Neurovascular Pathophysiology and Emerging Biomarkers in Cerebral Malaria: An Integrative Perspective
Abstract
1. Introduction
2. Neurovascular Pathophysiology
2.1. Parasite and Host Immune Interactions in Cerebral Malaria
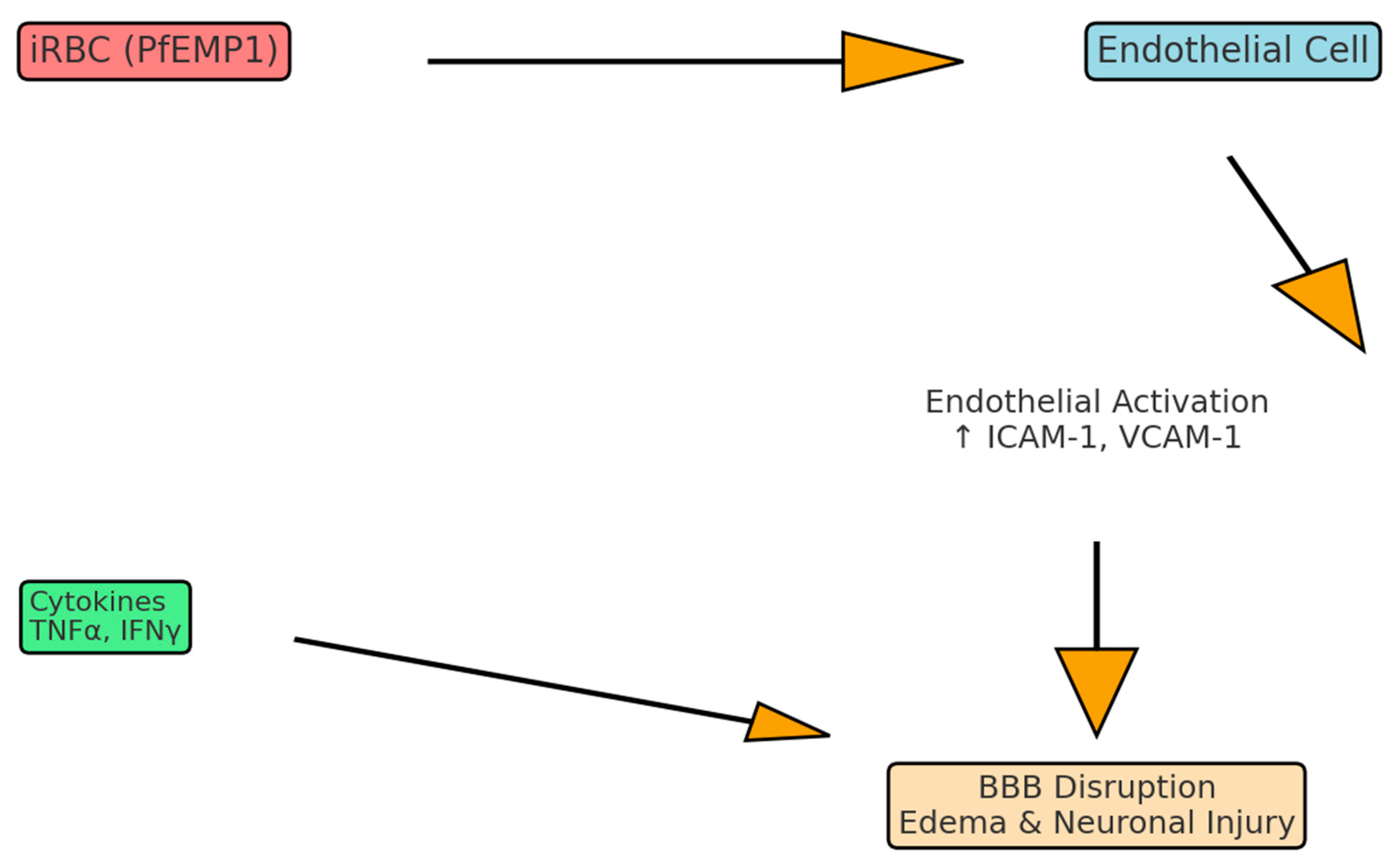
2.2. Endothelial Dysfunction and Blood–Brain Barrier Disruption
2.3. Parasite Adhesion and Microvascular Sequestration
2.4. Cytokine Milieu, Chemokines, and Immune Dysregulation
2.5. Translational Biomarkers and Adjunctive Therapy Development
3. Biomarkers
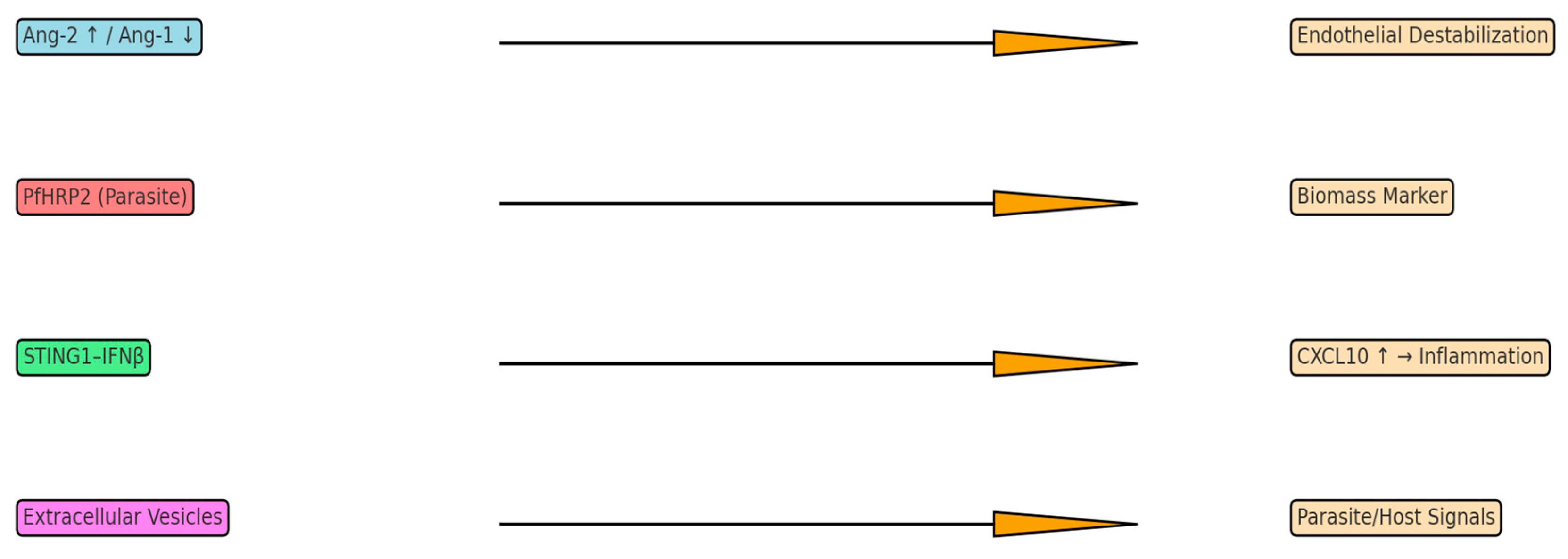
3.1. The Angiopoietin-Tie2 Regulatory System
3.2. Cell-Adhesion Molecules and Von Willebrand Factor
3.3. Chemokine Profiles and Immune Activation
3.4. STING1-IFNβ-CXCL10 Axis and Innate Immune Sensing
3.5. Parasite-Derived Biomass Indicators
3.6. Retinopathy Associated Patterns and Diagnostic Specificity
4. Integrative Perspective on Pathophysiology and Biomarkers
4.1. Conceptualizing Systematic Approaches to Cerebral Malaria
4.2. Extracellular Vesicles as a Window into Brain Pathophysiology
4.3. Revolution in Molecular Diagnostics
4.4. Novel Pathophysiological Mechanisms
4.5. Clinical Translation Challenges
4.6. Multi-Omic Data Integration
5. Clinical Implications and Future Research Directions
5.1. Preventive Strategies and Current Vaccines
5.2. A New Era of Precision Medicine in Cerebral Malaria
5.3. Diagnostic Revolution and Point-of-Care Applications
5.4. Therapeutic Target Identification and Drug Development
5.5. Immune Modulation and Neuroprotective Strategies
5.6. Long-Term Outcomes and Rehabilitation Strategies
5.7. Future Research Priorities and Technological Innovations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Idro, R.; Marsh, K.; John, C.C.; Newton, C.R.J.C. Cerebral malaria: Mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr. Res. 2010, 68, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Storm, J.; Craig, A.G. Pathogenesis of cerebral malaria—Inflammation and cytoadherence. Front. Cell. Infect. Microbiol. 2014, 4, 100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.S.; Sealy, B.C.; Combes, V. Extracellular vesicles, from pathogenesis to biomarkers: The case for cerebral malaria. Vessel. Plus 2020, 4, 17. [Google Scholar] [CrossRef]
- Darling, T.K.; Mimche, P.N.; Bray, C.; Umaru, B.; Brady, L.M.; Stone, C.; Moukoko, C.E.E.; Lane, T.E.; Ayong, L.S.; Lamb, T.J.; et al. EphA2 contributes to disruption of the blood-brain barrier in cerebral malaria. PLoS Pathog. 2020, 16, e1008261. [Google Scholar] [CrossRef]
- Seydel, K.B.; Kampondeni, S.D.; Valim, C.; Potchen, M.J.; Milner, D.A.; Muwalo, F.W.; Birbeck, G.L.; Bradley, W.G.; Fox, L.L.; Glover, S.J.; et al. Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 2015, 372, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, F.E.; Tangpukdee, N.; Opoka, R.O.; Lafferty, E.I.; Rajwans, N.; Hawkes, M.; Krudsood, S.; Looareesuwan, S.; John, C.C.; Liles, W.C.; et al. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict outcome in African children. PLoS ONE 2009, 4, e4912. [Google Scholar] [CrossRef]
- Boivin, M.J.; Bangirana, P.; Byarugaba, J.; Opoka, R.O.; Idro, R.; Jurek, A.M.; John, C.C. Cognitive impairment after cerebral malaria in children: A prospective study. Pediatrics 2007, 119, e360–e366. [Google Scholar] [CrossRef]
- Singh, M.; Mukherjee, P.; Narayanasamy, K.; Arora, R.; Sen, S.D.; Gupta, S.; Natarajan, K.; Malhotra, P. Proteome analysis of Plasmodium falciparum extracellular secretory antigens at asexual blood stages reveals a cohort of proteins with possible roles in immune modulation and signaling. Mol. Cell. Proteomics 2009, 8, 2102. [Google Scholar] [CrossRef]
- Milner, D.A., Jr.; Whitten, R.O.; Kamiza, S.; Carr, R.; Liomba, G.; Dzamalala, C.; Seydel, K.B.; Molyneux, M.E.; Taylor, T.E. The systemic pathology of cerebral malaria in African children. Front. Cell. Infect. Microbiol. 2014, 4, 104. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Kager, P.A.; Vreeken, J.; White, N.J. Abnormal blood flow and red blood cell deformability in severe malaria. Parasitol. Today 2000, 16, 228–232. [Google Scholar] [CrossRef]
- Albrecht-Schgoer, K.; Lackner, P.; Schmutzhard, E.; Baier, G. Cerebral malaria: Current clinical and immunological asects. Front. Immunol. 2022, 13, 863568. [Google Scholar] [CrossRef] [PubMed]
- Dunst, J.; Kamena, F.; Matuschewski, K. Cytokines and chemokines in cerebral malaria pathogenesis. Front. Cell. Infect. Microbiol. 2017, 7, 324. [Google Scholar] [CrossRef]
- Conroy, A.L.; Hawkes, M.; Glover, S.J.; Erdman, L.K.; Seydel, K.B.; Taylor, T.E.; Molyneux, M.E.; Kain, K.C. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: A retrospective case-control study. Crit. Care Med. 2012, 40, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.O.; Jain, V.; Roberts, C.E.; Lucchi, N.; Joel, P.K.; Singh, M.P.; Nagpal, A.C.; Dash, A.P.; Udhayakumar, V.; Singh, N.; et al. CXCL10 and IL-8 as biomarkers for disease severity in cerebral malaria. J. Infect. Dis. 2011, 203, 828–836. [Google Scholar]
- Lyke, K.E.; Burges, R.; Cissoko, Y.; Sangare, L.; Dao, M.; Diarra, I.; Kone, A.; Harley, R.; Plowe, C.V.; Doumbo, O.K.; et al. Serum levels of the proinflammatory cytokines interleukin-1β, IL-6, IL-8, IL-10, tumor necrosis factor-α and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria. Infect. Immun. 2004, 72, 5630–5637. [Google Scholar] [CrossRef]
- John, C.C.; Opika-Opoka, R.; Byarugaba, J.; Idro, R.; Boivin, M.J. Low levels of RANTES are associated with mortality in children with cerebral malaria. J. Infect. Dis. 2006, 194, 837–845. [Google Scholar] [CrossRef]
- Armah, H.B.; Wilson, N.O.; Sarfo, B.Y.; Powell, M.D.; Bond, V.C.; Anderson, W.; Adjei, A.A.; Gyasi, R.K.; Tettey, Y.; Wiredu, E.K.; et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar. J. 2007, 6, 147. [Google Scholar] [CrossRef]
- Medana, I.M.; Turner, G.D. Human cerebral malaria and the blood–brain barrier. Int. J. Parasitol. 2006, 36, 555–568. [Google Scholar] [CrossRef]
- van der Heyde, H.C.; Nolan, J.; Combes, V.; Gramaglia, I.; Grau, G.E. A unified hypothesis for the genesis of cerebral malaria: Sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006, 22, 503–508. [Google Scholar] [CrossRef]
- Riggle, B.A.; Manglani, M.; Maric, D.; Johnson, K.R.; Lee, M.H.; Neto, O.L.A.; Taylor, T.E.; Seydel, K.B.; Nath, A.; Miller, L.H.; et al. CD8+ T cells target cerebrovascular endothelial cells in human pediatric cerebral malaria. J. Clin. Investig. 2020, 130, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Erdman, L.K.; Dhabangi, A.; Musoke, C.; Conroy, A.L.; Hawkes, M.; Higgins, S.; Rajwans, N.; Wolofsky, K.T.; Streiner, D.L.; Liles, W.C.; et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: A retrospective case-control study. PLoS ONE 2011, 6, e17440. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, M.; Opoka, R.O.; Namasopo, S.; Miller, C.; Conroy, A.L.; Serghides, L.; Kim, H.; Thampi, N.; Liles, W.C.; John, C.C.; et al. Nitric oxide for adjunctive treatment of severe malaria: Hypothesis and rationale. Med. Hypotheses 2011, 77, 437–444. [Google Scholar] [CrossRef]
- Serghides, L.; Kim, H.; Lu, Z.; Kain, D.C.; Miller, C.; Francis, R.C.; Liles, W.C.; Zapol, W.M.; Kain, K.C.; Beeson, J.G. Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS ONE 2011, 6, e27714. [Google Scholar] [CrossRef]
- Turner, L.; Lavstsen, T.; Berger, S.S.; Wang, C.W.; Petersen, J.E.V.; Avril, M.; Brazier, A.J.; Freeth, J.; Jespersen, J.S.; Nielsen, M.A.; et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013, 498, 502–505. [Google Scholar] [CrossRef]
- Craig, A.G.; Grau, G.E.; Janse, C.; Kazura, J.W.; Milner, D.; Barnwell, J.W.; Turner, G.; Langhorne, J.; on behalf of the participants of the Hinxton Retreat meeting on “Animal Models for Research on Severe Malaria”; Rall, G.F. The role of animal models for research on severe malaria. PLoS Pathog. 2012, 8, e1002401. [Google Scholar] [CrossRef]
- Smith, J.D.; Craig, A.G.; Kriek, N.; Hudson-Taylor, D.; Kyes, S.; Fagen, T.; Pinches, R.; Baruch, D.I.; Newbold, C.I.; Miller, L.H. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: A parasite adhesion trait implicated in cerebral malaria. Proc. Natl. Acad. Sci. USA 2000, 97, 1766–1771. [Google Scholar] [CrossRef]
- Newbold, C.I.; Warn, P.; Black, G.; Snow, B.; Craig, A.; Berendt, A.; Marsh, K.; Peshu, N.; Msobo, M. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1997, 57, 389–398. [Google Scholar] [CrossRef]
- Jain, V.; Armah, H.B.; Tongren, J.E.; Ned, R.M.; Wilson, N.O.; Crawford, S.; Joel, P.K.; Singh, M.P.; Nagpal, A.C.; Dash, A.; et al. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar. J. 2008, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.M.; Fanello, C.I.; Hendriksen, I.C.; Gomes, E.; Seni, A.; Chhaganlal, K.D.; Bojang, K.; Olaosebikan, R.; Anunobi, N.; Maitland, K.; et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): An open-label, randomised trial. Lancet 2010, 376, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.W.; Lampah, D.A.; Gitawati, R.; Tjitra, E.; Kenangalem, E.; Piera, K.; Price, R.N.; Duffull, S.B.; Celermajer, D.S.; Anstey, N.M. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc. Natl. Acad. Sci. USA 2008, 105, 17097–17102. [Google Scholar] [CrossRef]
- Gomes, C.; Varo, R.; Duran-Frigola, M.; Sitoe, A.; Bila, R.; Machevo, S.; Mayor, A.; Bassat, Q.; Rodriguez, A. Endothelial transcriptomic analysis identifies biomarkers of severe and cerebral malaria. JCI Insight 2023, 8, e172845. [Google Scholar] [CrossRef]
- Ouma, B.J.; Bangirana, P.; Ssenkusu, J.M.; Datta, D.; Opoka, R.O.; Idro, R.; Kain, K.C.; John, C.C.; Conroy, A.L. Plasma angiopoietin-2 is associated with age-related deficits in cognitive sub-scales in Ugandan children following severe malaria. Malar. J. 2021, 20, 17. [Google Scholar] [CrossRef]
- Conroy, A.L.; Phiri, H.; Hawkes, M.; Glover, S.; Mallewa, M.; Seydel, K.B.; Taylor, T.E.; Molyneux, M.E.; Kain, K.C.; Borrmann, S. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children. PLoS ONE 2010, 5, e15291. [Google Scholar] [CrossRef]
- Higgins, S.J.; Purcell, L.A.; Silver, K.L.; Tran, V.; Crowley, V.; Hawkes, M.; Conroy, A.L.; Opoka, R.O.; Hay, J.G.; Quaggin, S.E.; et al. Dysregulation of angiopoietin-1 plays a mechanistic role in the pathogenesis of cerebral malaria. Sci. Transl. Med. 2016, 8, 358ra128. [Google Scholar] [CrossRef]
- Conroy, A.L.; Hawkes, M.; Hayford, K.; Namasopo, S.; Opoka, R.O.; John, C.C.; Liles, W.C.; Kain, K.C. Prospective validation of pediatric disease severity scores to predict mortality in Ugandan children presenting with malaria and non-malaria febrile illness. Crit. Care 2015, 19, 47. [Google Scholar] [CrossRef]
- Moxon, C.A.; Chisala, N.V.; Wassmer, S.C.; Taylor, T.E.; Seydel, K.B.; Molyneux, M.E.; Faragher, B.; Kennedy, N.; Toh, C.-H.; Craig, A.G.; et al. Persistent endothelial activation and inflammation after Plasmodium falciparum infection in Malawian children. J. Infect. Dis. 2014, 209, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Hollestelle, M.J.; Donkor, C.; Mantey, E.A.; Chakravorty, S.J.; Craig, A.; Akoto, A.O.; O’DOnnell, J.; Van Mourik, J.A.; Bunn, J. von Willebrand factor propeptide in malaria: Evidence of acute endothelial cell activation. Br. J. Haematol. 2006, 133, 562–569. [Google Scholar] [CrossRef]
- Barber, B.E.; William, T.; Grigg, M.J.; Parameswaran, U.; Piera, K.A.; Price, R.N.; Yeo, T.W.; Anstey, N.M.; Stevenson, M.M. Parasite biomass-related inflammation, endothelial activation, microvascular dysfunction and disease severity in vivax malaria. PLoS Pathog. 2015, 11, e1004558. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.O.; Jain, V.; Roberts, C.E.; Lucchi, N.; Joel, P.K.; Singh, M.P.; Nagpal, A.C.; Dash, A.P.; Udhayakumar, V.; Singh, N.; et al. CXCL4 and CXCL10 predict risk of fatal cerebral malaria. Dis. Markers 2011, 30, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, N.W.; Jain, V.; Wilson, N.O.; Singh, N.; Udhayakumar, V.; Stiles, J.K. Potential serological biomarkers of cerebral malaria. Dis. Markers 2011, 31, 327. [Google Scholar] [CrossRef]
- Kotepui, M.; Mahittikorn, A.; Masangkay, F.R.; Kotepui, K.U. A systematic review of circulating IP-10/CXCL10 in patients with Plasmodium infections in relation to disease severity. Sci. Rep. 2024, 14, 31723. [Google Scholar] [CrossRef] [PubMed]
- Pais, T.F.; Ali, H.; Moreira da Silva, J.; Duarte, N.; Neres, R.; Chhatbar, C.; Acúrcio, R.C.; Guedes, R.C.; Moraes, M.C.S.; Costa-Silva, B.; et al. Brain endothelial STING1 activation by Plasmodium-sequestered heme promotes cerebral malaria via type I IFN response. Proc. Natl. Acad. Sci. USA 2022, 119, e2206327119. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli, R.T.; Kalantari, P.; Fitzgerald, K.A.; Golenbock, D.T. Innate sensing of malaria parasites. Nat. Rev. Immunol. 2014, 14, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Doldan-Silvero, A.; Acevedo-Gadea, C.; Habib, C.; Freeman, J.; Johari, V. ADAMTS13 activity and inhibitor. Am. J. Hematol. 2008, 83, 811–814. [Google Scholar] [CrossRef]
- Wang, Y.; De Labastida Rivera, F.; Edwards, C.L.; Frame, T.C.; Engel, J.A.; Bukali, L.; Na, J.; Ng, S.S.; Corvino, D.; de Oca, M.M.; et al. STING activation promotes autologous type I interferon-dependent development of type 1 regulatory T cells during malaria. J. Clin. Investig. 2023, 133, e169417. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Su, X.Z.; Li, J.; Shi, J.-J.; Xie, L.-H. Multicohort transcriptome analysis of whole blood identifies robust human response signatures in Plasmodium falciparum infections. Malar. J. 2022, 21, 333. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Desakorn, V.; Pongtavornpinyo, W.; Sahassananda, D.; Silamut, K.; Chotivanich, K.; Newton, P.N.; Pitisuttithum, P.; Smithyman, A.M.; White, N.J.; et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005, 2, e204. [Google Scholar] [CrossRef]
- Hendriksen, I.C.; Mwanga-Amumpaire, J.; von Seidlein, L.; Mtove, G.; White, L.J.; Olaosebikan, R.; Lee, S.J.; Tshefu, A.K.; Woodrow, C.; Amos, B.; et al. Diagnosing severe falciparum malaria in parasitaemic African children: A prospective evaluation of plasma PfHRP2 measurement. PLoS Med. 2012, 9, e1001297. [Google Scholar] [CrossRef]
- Hendriksen, I.C.; White, L.J.; Veenemans, J.; Mtove, G.; Woodrow, C.; Amos, B.; Saiwaew, S.; Gesase, S.; Nadjm, B.; Silamut, K.; et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J. Infect. Dis. 2013, 207, 351–361. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Du, D.; Wychrij, D.; Cain, M.D.; Wu, Q.; Klein, R.S.; Russo, I.; Goldberg, D.E. Histidine-rich protein II nanoparticle delivery of heme iron load drives endothelial inflammation in cerebral malaria. Proc. Natl. Acad. Sci. USA 2023, 120, e2306318120. [Google Scholar] [CrossRef]
- Pal, P.; Daniels, B.P.; Oskman, A.; Diamond, M.S.; Klein, R.S.; Goldberg, D.E.; Johnson, P.J. Plasmodium falciparum histidine-rich protein II compromises brain endothelial barriers and may promote cerebral malaria pathogenesis. mBio 2016, 7, e00617-16. [Google Scholar] [CrossRef]
- Faille, D.; El-Assaad, F.; Mitchell, A.J.; Alessi, M.C.; Chimini, G.; Fusai, T.; Grau, G.E.; Combes, V. Endocytosis and intracellular processing of platelet microparticles by brain endothelial cells. J. Cell. Mol. Med. 2012, 16, 1731–1738. [Google Scholar] [CrossRef]
- Couper, K.N.; Barnes, T.; Hafalla, J.C.; Combes, V.; Ryffel, B.; Secher, T.; Grau, G.E.; Riley, E.M.; de Souza, J.B.; Kazura, J.W. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010, 6, e1000744. [Google Scholar] [CrossRef]
- El-Assaad, F.; Wheway, J.; Hunt, N.H.; Grau, G.E.; Combes, V. Production, fate and pathogenicity of plasma microparticles in murine cerebral malaria. PLoS Pathog. 2014, 10, e1003839. [Google Scholar] [CrossRef]
- Watson, J.A.; Uyoga, S.; Wanjiku, P.; Makale, J.; Nyutu, G.M.; Mturi, N.; George, E.C.; Woodrow, C.J.; Day, N.P.J.; Bejon, P.; et al. Improving the diagnosis of severe malaria in African children using platelet counts and plasma PfHRP2 concentrations. Sci. Transl. Med. 2022, 14, eabn5040. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Zhang, H.F.; Zhao, X.X.; Lai, Y.J.; Liu, F.F. Genomic analysis of host gene responses to cerebral Plasmodium falciparum malaria. Immun. Inflamm. Dis. 2021, 9, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Ikerionwu, C.; Ugwuishiwu, C.; Okpala, I.; James, I.; Okoronkwo, M.; Nnadi, C.; Orji, U.; Ebem, D.; Ike, A. Application of machine and deep learning algorithms in optical microscopic detection of Plasmodium: A malaria diagnostic tool for the future. Photodiagnosis Photodyn. Ther. 2022, 40, 103198. [Google Scholar] [CrossRef] [PubMed]
- Loh, R.; Yong, W.X.; Yapeter, J.; Subburaj, K.; Chandramohanadas, R. A deep learning approach to the screening of malaria infection: Automated and rapid cell counting, object detection and instance segmentation using Mask R-CNN. Comput. Med. Imaging Graph. 2021, 88, 101845. [Google Scholar] [CrossRef] [PubMed]
- Regev-Rudzki, N.; Wilson, D.W.; Carvalho, T.G.; Sisquella, X.; Coleman, B.M.; Rug, M.; Bursac, D.; Angrisano, F.; Gee, M.; Hill, A.F.; et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013, 153, 1120–1133. [Google Scholar] [CrossRef]
- Mantel, P.-Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef]
- Abdi, A.; Mwikali, K.; Mwangi, S.; Pance, A.; Ochola-Oyier, L.I.; Kariuki, S.; Newton, C.; Bejon, P.; Rayner, J.C. The mRNA content of plasma extracellular vesicles provides a window into the brain during cerebral malaria disease progression. Res. Sq. 2023; preprint. [Google Scholar]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2015, 44, D457–D462. [Google Scholar] [CrossRef]
- Beare, N.A.; Taylor, T.E.; Harding, S.P.; Lewallen, S.; Molyneux, M.E. Malarial retinopathy: A newly established diagnostic sign in severe malaria. Am. J. Trop. Med. Hyg. 2006, 75, 790–797. [Google Scholar] [CrossRef]
- Kutmon, M.; Riutta, A.; Nunes, N.; Hanspers, K.; Willighagen, E.L.; Bohler, A.; Mélius, J.; Waagmeester, A.; Sinha, S.R.; Miller, R.; et al. WikiPathways: Capturing the full diversity of pathway knowledge. Nucleic Acids Res. 2016, 44, D488–D494. [Google Scholar] [CrossRef]
- Sahu, P.K.; Duffy, F.J.; Dankwa, S.; Vishnyakova, M.; Majhi, M.; Pirpamer, L.; Vigdorovich, V.; Bage, J.; Maharana, S.; Mandala, W.; et al. Determinants of brain swelling in pediatric and adult cerebral malaria. JCI Insight 2021, 6, e145823. [Google Scholar] [CrossRef]
- Strangward, P.; Haley, M.J.; Albornoz, M.G.; Barrington, J.; Shaw, T.; Dookie, R.; Zeef, L.; Baker, S.M.; Winter, E.; Tzeng, T.-C.; et al. Targeting the IL33-NLRP3 axis improves therapy for experimental cerebral malaria. Proc. Natl. Acad. Sci. USA 2018, 115, 7404–7409. [Google Scholar] [CrossRef]
- Kalantari, P.; DeOliveira, R.B.; Chan, J.; Corbett, Y.; Rathinam, V.; Stutz, A.; Latz, E.; Gazzinelli, R.T.; Golenbock, D.T.; Fitzgerald, K.A. Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. Cell Rep. 2014, 6, 196–210. [Google Scholar] [CrossRef]
- Muppidi, P.; Wright, E.; Wassmer, S.C.; Gupta, H. Diagnosis of cerebral malaria: Tools to reduce Plasmodium falciparum associated mortality. Front. Cell. Infect. Microbiol. 2023, 13, 1090013. [Google Scholar] [CrossRef] [PubMed]
- Barrera, V.; MacCormick, I.; Czanner, G.; Hiscott, P.S.; White, V.A.; Craig, A.G.; Beare, N.A.V.; Culshaw, L.H.; Zheng, Y.; Biddolph, S.C.; et al. Neurovascular sequestration in paediatric Plasmodium falciparum malaria is visible clinically in the retina. eLife 2018, 7, e29578. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Matsuo, Y.; Fujii, Y.; Ohta, A.; Hirota, K. Rapid detection of single nucleotide polymorphisms using the MinION nanopore sequencer: A feasibility study for perioperative precision medicine. JA Clin. Rep. 2022, 8, 17. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk. Press Release. 2021. Available online: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk (accessed on 6 October 2021).
- World Health Organization. WHO Recommends R21/Matrix-M Vaccine for Malaria Prevention in Updated Advice on Immunization. Press Release. 2023. Available online: https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vaccine-for-malaria-prevention-in-updated-advice-on-immunization (accessed on 2 October 2023).
- Bruneel, F. Human cerebral malaria: 2019 mini review. Rev. Neurol. 2019, 175, 445–450. [Google Scholar] [CrossRef]
- Langfitt, J.T.; McDermott, M.P.; Brim, R.; Mboma, S.; Potchen, M.J.; Kampondeni, S.D.; Seydel, K.B.; Semrud-Clikeman, M.; Taylor, T.E. Neurodevelopmental impairments 1 year after cerebral malaria. Pediatrics 2019, 143, e20181026. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Satpathi, S.; Behera, P.K.; Mishra, S.K.; Mohanty, S.; Wassmer, S.C. Pathogenesis of cerebral malaria: New diagnostic tools, biomarkers, and therapeutic approaches. Front. Cell. Infect. Microbiol. 2015, 5, 75. [Google Scholar] [CrossRef]
- Dietmann, A.; Helbok, R.; Lackner, P.; Issifou, S.; Lell, B.; Matsiegui, P.; Reindl, M.; Schmutzhard, E.; Kremsner, P.G. Matrix metalloproteinases and their tissue inhibitors (TIMPs) in Plasmodium falciparum malaria: Serum levels of TIMP-1 are associated with disease severity. J. Infect. Dis. 2008, 197, 1614–1620. [Google Scholar] [CrossRef]
- Georgiadou, A.; Naidu, P.; Walsh, S.; Kamiza, S.; Barrera, V.; Harding, S.P.; Moxon, C.A.; Cunnington, A.J. Localised release of matrix metallopeptidase-8 in fatal cerebral malaria. Clin. Transl. Immunol. 2021, 10, e1263. [Google Scholar] [CrossRef]
- Rénia, L.; Grau, G.E.; Wassmer, S.C. CD8+ T cells and human cerebral malaria: A shifting episteme. J. Clin. Investig. 2020, 130, 1109–1111. [Google Scholar] [CrossRef]
- Lima, M.N.; Oliveira, H.A.; Fagundes, P.M.; Estato, V.; Silva, A.Y.O.; Freitas, R.J.R.X.; Passos, B.A.B.R.; Oliveira, K.S.; Batista, C.N.; Vallochi, A.L.; et al. Mesenchymal stromal cells protect against vascular damage and depression-like behavior in mice surviving cerebral malaria. Stem Cell Res. Ther. 2020, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Idro, R.; Carter, J.A.; Fegan, G.; Neville, B.G.; Newton, C.R. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch. Dis. Child. 2006, 91, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ouma, B.J.; Ssenkusu, J.M.; Shabani, E.; Datta, D.; Opoka, R.O.M.; Idro, R.M.; Bangirana, P.; Park, G.; Joloba, M.L.; Kain, K.C.M.; et al. Endothelial activation, acute kidney injury, and cognitive impairment in pediatric severe malaria. Crit. Care Med. 2020, 48, e734–e743. [Google Scholar] [CrossRef]
- Omar, M.; Marchionni, L.; Häcker, G.; Badr, M.T. Host blood gene signatures can detect the progression to severe and cerebral malaria. Front. Cell. Infect. Microbiol. 2021, 11, 743616. [Google Scholar] [CrossRef]
| Biomarker | Source | Mechanism | Diagnostic/Prognostic Value |
|---|---|---|---|
| Ang-2 ↑/Ang-1 ↓ | Endothelium | Tie2 imbalance → BBB leak | Predicts mortality |
| sICAM-1, sVCAM-1, vWF | Endothelium | Endothelial activation | Correlate with severity |
| CXCL10 (IP-10) | Endothelium/immune | T-cell recruitment | Predicts fatal CM |
| PfHRP2 | Parasite | Parasite biomass marker | Improves diagnostic accuracy |
| Extracellular Vesicles | iRBCs, Endothelium | Carry parasite/host signals | Potential precision biomarker |
| Target Pathway | Candidate Intervention | Evidence |
|---|---|---|
| Endothelial stabilization (Ang–Tie2) | Ang-1 mimetics, Tie2 agonists | Preclinical success |
| Nitric Oxide bioavailability | Inhaled NO | Limited trial benefit |
| Cytokine modulation | Anti-CXCL10, CXCR3 blockade | Preclinical promise |
| Inflammasome (IL-33–NLRP3) | IL-33, MCC950 | Improves survival in mice |
| PfEMP1 adhesion | Adhesion blockers | High potential |
| Cell therapy | MSCs | Neuroprotection in models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikor, D.; Hurła, M.; Banaszek-Hurła, N.; Drelichowska, A.; Paul, M. Neurovascular Pathophysiology and Emerging Biomarkers in Cerebral Malaria: An Integrative Perspective. Neurol. Int. 2025, 17, 149. https://doi.org/10.3390/neurolint17090149
Pikor D, Hurła M, Banaszek-Hurła N, Drelichowska A, Paul M. Neurovascular Pathophysiology and Emerging Biomarkers in Cerebral Malaria: An Integrative Perspective. Neurology International. 2025; 17(9):149. https://doi.org/10.3390/neurolint17090149
Chicago/Turabian StylePikor, Damian, Mikołaj Hurła, Natalia Banaszek-Hurła, Alicja Drelichowska, and Małgorzata Paul. 2025. "Neurovascular Pathophysiology and Emerging Biomarkers in Cerebral Malaria: An Integrative Perspective" Neurology International 17, no. 9: 149. https://doi.org/10.3390/neurolint17090149
APA StylePikor, D., Hurła, M., Banaszek-Hurła, N., Drelichowska, A., & Paul, M. (2025). Neurovascular Pathophysiology and Emerging Biomarkers in Cerebral Malaria: An Integrative Perspective. Neurology International, 17(9), 149. https://doi.org/10.3390/neurolint17090149






