1. Introduction
Vertigo is one of the most common complaints in medical practice. It is associated with various underlying conditions, including neurological, otolaryngological, and psychological disorders. The prevalence of dizziness in the adult population is in the range of 20–30%, and it is particularly common among the elderly, significantly impacting their quality of life and daily functioning. Among those over 65 years of age, the prevalence of dizziness reaches 30%, while in individuals over 75, it increases to 50%, highlighting the public health significance of this issue in aging populations [
1,
2].
The etiology of dizziness is often multifactorial, involving degenerative changes in the vestibular system and neurological disorders, as well as circulatory and musculoskeletal conditions. The most frequently diagnosed causes include benign paroxysmal positional vertigo (BPPV), Ménière’s disease, vestibular neuritis, and central nervous system vascular diseases [
3,
4].
Dizziness is often accompanied by other symptoms, such as headache, imbalance, psychological issues, and nausea/vomiting, which are also critical for establishing an accurate diagnosis. Detailed examination of these symptoms, as well as preliminary imaging studies such as CT (Computer Tomography), cervical spine X-rays, and carotid/vertebral artery ultrasound, aids in precise diagnosis and targeted therapeutic decisions. However, it is important to recognize that routine MRI (Magnetic Resonance Imaging) often fails to identify specific causes of dizziness, as structural abnormalities—such as white matter lesions—are common in both symptomatic and asymptomatic populations [
5,
6].
MRI is currently one of the most effective imaging modalities for examining the brain. MRI enables the assessment of soft tissues, including clear visualization of gray and white matter, ventricles, blood vessels, and nerves (such as the VIII. cranial nerve—the vestibulocochlear nerve, damage of which can result in dizziness). The standard protocol includes the following sequences: sagittal T1-weighted, coronal FLAIR (Fluid-Attenuated Inversion Recovery), axial T2-weighted, axial DWI (Diffusion Weighted Imaging), and axial SWI (Susceptibility Weighted Imaging). When necessary, post-contrast 3D sagittal T1-weighted sequences are performed. For dizziness, a 3D axial balanced steady-state gradient echo sequence (FIESTA/TrueFISP/balanced-FFE on GE, Siemens, and Philips, respectively) is always acquired to examine the inner ear, while for headaches, TOF (Time-of-Flight) sequences are added to the protocol [
7,
8,
9].
This study aims to identify the brain structural abnormalities in vertigo that can be diagnosed through brain MRI. Additionally, it provides a detailed overview of diagnostic procedures related to dizziness, with a particular focus on pre-MRI investigations, and analyzes the most common symptoms and therapeutic approaches. Through this analysis, the study seeks to advance the understanding and management of dizziness in clinical practice.
2. Materials and Methods
2.1. Participants and Design
Patients were enrolled from the outpatient clinic of Szent Margit Hospital between September 2020 and December 2022.
In the control group, vertigo was not reported as a symptom. Exclusion criteria included the presence of any neurological disease or complaint in the patient’s medical history. A total of 232 participants were enrolled. The mean age of the participants was 56.77 ± 17.14 years (age range 18–91 years). There were no clinical evaluations in the control group, only the MRI diagnosis was examined.
In the vertigo group, the inclusion criteria were that the patient reported dizziness as a symptom and that the attending physician had requested a brain MRI examination. Patients were not examined in the emergency setting for acute vertigo; instead, they suffered from longer-standing or recurrent dizziness. Exclusion criteria included the presence of any neurological disease or complaint in the patient’s medical history (other than vertigo or dizziness). A total of 232 participants were enrolled. The mean age of the participants was 59.41 ± 17.43 years (age range 19–93 years). We examined the medical history, including symptoms other than dizziness and the examinations conducted prior to the MRI. The most common abnormalities observed in the MRI results were categorized, including cortical atrophy, white matter lesions, vascular lesions, lacunar lesions, vascular encephalopathy, Circle of Willis variation, and sinusitis. Additionally, we collected the final diagnoses and treatments administered following the MRI.
2.2. MRI Protocol
The MRI parameters are summarized in
Table 1. The brain MRI protocol consisted of the following measurements:
sagittal T1-weighted
coronal FLAIR
axial T2-weighted
axial DWI
axial SWI (Susceptibility Weighted Imaging)
axial TOF
axial 3D FIESTA (this is a supplementary measurement for the inner ear, which is always included in the protocol in cases of vertigo)
optional: contrast enhanced 3D sagittal T1-weighted.
2.3. Outcomes
The primary outcomes are the structural changes described during MRI. Throughout the study, all findings were systematically evaluated by a neuroradiologist. To diagnose various pathologies, neurologists in Hungary apply local professional guidelines and the recommendations of the National Health Professional College. The most common pathologies were diagnosed based on the following criteria (
Figure 1).
Cortical atrophy: The Scheltens scale aids in the standardized grading of atrophy severity. It is characterized by a reduction in cortical thickness, widening of the sulci, and dilation of the ventricular system, particularly the lateral ventricles. These changes are typically observed on T1-weighted and FLAIR images, where thinning of the cortical structures is evident [
10].
White matter lesions: The Fazekas scale and the STRIVE (Standards for Reporting Vascular Changes on Neuroimaging) criteria are the most commonly used standards. White matter lesions are identified on FLAIR and T2-weighted images as bright (hyperintense) areas located in the periventricular or deep white matter. These may appear as discrete spots or confluent regions, typically indicating small vessel disease [
11].
Vascular lesions: In Hungary, the European Stroke Organization Guidelines serve as the primary reference. MR angiography is routinely used to assist in the diagnosis of vascular abnormalities. On DWI, acute ischemic lesions appear as hyperintense signals. T1/T2-weighted and MRA (Magnetic Resonance Aniography) images can identify stenosis, occlusions, or vascular wall abnormalities. In cases of haemorrhage, hemosiderin deposits can be detected using SWI techniques [
12].
Lacunar lesions: The STRIVE criteria assist in identifying these lesions. They are characterized by small (<15 mm) round or oval hyperintense areas on T2-weighted/FLAIR images. Common locations include the thalamus, basal ganglia, and internal capsule. On T1-weighted images, they appear as hypointense signals [
11].
Vascular encephalopathy: The Hungarian Stroke Guidelines and the recommendations of the MSKT (Hungarian Stroke Consensus Council), along with the application of the Fazekas scale, are essential for evaluation. Diffuse white matter hyperintensities are observed on FLAIR images. Subcortical infarcts, dilated perivascular spaces, or signs of microvascular disease may also be present. Cortical and subcortical atrophy is frequently associated with ventricular enlargement [
13].
Circle of Willis variation: The evaluation of the Circle of Willis focuses on assessing its completeness, anatomical variations, and flow characteristics. TOF-MRA is commonly used for visualization, allowing the detection of stenosis, occlusion, aneurysms, and collateral flow patterns. Variations or abnormalities are identified based on flow signal uniformity and vessel connections [
14].
Sinusitis: The ACR (American College of Radiology) Appropriateness Criteria and the Lund-Mackay scoring system are utilized. Sinus opacification, mucosal thickening, or fluid levels are identified on T2-weighted images. In acute inflammation, high-intensity signals and fluid levels are observed on T2. In chronic sinusitis, findings include thickened bony walls, fibrosis, or the presence of mucoceles [
15].
Secondary outcomes are other symptoms appearing in addition to vertigo, the examinations preceding the MRI, diagnosis after MRI, and treatments applied after the MRI examination.
2.4. Statistical Analyses
Demographic characteristics of the groups were compared to prove that age and gender distribution were similar. Age was analyzed using an independent samples t-test, while gender distribution was assessed with a chi-square test. Normality was assessed using the Shapiro–Wilk test, and the data followed a normal distribution.
Descriptive statistics were used to summarize the baseline characteristics. Data are reported as mean ± SD (Standard Deviation).
To evaluate differences in MRI findings between the control and vertigo groups, chi-square tests were conducted for cortical atrophy, white matter lesions, vascular lesions, lacunar infarcts, vascular encephalopathy, Circle of Willis variation, and sinusitis/sinus abnormalities.
Since the examinations preceding the MRI, MRI-related diagnoses, and therapies were not assessed in the control group, we focused on analyzing the frequencies of secondary findings statistically to provide insights into their distribution and potential relevance.
The significance level was set at p < 0.05 for all analyses.
Statistical calculations were conducted using IBM SPSS Statistics for Windows, Version 26.0., IBM Corp., Armonk, NY, USA.
2.5. Ethical Considerations
This study was registered at ClinicalTrials.gov under the identifier NCT06848712 on 22 February 2025. The study was approved by the Regional Ethical Committee of Szent Margit Hospital (IG/296-1/2025/2) on 4 February 2025. The study was conducted according to the World Medical Association Declaration of Helsinki. As it was a retrospective study, informed consent was waived in accordance with institutional guidelines. Data collection complied with GDPR (General Data Protection Regulation) and patient confidentiality regulations.
3. Results
3.1. Baseline Characteristics
Table 2 shows the descriptive data in the vertigo group. The study involved 68 male and 164 female participants, with an average age of 59.41 (±17.43) years. The participants’ ages ranged from 19 to 93 years. Examinations preceding the MRI, other symptoms, and MRI results are discussed in detail in the outcome results.
Table 3 shows the descriptive data in the control group. The study involved 76 male and 156 female participants, with an average age of 56.77 (±17.43) years. The participants’ ages ranged from 18 to 91 years. MRI results are discussed in detail in the outcome results.
No significant differences were found between the control and vertigo groups for age (t(462) = −1.646, p = 0.100) or gender distribution (χ2(1, n= 464) = 0.644, p = 0.422).
3.2. Primary Outcome
Of the enrolled subjects, 75% had positive MRI findings (structural changes). The MRI results are summarized in
Table 4.
In the vertigo group, white matter lesions (39.2%) were described the most frequently as a result of brain MRI, followed by cortical atrophy (25.9%) and vascular encephalopathy (31.5%).
Significant differences were detected in white matter lesions (
p = 0.001, see
Figure 2), lacunar lesions (
p = 0.047), Circle of Willis variation (
p = 0.03), and sinusitis/sinus abnormalities (
p = 0.005). Other MRI findings, including cortical atrophy, vascular lesion, and vascular encephalopathy, did not show statistically significant differences (
p > 0.05).
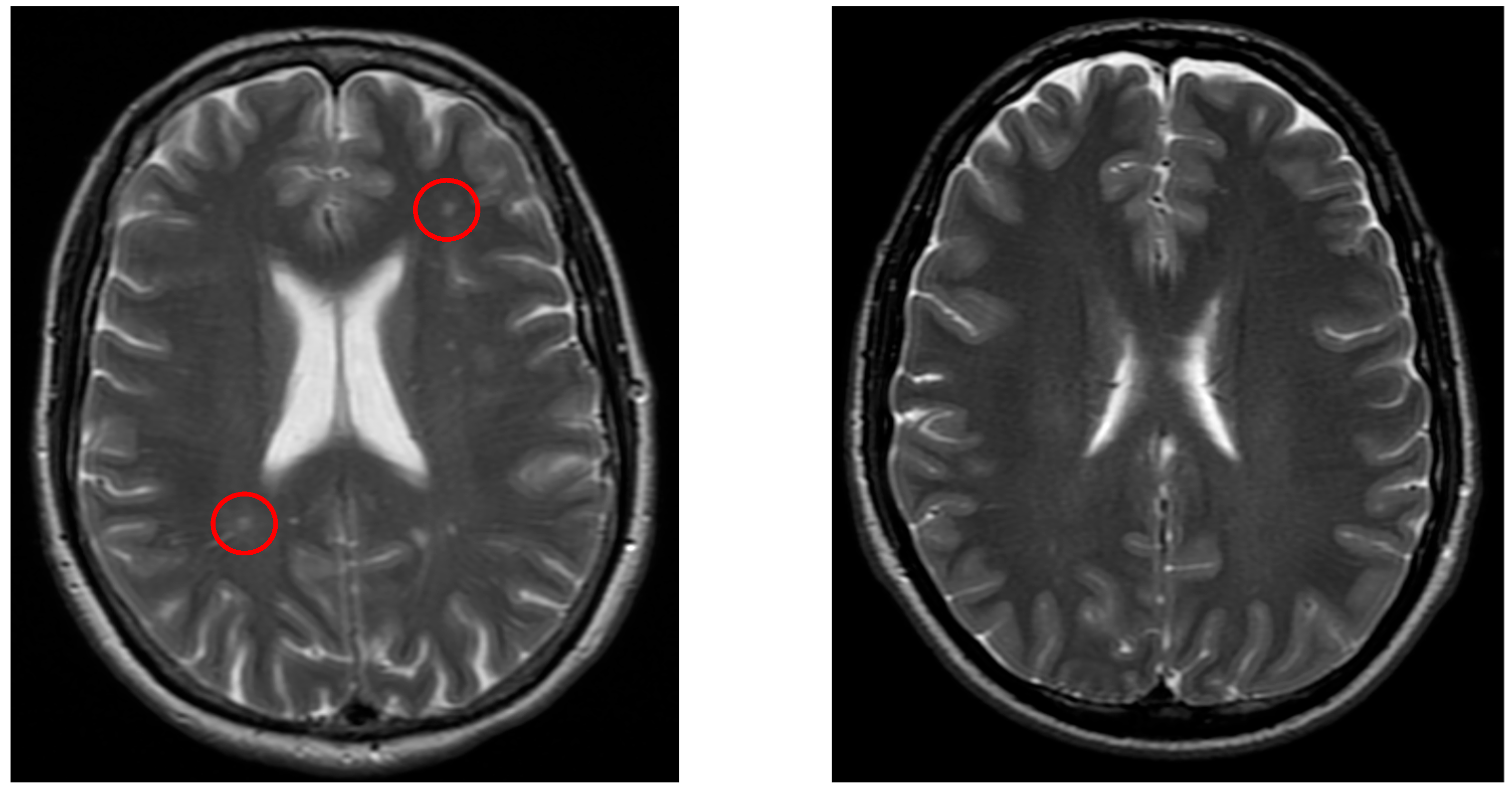
Figure 3 demonstrates a typical lacunar infarct detected in the vertigo group, in contrast with controls.
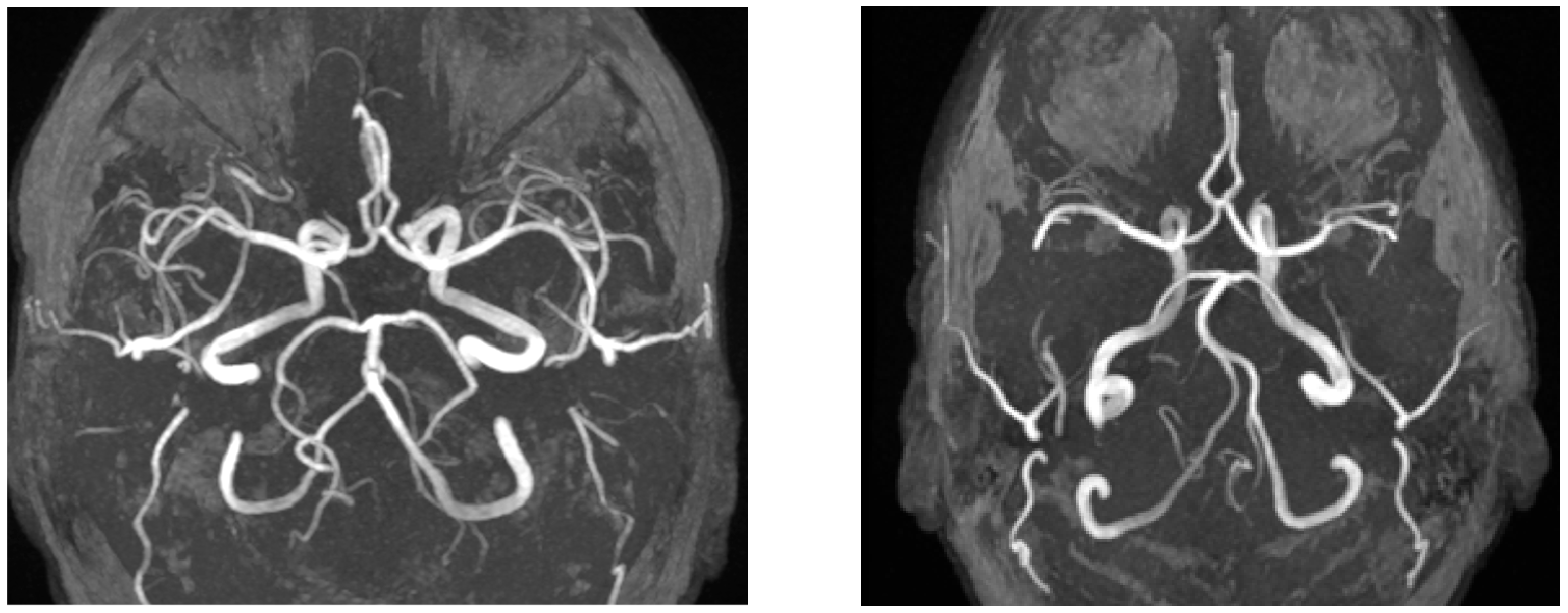
Representative images of Circle of Willis variations are shown in
Figure 4.
As illustrated in
Figure 5, patients from the vertigo group often showed sinus abnormalities, whereas no such abnormalities were observed in the control group.
3.3. Secondary Outcome
Table 5 presents the types of examinations performed prior to MRI, the number of cases for each examination type, and the number of positive findings. Among the 31 CT scans, 18 showed positive results. For carotis/vertebralis ultrasound, 75 cases were conducted, with 34 yielding positive findings. The most common finding in carotis/vertebralis ultrasound examinations was evidence of atherosclerosis (
n = 26). Cervical spine X-rays were performed in 23 cases, with 21 positive results. The most common finding in cervical spine X-rays was spondylosis (
n = 13). Additionally, 36 cases involved other types of examinations, of which 29 were positive. Other imaging examinations included for example cervical spine MRI, paranasal sinus X-rays, and EEG (Electroencephalogram).
Table 6 summarizes the symptoms experienced by patients, highlighting that headache was the most common symptom (24.14%), followed by imbalance (15.5%). Vomiting and nausea were reported in 11.2%, tinnitus in 8.6%, and psychological symptoms in 5.2%. Among the other symptoms, the most common ones were vision-related issues (
n = 15) and numbness (
n = 11).
The diagnoses after MRI are summarized in
Table 7. In 44 patients, no final diagnosis was provided. Among the remaining 188 cases, the most frequently diagnosed conditions were BPPV (34.5%) and Vertebrobasilar Syndrome (VBS, 11.6%).
The applied treatments are summarized in
Table 8. The most common interventions included lifestyle advice (21.1%) and physical training (15.01%). Physical training includes interventions such as the Epley maneuver and spinal exercises. Pharmacological treatments were less frequent, with cognitive enhancers (10.3%), anxiolytics, hypnotics, and antidepressants (6.5%), and anticoagulants (7.3%) being the most common. Other treatments, such as lipid-lowering medications, herbal medicines, and betahistin, were used in 2–6% of cases. Rare interventions included antimigraine medications (1.7%) and steroids (0.4%).
4. Discussion
This study investigates the underlying structural brain changes associated with vertigo.
Our primary outcome revealed that white matter lesions, lacunar lesions, variations of the Circle of Willis, and sinusitis were significantly more prevalent in patients presenting with vertigo compared to the control group. Midbrain white matter lesions have been observed more frequently in patients with dizziness. In our study, we did not examine the precise localization of white matter lesions. In another study, similar to ours, white matter lesions were also found to be significantly more frequent in the dizziness group. This research also highlighted that non-specific white matter lesions were more common [
16].
Brain MRI examinations were frequently preceded by carotid/vertebral artery ultrasound, where the findings suggested that atherosclerosis could be a contributing factor to dizziness. Among cervical spine X-rays, the majority showed abnormal findings, with spondylosis being the most common diagnosis [
17].
In addition to dizziness, the most frequently reported symptoms were headache, imbalance, visual disturbances, and numbness. In some publications, tinnitus and hearing loss are mentioned as symptoms accompanying dizziness, in contrast to the symptoms observed in our study [
18].
Following the MRI results, the treating physicians most frequently diagnosed patients with BPPV. Other publications have also found BPPV to be one of the most common peripheral vestibular disorders. Similar to our results, in Whitman’s article, the most common causes of dizziness included BPPV, vestibular migraine, anxiety disorders, orthostatic hypotension, and medication side effects [
19]. Although not observed in our cohort, it is important to mention vestibular schwannoma, which can also present with vertigo. Vestibular schwannoma is a benign tumor arising from Schwann cells of the vestibular nerve, most commonly from its inferior division [
20]. For example, in the case report by Joon Yong Park and Chang-Hee Kim, a vestibular schwannoma caused vertigo to a 57-year-old patient [
21].
In the treatment of dizziness, lifestyle modifications and various physical exercises were the most commonly recommended interventions, including techniques such as the Epley maneuver and spinal exercises. Among pharmacological therapies, the use of betahistine and anxiolytics, hypnotics, and antidepressants were the most widespread, aiming to address both the vestibular symptoms and the psychological burden associated with chronic vertigo. Other studies have also identified physical exercises targeting BPPV, such as repositioning maneuvers, and the use of betahistine as the most common and effective therapeutic approaches [
22].
The nature of dizziness was not recorded by referring physicians in 155 cases, preventing us from examining this aspect in detail. However, other studies have identified significant associations between dizziness and the structural characteristics of specific brain regions [
23].
5. Limitation
A limitation of our study is that we did not examine the precise localization of structural brain abnormalities. Additionally, since we only collected MRI diagnoses for the control group, we could only report frequencies for other parameters we analyzed (such as prior examinations and treatments). Another limitation is that we did not conduct a detailed examination of the variations in the Circle of Willis.
6. Conclusions
In addition to dizziness, the most commonly reported symptoms are headache, imbalance, and visual disturbances. Prior to MRI, vertebral/carotid artery ultrasound is often indicated to rule out atherosclerosis, while cervical spine X-rays are used to exclude spondylosis. Based on MRI findings, in addition to various white matter lesions, malformations of the Circle of Willis and inflammations of the paranasal sinuses may also contribute to dizziness symptoms. In terms of treatment, alongside symptomatic therapies (e.g., BPPV exercises, cervical spine exercises, Epley maneuver, and betahistine), addressing lifestyle-related triggers (e.g., using antidepressants or providing lifestyle counseling) is also essential.
Author Contributions
Conceptualization, K.S. and J.T.; methodology, K.S. and J.T.; software, K.S. and S.K.; validation, J.T.; formal analysis, K.S., S.K., G.O., and J.T.; investigation, K.S.; resources, K.S.; data curation, K.S. and S.K.; writing—original draft preparation, K.S.; writing—review and editing, S.K., G.O., and J.T.; visualization, S.K., G.O., and J.T.; supervision, S.K., G.O., and J.T.; project administration, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Ethical Committee of Szent Margit Hospital (Approval number: IG/296-1/2025/2) on 4 February 2025. Additionally, the study was registered at ClinicalTrials.gov under the identifier NCT06848712 on 22 February 2025.
Informed Consent Statement
Patient consent was waived because this study is retrospective. The informed consent was waived in accordance with institutional guidelines. Data collection complied with GDPR (General Data Protection Regulation) and patient confidentiality regulations.
Data Availability Statement
Authors are committed to making the data available upon request.
Conflicts of Interest
The authors declare no conflicts of interest. The authors state that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Abbreviations
The following abbreviations are used in this manuscript:
| BPPV | Benign Paroxysmal Positional Vertigo |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| FLAIR | Fluid-Attenuated Inversion Recovery |
| DWI | Diffusion Weighted Imaging |
| SWI | Susceptibility Weighted Imaging |
| TOF | Time-of-Flight |
| TR | Repetition Time |
| ms | milliseconds |
| TE | Echo Time |
| TI | Inversion Time |
| FOV | Field of View |
| mm | millimeters |
| FS | Fat Saturation |
| min | minutes |
| NEX | Number of Excitations |
| s/mm2 | seconds per square millimeter |
| kHz | kilohertz |
| FSE | Fast Spin Echo |
| SE EPI | Spin Echo Echo Planar Imaging |
| GRE | Gradient Echo |
| SPGR | Spoiled Gradient Echo |
| FSPGR | Fast Spoiled Gradient Echo |
| FIESTA GRE | Fast Imaging Employing Steady-State Acquisition Gradient Echo |
| STRIVE | Standards for Reporting Vascular Changes on Neuroimaging |
| MRA | Magnetic Resonance Angiography |
| MSKT | Magyar Stroke Konszenzus Tanács (Hungarian Stroke Consensus Panel) |
| ACR | American College of Radiology |
| GDPR | General Data Protection Regulation |
| US | Ultrasound |
| EEG | Electroencephalogram |
| VBS | Vertebrobasilar Syndrome |
References
- Neuhauser, H.K. The epidemiology of dizziness and vertigo. Handb. Clin. Neurol. 2016, 137, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Forster, M.; Maihoub, S.; Tamás, L.; Szirmai, Á. Az időskori szédülés életminőségre kifejtett hatása. Orvosi Hetil. 2021, 162, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Bisdorff, A.; Von Brevern, M.; Lempert, T.; Newman-Toker, D.E. Classification of vestibular symptoms: Towards an international classification of vestibular disorders. J. Vestib. Res. 2009, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Edlow, J.A.; Newman-Toker, D. Using the Physical Examination to Diagnose Patients with Acute Dizziness and Vertigo. J. Emerg. Med. 2016, 50, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Colledge, N.; Lewis, S.; Mead, G.; Sellar, R.; Wardlaw, J.; Wilson, J. Magnetic resonance brain imaging in people with dizziness: A comparison with non-dizzy people. J. Neurol. Neurosurg. Psychiatry 2002, 72, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lin, C.; Sun, Y.; Shen, S.; Xiao, L.; Chen, Z.; Liu, Y.; Liu, T.; Rong, L. Altered cerebral gray matter volume and functional connectivity in patients with residual dizziness of benign paroxysmal positional vertigo. Clin. Radiol. 2024, 82, 106780. [Google Scholar] [CrossRef] [PubMed]
- Happonen, T.; Nyman, M.; Ylikotila, P.; Mattila, K.; Hirvonen, J. Imaging Outcomes of emergency MR imaging in dizziness and vertigo: A retrospective cohort study. Am. J. Neuroradiol. 2024, 45, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Schwedt, T.J.; Chong, C.D. Medication Overuse Headache: Pathophysiological Insights from Structural and Functional Brain MRI Research. Headache J. Head Face Pain 2017, 57, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.P.; Silva, L.O.J.E.; Farah, W.; Seisa, M.; Balla, A.K.; Christensen, A.; Farah, M.; Hasan, B.; Bellolio, F.; Murad, M.H. Diagnostic accuracy of neuroimaging in emergency department patients with acute vertigo or dizziness: A systematic review and meta-analysis for the guidelines for reasonable and appropriate care in the emergency department. Acad. Emerg. Med. 2022, 30, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Pasquier, F.; Weerts, J.G. Qualitative assessment of cerebral atrophy on MRI: Inter- and intra-observer reproductibility in dementia and normal aging. Eur. Neurol. 1997, 37, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed]
- European Stroke Organization (ESO). European Stroke Organization (ESO) Guidelines. Stroke Prevention and Management Recommendations; European Stroke Organization (ESO): Basel, Switzerland, 2020. [Google Scholar]
- Hungarian Stroke Consensus Council (MSKT). National Stroke Guidelines; Hungarian Stroke Consensus Council (MSKT): Budapest, Hungary, 2021. [Google Scholar]
- Naveen, S.R.; Bhat, V.; Karthik, G.A. Magnetic resonance angiographic evaluation of circle of Willis: A morphologic study in a tertiary hospital set up. Ann. Indian Acad. Neurol. 2015, 18, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Mackay, I.S. Scoring system for sinusitis assessment. Radiology 1993, 189, 393–398. [Google Scholar]
- Pollak, L.; Osherov, M.; Berkovitz, N.; Beckerman, I.; Stryjer, R.; Tal, S. Magnetic resonance brain imaging in patients with visual vertigo. Brain Behav. 2015, 5, e00402. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, S.; Raghvi, A.; Roselin, V.; Namasivaya, N.R.B.; Rajasekaran, S. Prevalence of Cervical Spondylosis among Cases with Vertigo in a Tertiary Care Center. Int. J. Recent Surg. Med. Sci. 2022, 9, 23–27. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Santina, C.C.D.; Blitz, A.M. Vertigo and Hearing Loss; Elsevier: Amsterdam, The Netherlands, 2016; p. 136. [Google Scholar]
- Whitman, G.T. Dizziness. Am. J. Med. 2018, 131, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Wassam, M.; Mari, H.; Vinh, N. Neuroimaging of Dizziness and Vertigo. Otolaryngol. Clin. N. Am. 2021, 54, 893–911. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, C.H. Vestibular Schwannoma Presenting as Acute Vertigo Mimicking Vestibular Neuritis. Case Rep. Neurol. 2022, 14, 464–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva Gameiro, B.; Silva Fonseca, A.C.; Guimarães, B.S.C.; Andrade, C.F.G.; Pereira, D.T.C.; Ferreira, R.I.M.; da Conceição Coelho Moreira, M. Betahistine in the treatment of peripheral vertigo: An evidence-based review. Egypt. J. Otolaryngol. 2024, 40, 108. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, X.; Yao, J.; Lu, W.; Zhu, Z. Mendelian Randomization Reveals Causal Links Between Vertigo Types and structural characteristics of specific brain regions. Res. Sq. 2024. [Google Scholar] [CrossRef]
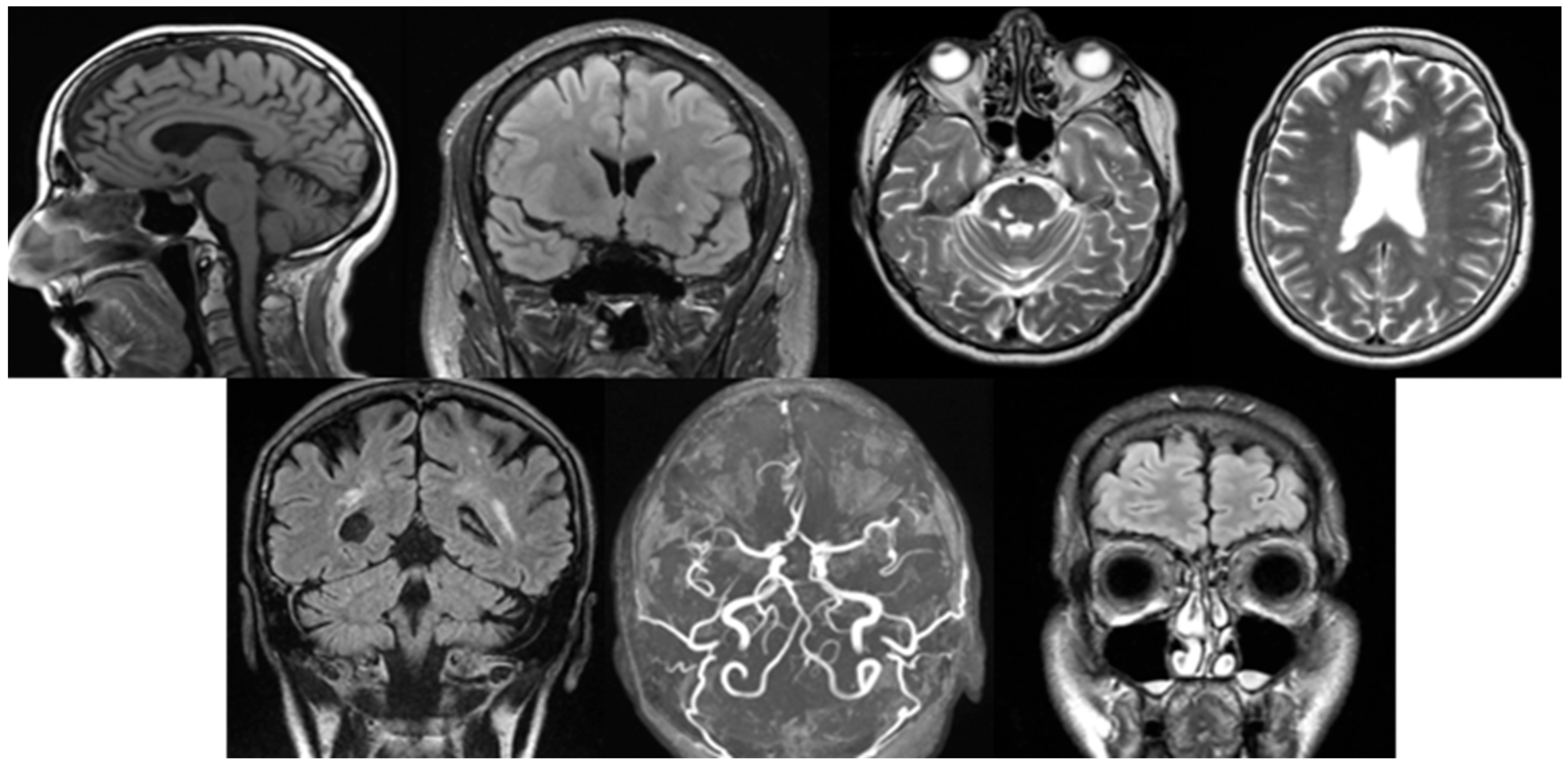
Figure 1.
From left to right in the first row are cortical atrophy (sagittal T1-weighted, cortical thickness and widening of the sulci), white matter lesion (coronal FLAIR, hyperintense periventricular changes), vascular lesions (axial T2-weighted, focal hyperintensity), and lacunar lesion (axial T2-weighted, hyperintense deep white matter changes), and in the second row are vascular encephalopathy (coronal FLAIR, diffuse white matter hyperintensities), circle of Willis variation (axial TOF-MRA, anatomical variation in the arterial configuration), sinusitis (coronal FLAIR, mucosal thickening and sinus opacification).
Figure 1.
From left to right in the first row are cortical atrophy (sagittal T1-weighted, cortical thickness and widening of the sulci), white matter lesion (coronal FLAIR, hyperintense periventricular changes), vascular lesions (axial T2-weighted, focal hyperintensity), and lacunar lesion (axial T2-weighted, hyperintense deep white matter changes), and in the second row are vascular encephalopathy (coronal FLAIR, diffuse white matter hyperintensities), circle of Willis variation (axial TOF-MRA, anatomical variation in the arterial configuration), sinusitis (coronal FLAIR, mucosal thickening and sinus opacification).
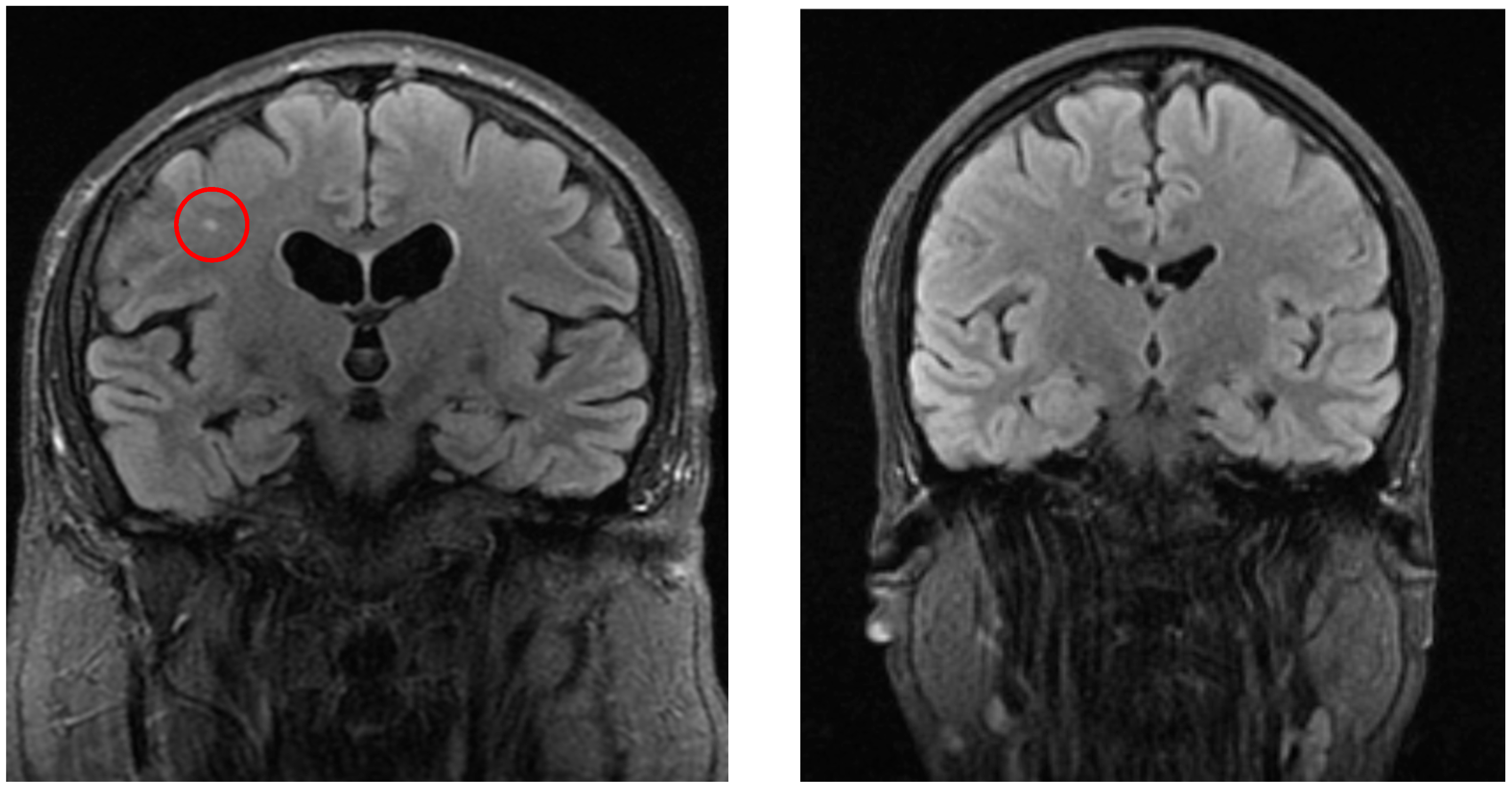
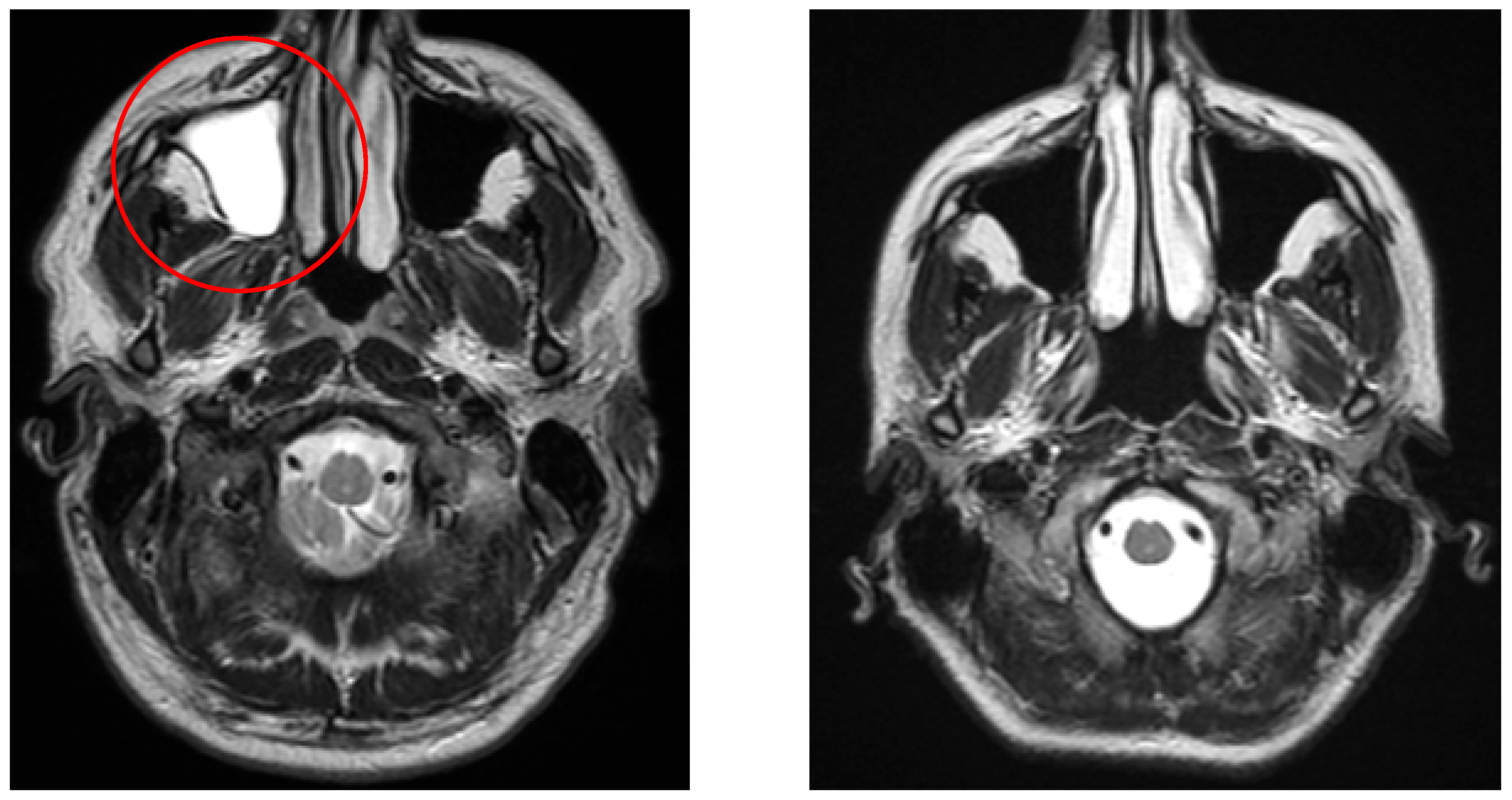
Figure 2.
The left image (coronal FLAIR) shows a white matter lesion (red circle) in a patient from the vertigo group, whereas the right image (coronal FLAIR) demonstrates a control patient without such lesions.
Figure 2.
The left image (coronal FLAIR) shows a white matter lesion (red circle) in a patient from the vertigo group, whereas the right image (coronal FLAIR) demonstrates a control patient without such lesions.
Figure 3.
The left image (axial T2-weighted) shows lacunar lesions (red circle) in a patient from the vertigo group, whereas the right image (axial T2-weighted) demonstrates a control patient without such lesions.
Figure 3.
The left image (axial T2-weighted) shows lacunar lesions (red circle) in a patient from the vertigo group, whereas the right image (axial T2-weighted) demonstrates a control patient without such lesions.
Figure 4.
The left image (axial TOF-MRA) shows a variation of the basilar artery in a patient from the vertigo group, whereas the right image (axial TOF-MRA) demonstrates a control patient with a normal Circle of Willis.
Figure 4.
The left image (axial TOF-MRA) shows a variation of the basilar artery in a patient from the vertigo group, whereas the right image (axial TOF-MRA) demonstrates a control patient with a normal Circle of Willis.
Figure 5.
The left image (axial T2-weighted) shows an extensive inflammation in the right maxillary sinus (red circle) in a patient from the vertigo group, whereas the right image (axial T2-weighted) demonstrates a control patient with a clear sinus.
Figure 5.
The left image (axial T2-weighted) shows an extensive inflammation in the right maxillary sinus (red circle) in a patient from the vertigo group, whereas the right image (axial T2-weighted) demonstrates a control patient with a clear sinus.
Table 1.
MRI pulse sequences parameters.
Table 1.
MRI pulse sequences parameters.
| | Sequence Type | TR 1 | TE 2 | TI 3 | FOV 4 | Matrix 5 | FS 6 |
Scan Time 7 |
NEX 8 |
b-Value 9 |
Bandwith 10 |
|---|
|
Sagittal T1
| FSE 11 | 577 | 12,34 | - | 240 | 512 × 512 | N | 3,5 | 31.25 | - | 1 |
|
Axial T2
| FSE | 7580 | 86.5 | - | 260 | 512 × 512 | N | 3.5 | 62.5 | - | 1.5 |
|
Coronal T2
| FLAIR | 9000 | 120 | 2580 | 240 | 256 × 256 | N | 3.5 | 70 | - | 1 |
|
Axial DWI
| SE EPI 12 | 7551 | 83.1 | - | 260 | 256 × 256 | N | 2.5 | 250 | 20/1000 | 1 |
|
Axial SWI
| GRE 13 | 73.7 | 47.07 | - | 260 | 512 × 512 | N | 3 | 41.67 | - | 1 |
|
Axial TOF
| SPGR 14 | 26 | 6.8 | - | 220 | 512 × 512 | Y | 3.5 | 250 | - | 1 |
|
+c Sagittal 3D T1
| FSPGR 15 | 6.884 | 2.06 | - | 240 | 512 × 512 | N | 3.5 | 83.33 | - | 1 |
|
Axial 3D FIESTA
| FIESTA GRE 16 | 6.90 | 2.54 | - | 270 | 512 × 512 | N | 3.5 | 125 | - | 1 |
Table 2.
Descriptive characteristics of the patients with vertigo.
Table 2.
Descriptive characteristics of the patients with vertigo.
| Variable | Count or Mean | ±SD |
|---|
| Number of subjects (males) | 232 (68) | |
| Age | 59.41 years | 17.43 years |
| Examinations preceding the MRI, n | | |
| Carotis/vertebralis US (Ultrasound) | 75 (32%) | |
| CT | 31 (13%) | |
| Cervical spine X-ray | 23 (9%) | |
| Other | 36 (15%) | |
| Other symptoms, n | | |
| Headache | 56 (24%) | |
| Imbalance | 36 (15%) | |
| Vomiting, nausea | 26 (11%) | |
| Tinnitus | 20 (9%) | |
| Psychological | 12 (5%) | |
| Other | 71 (31%) | |
| MRI results, n | | |
| White matte lesion | 91 (39%) | |
| Vascular encephalopathy | 73 (31%) | |
| Cortical atrophy | 60 (26%) | |
| Circle of Willis variation | 51 (22%) | |
| Sinusitis | 39 (17%) | |
| Lacunar lesion | 31 (13%) | |
| Vascular lesion | 13 (6%) | |
Table 3.
Descriptive characteristics of the control subjects.
Table 3.
Descriptive characteristics of the control subjects.
| Variable | Count or Mean | ±SD |
|---|
| Number of subjects (males) | 232 (76) | |
| Age | 56.77 years | 17.43 years |
| MRI results, n | | |
| Vascular encephalopathy | 62 (27%) | |
| White matte lesion | 58 (25%) | |
| Cortical atrophy | 52 (22%) | |
| Lacunar lesion | 47 (20%) | |
| Circle of Willis variation | 33 (14%) | |
| Sinusitis | 19 (8%) | |
| Vascular lesion | 8 (3, 4%) | |
Table 4.
Incidence of observed structural changes.
Table 4.
Incidence of observed structural changes.
| Results | Vertigo Group (%) | Control Group (%) |
|---|
| White matter lesion | 39.2 | 25 |
| Vascular encephalopathy | 31.5 | 26.7 |
| Cortical atrophy | 25.9 | 22.4 |
| Circle of Willis variation | 22 | 14.2 |
| Sinusitis | 16.8 | 8.2 |
| Lacunar lesion | 13.4 | 20.3 |
| Vascular lesion | 5.6 | 3.4 |
Table 5.
Examinations preceding the MRI.
Table 5.
Examinations preceding the MRI.
| Examinations | Number of Cases | Positive Cases |
|---|
| Carotis/vertebralis US | 75 | 34 (45%) |
| CT | 31 | 18 (58%) |
| Cervical spine X-ray | 23 | 21 (91%) |
| Other | 36 | 29 (81%) |
Table 6.
Other symptoms.
| Symptoms | % |
|---|
| Headache | 24.14 |
| Imbalance | 15.5 |
| Vomiting, nausea | 11.2 |
| Tinnitus | 8.6 |
| Psychological | 5.2 |
| Other | 30.6 |
Table 7.
Final diagnosis after MRI.
Table 7.
Final diagnosis after MRI.
| Final Diagnosis | % |
|---|
| BPPV | 34.5 |
| VBS | 11.6 |
| Psychological | 5.6 |
| Encephalopathy | 4.7 |
| Sclerosis | 2.6 |
| Brain infarction | 4.3 |
| Headache | 4.3 |
| Sinusitis | 2.2 |
| Central Balance Syndrome | 2.2 |
| Not specified | 19 |
| Other | 9.1 |
Table 8.
Treatments.
| Treatments | % | Treatments | % |
|---|
| Lifestyle advance | 21.1 | Anti-inflammatory | 2.2 |
| Physical training | 15.01 | Lipid-lowering medications | 2.2 |
| Cognitive enhancers | 10.3 | Antimigraine medications | 1.7 |
| Anticoagulant | 7.3 | Antiepileptics | 1.3 |
| Anxiolytics, hypnotics, antidepressants | 6.5 | Antiparkinson medications | 0.9 |
| Betahistin | 6 | Steroid | 0,4 |
| Herbal medicine | 3 | Other drugs | 6 |
| Vitamins and minerals | 3 | Other non—pharmacological | 5.17 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).