompson, R.P. Bone Density Improves with Disease Remission in Patients with Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2003, 15, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Stockbrügger, R.W.; Schoon, E.J.; Bollani, S.; Mills, P.R.; Israeli, E.; Landgraf, L.; Felsenberg, D.; Ljunghall, S.; Nygard, G.; Persson, T.; et al. Discordance between the Degree of Osteopenia and the Prevalence of Spontaneous Vertebral Fractures in Crohn’s Disease: OSTEOPENIA AND VERTEBRAL FRACTURES IN CROHN’S DISEASE. Aliment. Pharmacol. Ther. 2002, 16, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Szafors, P.; Che, H.; Barnetche, T.; Morel, J.; Gaujoux-Viala, C.; Combe, B.; Lukas, C. Risk of Fracture and Low Bone Mineral Density in Adults with Inflammatory Bowel Diseases. A Systematic Literature Review with Meta-Analysis. Osteoporos. Int. 2018, 29, 2389–2397. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic Screening for Osteoporosis Using Abdominal Computed Tomography Scans Obtained for Other Indications. Ann. Intern. Med. 2013, 158, 588. [Google Scholar] [CrossRef]
| Clinical Features | Affected Sites | |
|---|---|---|
| Peripheral arthritis | ||
| Type 1 |
|
|
| Type 2 |
|
|
| Enthesitis |
|
|
| Dactylitis |
|
|
| Axial involvement |
|
|
| Osteoporotic fracture |
|
|
| Imaging Techniques | Structures Assessed | Pathological Findings | Advantages | Disadvantages |
|---|---|---|---|---|
| Conventional radiography | Peripheral joints Axial joints Vertebral bodies Entheses | Erosion, vertebral syndesmophyte, sclerosis, ankylosis, new bone formation | Low cost, reference technique, short testing time, wide availability, baseline assessment | Ionizing radiation, limited in detection of inflammatory lesions, low sensitivity |
| Magnetic resonance imaging | Peripheral joints Axial joints Vertebral bodies Tendon Entheses | Bone marrow edema, synovitis, tenosynovitis, enthesitis, bursitis, erosion | Sensitive, no radiation burden, high spatial resolution, excellent soft tissue visualization | High cost, limited availability, long scanning time, not dynamic, contraindicated in patients with metallic implants |
| Computed tomography | Peripheral joints Axial joints Vertebral bodies Tendon | Erosion, vertebral syndesmophyte, sclerosis, ankylosis, new bone formation | Sensitive, high spatial resolution | High cost, limited availability, ionizing radiation, no detection of inflammatory lesions |
| Ultrasound | Peripheral joints Tendon Entheses | Synovitis, tenosynovitis, enthesitis, erosion, new bone formation | Sensitive, no radiation burden, high spatial resolution, excellent soft tissue visualization, short testing time, bedside procedure | Limited acoustic windows, operator dependency, long learning curve |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becciolini, A.; Di Donato, E.; Lucchini, G.; Santilli, D.; Mozzani, F.; Riva, M.; Ariani, A. Imaging in the Assessment of Musculoskeletal Manifestations Associated with Inflammatory Bowel Disease. Gastroenterol. Insights 2021, 12, 100-110. https://doi.org/10.3390/gastroent12010010
Becciolini A, Di Donato E, Lucchini G, Santilli D, Mozzani F, Riva M, Ariani A. Imaging in the Assessment of Musculoskeletal Manifestations Associated with Inflammatory Bowel Disease. Gastroenterology Insights. 2021; 12(1):100-110. https://doi.org/10.3390/gastroent12010010
Chicago/Turabian StyleBecciolini, Andrea, Eleonora Di Donato, Gianluca Lucchini, Daniele Santilli, Flavio Mozzani, Michele Riva, and Alarico Ariani. 2021. "Imaging in the Assessment of Musculoskeletal Manifestations Associated with Inflammatory Bowel Disease" Gastroenterology Insights 12, no. 1: 100-110. https://doi.org/10.3390/gastroent12010010
APA StyleBecciolini, A., Di Donato, E., Lucchini, G., Santilli, D., Mozzani, F., Riva, M., & Ariani, A. (2021). Imaging in the Assessment of Musculoskeletal Manifestations Associated with Inflammatory Bowel Disease. Gastroenterology Insights, 12(1), 100-110. https://doi.org/10.3390/gastroent12010010






