Deciphering the Structural Biology of GFAP: Connotations of Its Potency in Presaging the Diagnosis for Traumatic Brain Injury and AD
Abstract
1. Background
Introduction Section
2. Methodology
3. Discussion—Astrocytes in AD
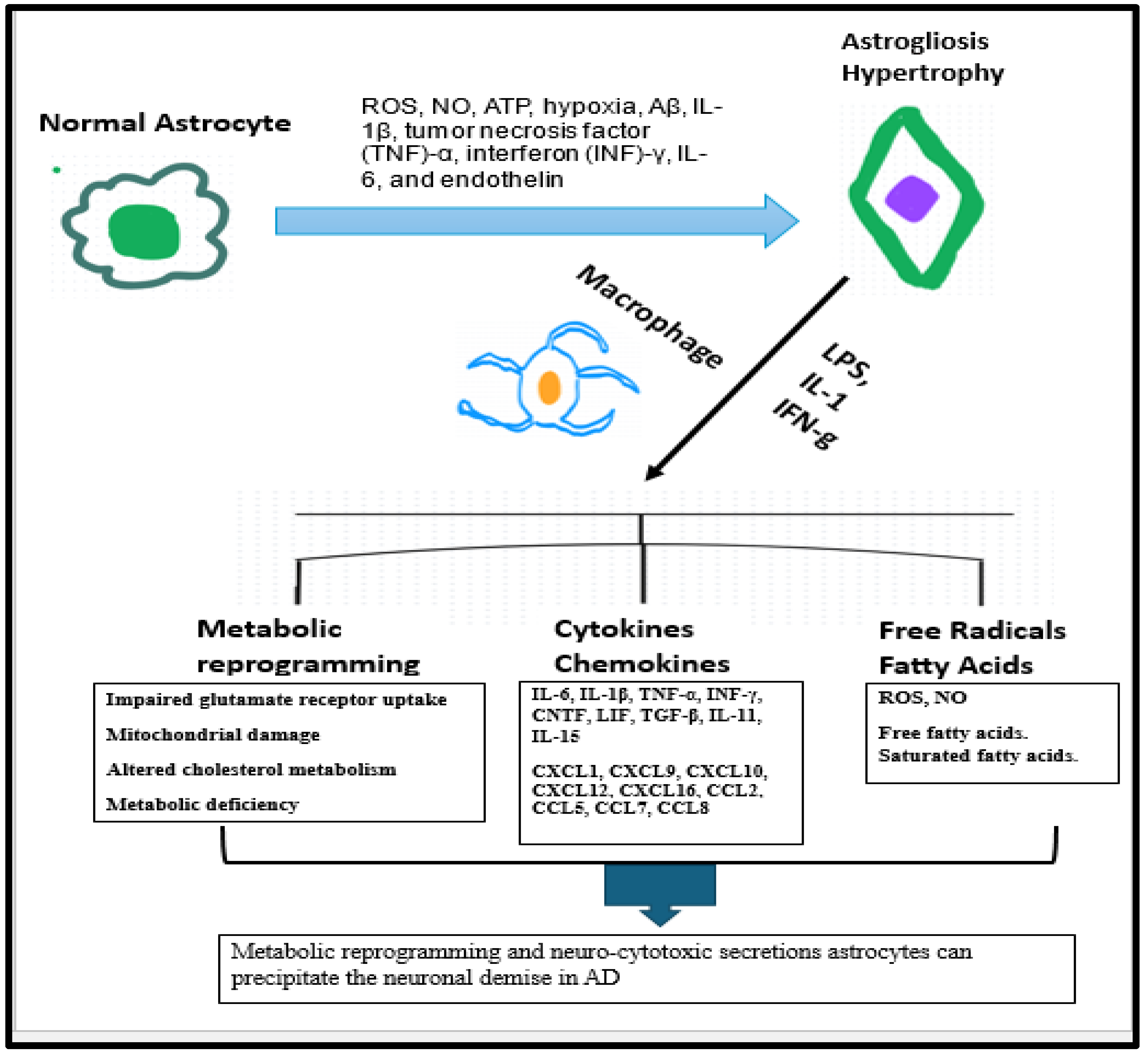
3.1. Astrocytes Are a Part of Innate Immune Response in AD
3.2. Astrogliosis Is an Innate Immune Response Secondary to Neuronal Death in AD
3.3. Astrogliosis Is a Primary Source for Assembly and Exudation of GFAP into the Systemic Circulation
4. Physiological Facets of GFAP
4.1. What Is This GFAP?
4.2. GFAP Expression
4.3. GFAP Structure
4.4. Physiological Functions of GFAP
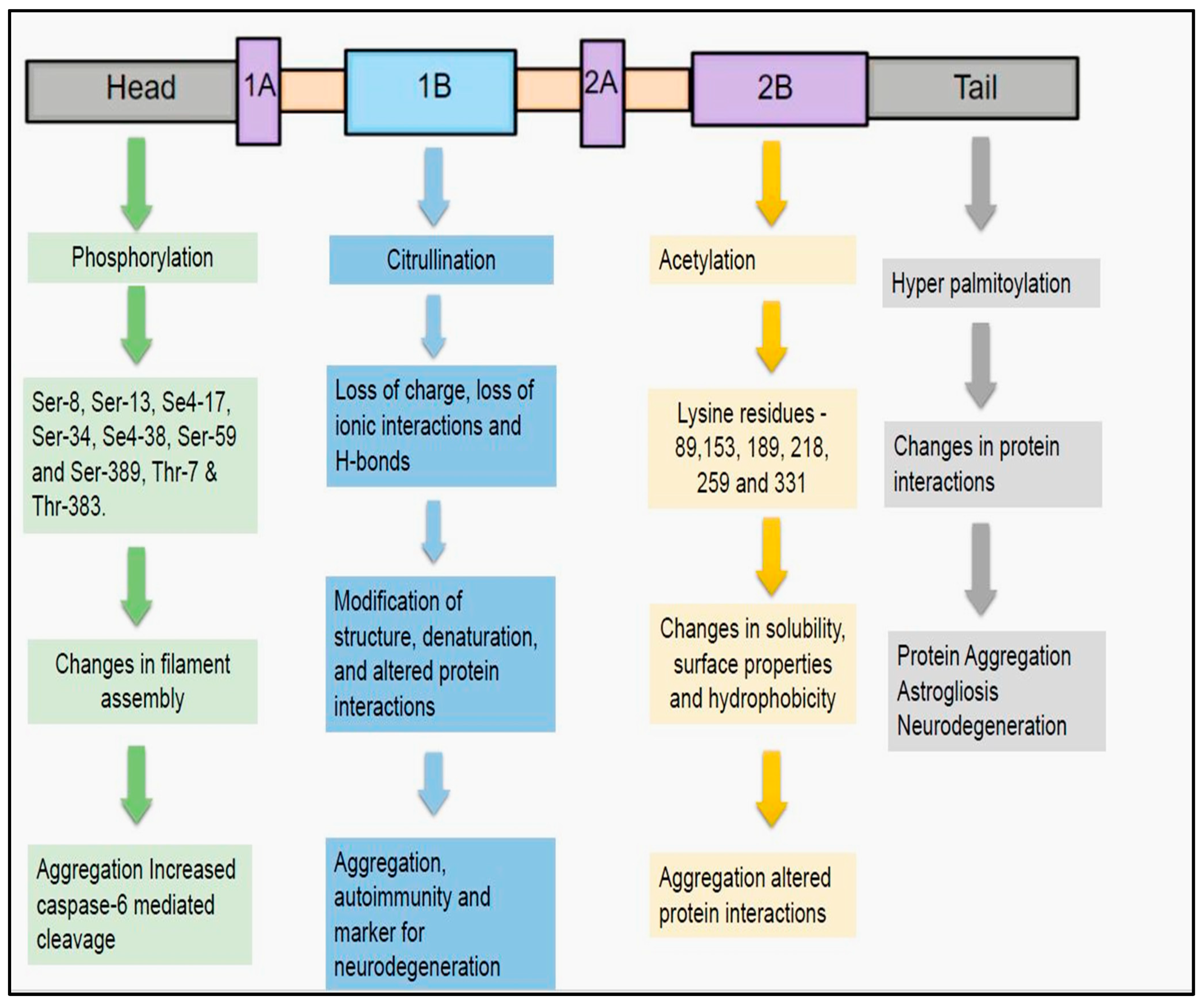
4.5. Post-Translational Modifications of GFAP
- Citrullination
- Acetylation
- Hyper-palmitoylation
4.6. How These Modifications Influence Detectability and Interpretation of GFAP Levels in CSF and Plasma
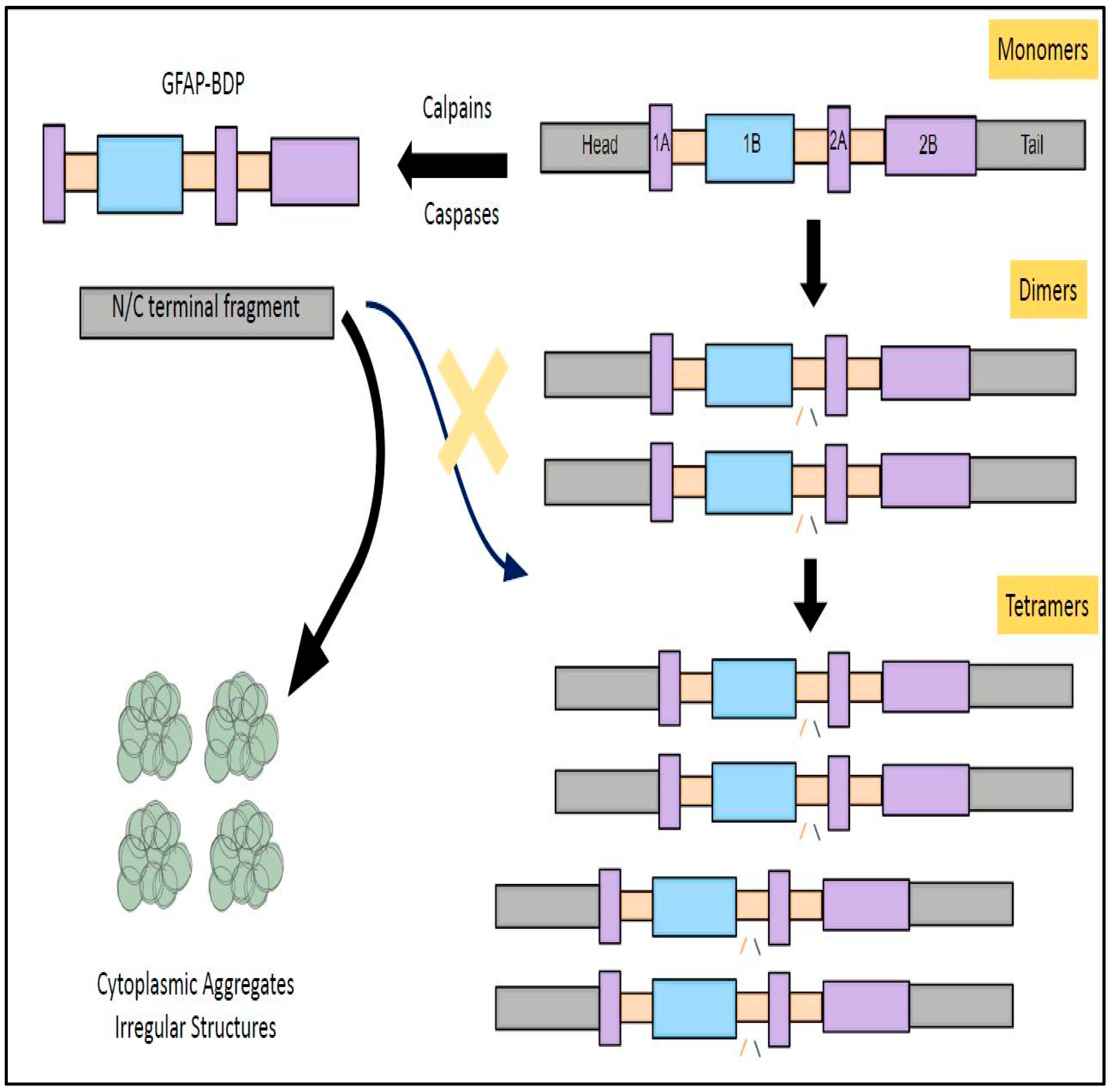
4.7. GFAP Cleavage into Subfractions and Its Clinical Significance
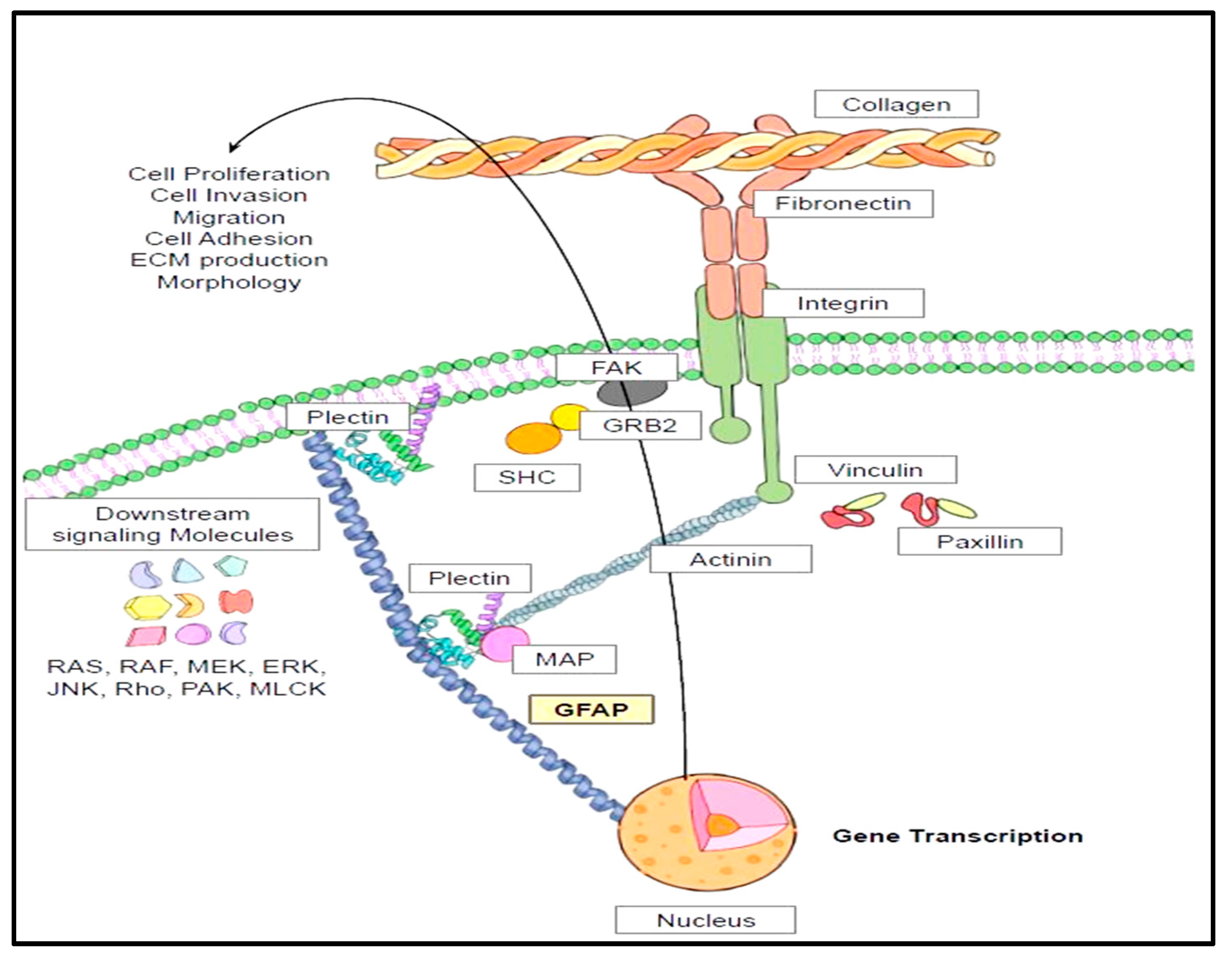
4.8. Cytoskeletal Interactions and Cell Signaling Pathways of GFAP
4.9. Half-Life and Clearance of GFAP
5. TBI and GFAP
5.1. TBI and Its Correlation with AD
5.2. Astrogliosis and TBI
5.3. GFAP and UCH-L1 (Ubiquitin C-Terminal Hydrolase-L1) Levels in TBI (Traumatic Brain Injury)
5.4. TBI Itself Might Derail the Release of Biomarkers into the Systemic Circulation
6. AD and GFAP
6.1. Astrocytic Damage in AD
6.2. GFAP as a Reliable Biomarker in Diagnosis and Prognosis of AD
S100 Implication in AD
6.3. Does GFAP Circumnavigate Seamlessly in AD Brain? Fallacies in Excretory Pathways
6.4. FDA-Approved Assay for the AD Diagnostics Focusing on Spatiotemporal GFAP Profiles in AD and TBI
7. Summary Points
- (a)
- Astrogliosis fomented fingerprint, GFAP, is a 50 kDa cytoskeletal protein whose gene can be traced to the chromosome 17.
- (b)
- From a structural standpoint, it is comprised of a head domain, rod domain, and tail domain.
- (c)
- The filamentous form of GFAP is influenced by the aggregation of individual monomers into dimers and tetramers.
- (d)
- Physiologically, there exists a dynamic equilibrium between assembly and disassembly of individual subunits.
- (e)
- GFAP participates in various physiological functions including having a trophic effect on neurons, BBB maintenance, myelination, synaptic plasticity, glutamine transport, and neuronal growth.
- (f)
- GFAP tends to exist in many isoforms. The principal isoform responsible for fostering many physiological functions in the brain is GFAP-α.
- (g)
- Under pathological conditions, GFAP mRNA can be jolted to endure alternative splicing, and this native mRNA eventually procreates to give birth to different isoforms namely GFAP-β, GFAP-γ, GFAP-δ, GFAP-κ, and GFAP-ζ.
- (h)
- The stability of GFAP filament is regulated by four types of post-translational modifications namely phosphorylation, citrullination, acetylation, and hyper-palmitoylation.
- (i)
- Unfortunately, GFAP (50 kDa) is assailable and vulnerable to degradation by calpain and caspases resulting in bringing forth petite fragments known as breakdown products (BDPs) with a lower molecular weight ranging from 26 to 44 kDa. These BDPs can fall outside of detection range and sometimes evade detection by standard antibody detection kits.
- (j)
- GFAP direct cytoskeletal interactions mainly with plectins and actin microfilaments foster indirect communications with integrins and extracellular matrix proteins. These interactions are decisive in executing cardinal cellular functions such as survival, proliferation, invasion, and migration.
- (k)
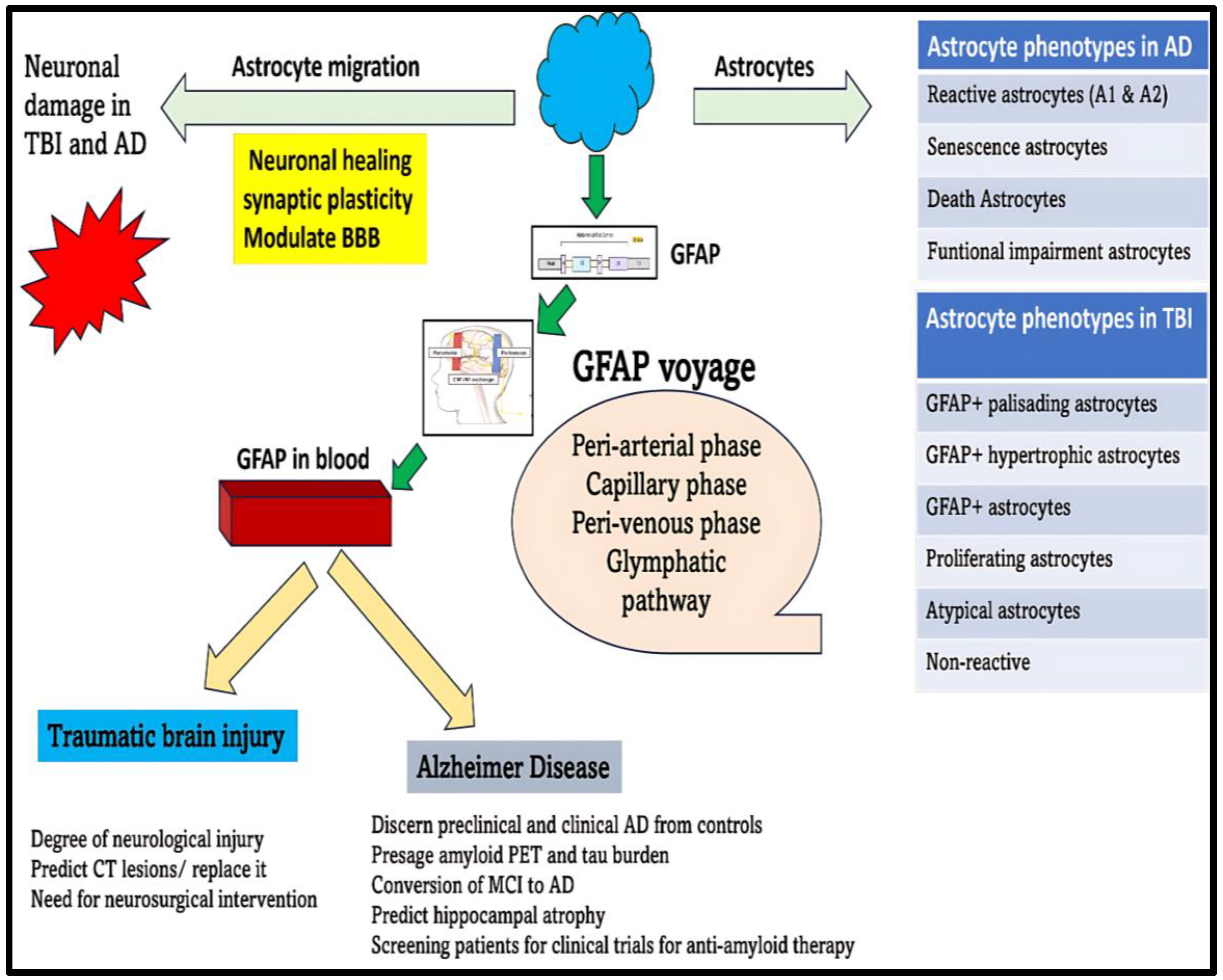
- GFAP can be a future blood-based biomarker that can reliably forecast brain damage and reduce the need for CT scans for diagnosis and prognosis of TBI, a factor making a huge difference in primary care centers (Figure 6).
- (l)
- GFAP can also perform as an excellent marker for auguring the diagnosis of preclinical AD, stockpiling of toxic aggregates, as well as disease progression in AD (Figure 6).
- (m)
- The potency of GFAP to function as a trustworthy marker in blood is primarily dependent on its successful escape from the brain tissues through the peri-arterial, peri-venous, and glymphatic pathways into the systemic circulation. Unfortunately, various quagmires due to TBI and AD, which might be operational, can curb its smooth transport into the blood. Understanding these potential fallibilities is essential before we relate the fluctuations of GFAP in the blood to disease changes in TBI and AD.
8. Future Directions
9. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [CrossRef]
- Hudomiet, P.; Hurd, M.D.; Rohwedder, S. Trends in inequalities in the prevalence of dementia in the United States. Proc. Natl. Acad. Sci. USA 2022, 119, e2212205119. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Mobley, W.C. Alzheimer Disease Pathogenesis: Insights from Molecular and Cellular Biology Studies of Oligomeric Aβ and Tau Species. Front. Neurosci. 2019, 13, 659. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Arriagada, P.V.; Growdon, J.H.; Hedley-Whyte, E.T.; Hyman, B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992, 42, 631–639. [Google Scholar] [CrossRef]
- Bierer, L.M.; Hof, P.R.; Purohit, D.P.; Carlin, L.; Schmeidler, J.; Davis, K.L.; Perl, D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch. Neurol. 1995, 52, 81–88. [Google Scholar] [CrossRef]
- Singh, V.; Chertkow, H.; Lerch, J.P.; Evans, A.C.; Dorr, A.E.; Kabani, N.J. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain 2006, 129, 2885–2893. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, H.M.; Yoon, B.E. Exploring glia to better understand Alzheimer’s disease. Anim. Cells Syst. 2018, 22, 213–218. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Duchen, M.R. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 2309–2314. [Google Scholar] [CrossRef]
- DeWitt, D.A.; Perry, G.; Cohen, M.; Doller, C.; Silver, J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer’s disease. Exp. Neurol. 1998, 149, 329–340. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef]
- Bezzi, P.; Domercq, M.; Brambilla, L.; Galli, R.; Schols, D.; De Clercq, E.; Vescovi, A.; Bagetta, G.; Kollias, G.; Meldolesi, J.; et al. CXCR4-activated astrocyte glutamate release via TNFα: Amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2001, 4, 702–710. [Google Scholar] [CrossRef]
- Singh, N.; Benoit, M.R.; Zhou, J.; Das, B.; Davila-Velderrain, J.; Kellis, M.; Tsai, L.H.; Hu, X.; Yan, R. BACE-1 inhibition facilitates the transition from homeostatic microglia to DAM-1. Sci. Adv. 2022, 8, eabo1286. [Google Scholar] [CrossRef]
- Zhou, J.; Singh, N.; Galske, J.; Hudobenko, J.; Hu, X.; Yan, R. BACE1 regulates expression of Clusterin in astrocytes for enhancing clearance of β-amyloid peptides. Mol. Neurodegener. 2023, 18, 31. [Google Scholar] [CrossRef]
- Kumar, A.; Fontana, I.C.; Nordberg, A. Reactive astrogliosis: A friend or foe in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2023, 164, 309–324. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Olabarria, M.; Chvatal, A.; Verkhratsky, A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. 2009, 16, 378–385. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S. GFAP and astrogliosis. Brain Pathol. 1994, 4, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-o.; Holtzman, D.M. Current understanding of the Alzheimer’s disease-associated microbiome and therapeutic strategies. Exp. Mol. Med. 2024, 56, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Shah, R.; Alford, N.; Mishra, S.P.; Jain, S.; Hansen, B.; Sanberg, P.; Molina, A.J.A.; Yadav, H. The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer’s Disease. J. Gerontol. Ser. A 2023, 78, 2187–2202. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Pathak, A.; Samaiya, P.K. Alzheimer’s disease: From early pathogenesis to novel therapeutic approaches. Metab. Brain Dis. 2024, 39, 1231–1254. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, I.D.; Lim, J.; Moon, J.-S. Pathological phenotypes of astrocytes in Alzheimer’s disease. Exp. Mol. Med. 2024, 56, 95–99. [Google Scholar] [CrossRef]
- Edison, P. Astroglial activation: Current concepts and future directions. Alzheimer’s Dement. 2024, 20, 3034–3053. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Beach, T.G.; Walker, R.; McGeer, E.G. Patterns of gliosis in Alzheimer’s disease and aging cerebrum. Glia 1989, 2, 420–436. [Google Scholar] [CrossRef]
- Beach, T.G.; McGeer, E. Lamina-specific arrangement of astrocytic gliosis and senile plaques in Alzheimer’s disease visual cortex. Brain Res. 1988, 463, 357–361. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V.; Rodriguez-Arellano, J.J.; Zorec, R. Astroglia in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 273–324. [Google Scholar]
- Bellaver, B.; Ferrari-Souza, J.P.; da Ros, L.U.; Carter, S.F.; Rodriguez-Vieitez, E.; Nordberg, A.; Pellerin, L.; Rosa-Neto, P.; Leffa, D.T.; Zimmer, E.R. Astrocyte biomarkers in Alzheimer disease: A systematic review and meta-analysis. Neurology 2021, 96, e2944–e2955. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a020420. [Google Scholar] [CrossRef]
- Chun, H.; Im, H.; Kang, Y.J.; Kim, Y.; Shin, J.H.; Won, W.; Lim, J.; Ju, Y.; Park, Y.M.; Kim, S.; et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2− production. Nat. Neurosci. 2020, 23, 1555–1566. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Loike, J.D.; Brionne, T.C.; Lu, E.; Anankov, R.; Yan, F.; Silverstein, S.C.; Husemann, J. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat. Med. 2003, 9, 453–457. [Google Scholar] [CrossRef]
- Jiang, Q.; Lee, C.D.; Mandrekar, S.; Wilkinson, B.; Cramer, P.; Zelcer, N.; Mann, K.; Lamb, B.; Willson, T.M.; Collins, J.L. ApoE promotes the proteolytic degradation of Aβ. Neuron 2008, 58, 681–693. [Google Scholar] [CrossRef]
- Apelt, J.; Ach, K.; Schliebs, R. Aging-related down-regulation of neprilysin, a putative β-amyloid-degrading enzyme, in transgenic Tg2576 Alzheimer-like mouse brain is accompanied by an astroglial upregulation in the vicinity of β-amyloid plaques. Neurosci. Lett. 2003, 339, 183–186. [Google Scholar] [CrossRef]
- Diedrich, J.; Wietgrefe, S.; Zupancic, M.; Staskus, K.; Retzel, E.; Haase, A.T.; Race, R. The molecular pathogenesis of astrogliosis in scrapie and Alzheimer’s disease. Microb. Pathog. 1987, 2, 435–442. [Google Scholar] [CrossRef]
- Kamphuis, W.; Mamber, C.; Moeton, M.; Kooijman, L.; Sluijs, J.A.; Jansen, A.H.P.; Verveer, M.; de Groot, L.R.; Smith, V.D.; Rangarajan, S.; et al. GFAP Isoforms in Adult Mouse Brain with a Focus on Neurogenic Astrocytes and Reactive Astrogliosis in Mouse Models of Alzheimer Disease. PLoS ONE 2012, 7, e42823. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Brahmachari, S.; Fung, Y.K.; Pahan, K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J. Neurosci. 2006, 26, 4930–4939. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Eng, L.F.; Vanderhaeghen, J.; Bignami, A.; Gerstl, B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971, 28, 351–354. [Google Scholar] [CrossRef]
- Rutka, J.T.; Murakami, M.; Dirks, P.B.; Hubbard, S.L.; Becker, L.E.; Fukuyama, K.; Jung, S.; Tsugu, A.; Matsuzawa, K. Role of glial filaments in cells and tumors of glial origin: A review. J. Neurosurg. 1997, 87, 420–430. [Google Scholar] [CrossRef]
- Russell, D.; Rubinstein, L. Pathology of Tumours of the Nervous System; Williams & Wilkins: Philadelphia, PA, USA, 1989. [Google Scholar]
- Ralton, J.E.; Lu, X.; Hutcheson, A.M.; Quinlan, R.A. Identification of two N-terminal non-alpha-helical domain motifs important in the assembly of glial fibrillary acidic protein. J. Cell Sci. 1994, 107, 1935–1948. [Google Scholar] [CrossRef]
- Rodnight, R.; Goncalves, C.; Wofchuk, S.; Leal, R. Control of the phosphorylation of the astrocyte marker glial fibrillary acidic protein (GFAP) in the immature rat hippocampus by glutamate and calcium ions: Possible key factor in astrocytic plasticity. Braz. J. Med. Biol. Res. 1997, 30, 325–338. [Google Scholar] [CrossRef]
- Yang, A.-W.; Lin, N.-H.; Yeh, T.-H.; Snider, N.; Perng, M.-D. Effects of Alexander disease–associated mutations on the assembly and organization of GFAP intermediate filaments. Mol. Biol. Cell 2022, 33, ar69. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takeda, M.; Angelides, K.J.; Tada, K.; Hariguchi, S.; Nishimura, T. Assembly, disassembly, and exchange of glial fibrillary acidic protein. Glia 1991, 4, 101–110. [Google Scholar] [CrossRef]
- Inagaki, M.; Nakamura, Y.; Takeda, M.; Nishimura, T.; Inagaki, N. Glial fibrillary acidic protein: Dynamic property and regulation by phosphorylation. Brain Pathol. 1994, 4, 239–243. [Google Scholar] [CrossRef]
- Chen, W.J.; Liem, R.K. The endless story of the glial fibrillary acidic protein. J. Cell Sci. 1994, 107 Pt 8, 2299–2311. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lim, S.C.; Chen, M.H.; Quinlan, R.A.; Perng, M.D. Alexander disease causing mutations in the C-terminal domain of GFAP are deleterious both to assembly and network formation with the potential to both activate caspase 3 and decrease cell viability. Exp. Cell Res. 2011, 317, 2252–2266. [Google Scholar] [CrossRef]
- McKeon, A.; Benarroch, E.E. Glial fibrillary acid protein. Funct. Involv. Dis. 2018, 90, 925–930. [Google Scholar] [CrossRef]
- Liedtke, W.; Edelmann, W.; Bieri, P.L.; Chiu, F.-C.; Cowan, N.J.; Kucherlapati, R.; Raine, C.S. GFAP Is Necessary for the Integrity of CNS White Matter Architecture and Long-Term Maintenance of Myelination. Neuron 1996, 17, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Inagaki, M.; Gonda, Y.; Nishizawa, K.; Kitamura, S.; Sato, C.; Ando, S.; Tanabe, K.; Kikuchi, K.; Tsuiki, S.; Nishi, Y. Phosphorylation sites linked to glial filament disassembly in vitro locate in a non-alpha-helical head domain. J. Biol. Chem. 1990, 265, 4722–4729. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takeda, M.; Aimoto, S.; Hojo, H.; Takao, T.; Shimonishi, Y.; Hariguchi, S.; Nishimura, T. Assembly regulatory domain of glial fibrillary acidic protein. A single phosphorylation diminishes its assembly-accelerating property. J. Biol. Chem. 1992, 267, 23269–23274. [Google Scholar] [CrossRef]
- Almazan, G.; Afar, D.E.; Bell, J.C. Phosphorylation and disruption of intermediate filament proteins in oligodendrocyte precursor cultures treated with calyculin A. J. Neurosci. Res. 1993, 36, 163–172. [Google Scholar] [CrossRef]
- Battaglia, R.A.; Beltran, A.S.; Delic, S.; Dumitru, R.; Robinson, J.A.; Kabiraj, P.; Herring, L.E.; Madden, V.J.; Ravinder, N.; Willems, E.; et al. Site-specific phosphorylation and caspase cleavage of GFAP are new markers of Alexander disease severity. eLife 2019, 8, e47789. [Google Scholar] [CrossRef]
- Jin, Z.; Fu, Z.; Yang, J.; Troncosco, J.; Everett, A.D.; Van Eyk, J.E. Identification and characterization of citrulline-modified brain proteins by combining HCD and CID fragmentation. Proteomics 2013, 13, 2682–2691. [Google Scholar] [CrossRef]
- György, B.; Tóth, E.; Tarcsa, E.; Falus, A.; Buzás, E.I. Citrullination: A posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef]
- Tarcsa, E.; Marekov, L.N.; Mei, G.; Melino, G.; Lee, S.-C.; Steinert, P.M. Protein Unfolding by Peptidylarginine Deiminase: Substrate Specificity and Structural Relationships of the Natural Substrates Trichohyalin and Filaggrin. J. Biol. Chem. 1996, 271, 30709–30716. [Google Scholar] [CrossRef]
- Romero, V.; Fert-Bober, J.; Nigrovic, P.; Darrah, E.; Haque, U.; Lee, D.; Eyk, J.; Rosen, A.; Andrade, F. Immune-Mediated Pore-Forming Pathways Induce Cellular Hypercitrullination and Generate Citrullinated Autoantigens in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 209ra150. [Google Scholar] [CrossRef]
- Zhang, Z.; Zoltewicz, J.S.; Mondello, S.; Newsom, K.J.; Yang, Z.; Yang, B.; Kobeissy, F.; Guingab, J.; Glushakova, O.; Robicsek, S.; et al. Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS ONE 2014, 9, e92698. [Google Scholar] [CrossRef]
- Vincent, S.R.; Leung, E.; Watanabe, K. Immunohistochemical localization of peptidylarginine deiminase in the rat brain. J. Chem. Neuroanat. 1992, 5, 159–168. [Google Scholar] [CrossRef]
- Inagaki, M.; Takahara, H.; Nishi, Y.; Sugawara, K.; Sato, C. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J. Biol. Chem. 1989, 264, 18119–18127. [Google Scholar] [CrossRef]
- Asaga, H.; Ishigami, A. Protein deimination in the rat brain: Generation of citrulline-containing proteins in cerebrum perfused with oxygen-deprived media. Biomed. Res. 2000, 21, 197–205. [Google Scholar] [CrossRef]
- Nicholas, A.P.; Sambandam, T.; Echols, J.D.; Barnum, S.R. Expression of citrullinated proteins in murine experimental autoimmune encephalomyelitis. J. Comp. Neurol. 2005, 486, 254–266. [Google Scholar] [CrossRef]
- Ishigami, A.; Ohsawa, T.; Hiratsuka, M.; Taguchi, H.; Kobayashi, S.; Saito, Y.; Murayama, S.; Asaga, H.; Toda, T.; Kimura, N. Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer’s disease. J. Neurosci. Res. 2005, 80, 120–128. [Google Scholar] [CrossRef]
- Drazic, A.; Myklebust, L.M.; Ree, R.; Arnesen, T. The world of protein acetylation. Biochim. Biophys. Acta (BBA—Proteins Proteom.) 2016, 1864, 1372–1401. [Google Scholar] [CrossRef]
- Christensen, D.; Xie, X.; Basisty, N.; Byrnes, J.; McSweeney, S.; Schilling, B.; Wolfe, A. Post-translational Protein Acetylation: An Elegant Mechanism for Bacteria to Dynamically Regulate Metabolic Functions. Front. Microbiol. 2019, 10, 1604. [Google Scholar] [CrossRef]
- Liu, D.; Liu, C.; Li, J.; Azadzoi, K.; Yang, Y.; Fei, Z.; Dou, K.; Kowall, N.W.; Choi, H.-P.; Vieira, F.; et al. Proteomic Analysis Reveals Differentially Regulated Protein Acetylation in Human Amyotrophic Lateral Sclerosis Spinal Cord. PLoS ONE 2013, 8, e80779. [Google Scholar] [CrossRef]
- Kanski, R.; Sneeboer, M.A.; van Bodegraven, E.J.; Sluijs, J.A.; Kropff, W.; Vermunt, M.W.; Creyghton, M.P.; De Filippis, L.; Vescovi, A.; Aronica, E.; et al. Histone acetylation in astrocytes suppresses GFAP and stimulates a reorganization of the intermediate filament network. J. Cell Sci. 2014, 127 Pt 20, 4368–4380. [Google Scholar] [CrossRef]
- Lin, D.T.S.; Davis, N.G.; Conibear, E. Targeting the Ras palmitoylation/depalmitoylation cycle in cancer. Biochem. Soc. Trans. 2017, 45, 913–921. [Google Scholar] [CrossRef]
- Salaun, C.; Greaves, J.; Chamberlain, L.H. The intracellular dynamic of protein palmitoylation. J. Cell Biol. 2010, 191, 1229–1238. [Google Scholar] [CrossRef]
- Sadeghi, R.S.; Kulej, K.; Kathayat, R.S.; Garcia, B.A.; Dickinson, B.C.; Brady, D.C.; Witze, E.S. Wnt5a signaling induced phosphorylation increases APT1 activity and promotes melanoma metastatic behavior. eLife 2018, 7, e34362. [Google Scholar] [CrossRef]
- Sharma, C.; Wang, H.-X.; Li, Q.; Knoblich, K.; Reisenbichler, E.S.; Richardson, A.L.; Hemler, M.E. Protein Acyltransferase DHHC3 Regulates Breast Tumor Growth, Oxidative Stress, and Senescence. Cancer Res. 2017, 77, 6880–6890. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Barren, C.; Kovacs, D.M. Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. J. Neurosci. 2013, 33, 11169–11183. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Fenn, R.H.; Barren, C.; Tanzi, R.E.; Kovacs, D.M. Palmitoylated APP Forms Dimers, Cleaved by BACE1. PLoS ONE 2016, 11, e0166400. [Google Scholar] [CrossRef]
- Andrew, R.J.; Fernandez, C.G.; Stanley, M.; Jiang, H.; Nguyen, P.; Rice, R.C.; Buggia-Prévot, V.; De Rossi, P.; Vetrivel, K.S.; Lamb, R.; et al. Lack of BACE1 S-palmitoylation reduces amyloid burden and mitigates memory deficits in transgenic mouse models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, E9665–E9674. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, L.; Rao, M.; Huang, Y.; Liu, C.-e.; Liu, S.; Zhao, Y.; Liu, H.; Zhu, J.; Chao, T.; et al. GFAP hyperpalmitoylation exacerbates astrogliosis and neurodegenerative pathology in PPT1-deficient mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2022261118. [Google Scholar] [CrossRef]
- Gogishvili, D.; Honey, M.I.J.; Verberk, I.M.W.; Vermunt, L.; Hol, E.M.; Teunissen, C.E.; Abeln, S. The GFAP proteoform puzzle: How to advance GFAP as a fluid biomarker in neurological diseases. J. Neurochem. 2025, 169, e16226. [Google Scholar] [CrossRef]
- Yang, Z.; Arja, R.D.; Zhu, T.; Sarkis, G.A.; Patterson, R.L.; Romo, P.; Rathore, D.S.; Moghieb, A.; Abbatiello, S.; Robertson, C.S.; et al. Characterization of Calpain and Caspase-6-Generated Glial Fibrillary Acidic Protein Breakdown Products Following Traumatic Brain Injury and Astroglial Cell Injury. Int. J. Mol. Sci. 2022, 23, 8960. [Google Scholar] [CrossRef] [PubMed]
- Zoltewicz, J.S.; Mondello, S.; Yang, B.; Newsom, K.J.; Kobeissy, F.; Yao, C.; Lu, X.C.; Dave, J.R.; Shear, D.A.; Schmid, K.; et al. Biomarkers track damage after graded injury severity in a rat model of penetrating brain injury. J. Neurotrauma 2013, 30, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Guingab-Cagmat, J.D.; Newsom, K.; Vakulenko, A.; Cagmat, E.B.; Kobeissy, F.H.; Zoltewicz, S.; Wang, K.K.; Anagli, J. In vitro MS-based proteomic analysis and absolute quantification of neuronal-glial injury biomarkers in cell culture system. Electrophoresis 2012, 33, 3786–3797. [Google Scholar] [CrossRef]
- Zoltewicz, J.S.; Scharf, D.; Yang, B.; Chawla, A.; Newsom, K.J.; Fang, L. Characterization of antibodies that detect human GFAP after traumatic brain injury. Biomark. Insights 2012, 7, 71–79. [Google Scholar] [CrossRef]
- Papa, L.; Lewis, L.M.; Falk, J.L.; Zhang, Z.; Silvestri, S.; Giordano, P.; Brophy, G.M.; Demery, J.A.; Dixit, N.K.; Ferguson, I.; et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 2012, 59, 471–483. [Google Scholar] [CrossRef]
- Okonkwo, D.O.; Yue, J.K.; Puccio, A.M.; Panczykowski, D.M.; Inoue, T.; McMahon, P.J.; Sorani, M.D.; Yuh, E.L.; Lingsma, H.F.; Maas, A.I.; et al. GFAP-BDP as an acute diagnostic marker in traumatic brain injury: Results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J. Neurotrauma 2013, 30, 1490–1497. [Google Scholar] [CrossRef]
- Fujita, K.; Kato, T.; Yamauchi, M.; Ando, M.; Honda, M.; Nagata, Y. Increases in fragmented glial fibrillary acidic protein levels in the spinal cords of patients with amyotrophic lateral sclerosis. Neurochem. Res. 1998, 23, 169–174. [Google Scholar] [CrossRef]
- Boutté, A.M.; Deng-Bryant, Y.; Johnson, D.; Tortella, F.C.; Dave, J.R.; Shear, D.A.; Schmid, K.E. Serum Glial Fibrillary Acidic Protein Predicts Tissue Glial Fibrillary Acidic Protein Break-Down Products and Therapeutic Efficacy after Penetrating Ballistic-Like Brain Injury. J. Neurotrauma 2016, 33, 147–156. [Google Scholar] [CrossRef]
- Pouw, M.H.; Hosman, A.J.F.; van Middendorp, J.J.; Verbeek, M.M.; Vos, P.E.; van de Meent, H. Biomarkers in spinal cord injury. Spinal Cord. 2009, 47, 519–525. [Google Scholar] [CrossRef]
- Fujita, K.; Yamauchi, M.; Matsui, T.; Titani, K.; Takahashi, H.; Kato, T.; Isomura, G.; Ando, M.; Nagata, Y. Increase of glial fibrillary acidic protein fragments in the spinal cord of motor neuron degeneration mutant mouse. Brain Res. 1998, 785, 31–40. [Google Scholar] [CrossRef]
- Mouser, P.E.; Head, E.; Ha, K.H.; Rohn, T.T. Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer’s disease brain. Am. J. Pathol. 2006, 168, 936–946. [Google Scholar] [CrossRef]
- Chen, M.H.; Hagemann, T.L.; Quinlan, R.A.; Messing, A.; Perng, M.D. Caspase cleavage of GFAP produces an assembly-compromised proteolytic fragment that promotes filament aggregation. ASN Neuro 2013, 5, e00125. [Google Scholar] [CrossRef]
- Tzeng, T.-T.; Tsay, H.-J.; Chang, L.; Hsu, C.-L.; Lai, T.-H.; Huang, F.-L.; Shiao, Y.-J. Caspase 3 involves in neuroplasticity, microglial activation and neurogenesis in the mice hippocampus after intracerebral injection of kainic acid. J. Biomed. Sci. 2013, 20, 90. [Google Scholar] [CrossRef]
- Rohn, T.T.; Mouser, P. [P-182]: Caspase cleavage of GFAP within degenerating astrocytes may lead to a compromised blood-brain-barrier in the Alzheimer’s disease brain. Alzheimer’s Dement. 2005, 1, S66. [Google Scholar] [CrossRef]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Seifert, G.J.; Lawson, D.; Wiche, G. Immunolocalization of the intermediate filament-associated protein plectin at focal contacts and actin stress fibers. Eur. J. Cell Biol. 1992, 59, 138–147. [Google Scholar]
- Yousefi, H.; Vatanmakanian, M.; Mahdiannasser, M.; Mashouri, L.; Alahari, N.V.; Monjezi, M.R.; Ilbeigi, S.; Alahari, S.K. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene 2021, 40, 1043–1063. [Google Scholar] [CrossRef]
- Schwartz, M.A. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2010, 2, a005066. [Google Scholar] [CrossRef]
- Guan, J.L. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life 2010, 62, 268–276. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Chastney, M.R.; Conway, J.R.W.; Ivaska, J. Integrin adhesion complexes. Curr. Biol. 2021, 31, R536–R542. [Google Scholar] [CrossRef]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef]
- Price, J.C.; Guan, S.; Burlingame, A.; Prusiner, S.B.; Ghaemmaghami, S. Analysis of proteome dynamics in the mouse brain. Proc. Natl. Acad. Sci. USA 2010, 107, 14508–14513. [Google Scholar] [CrossRef]
- DeArmond, S.J.; Lee, Y.L.; Kretzschmar, H.A.; Eng, L.F. Turnover of glial filaments in mouse spinal cord. J. Neurochem. 1986, 47, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Rolland, B.; Le Prince, G.; Fages, C.; Nunez, J.; Tardy, M. GFAP turnover during astroglial proliferation and differentiation. Brain Res. Dev. Brain Res. 1990, 56, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.C.; Goldman, J.E. Synthesis and turnover of cytoskeletal proteins in cultured astrocytes. J. Neurochem. 1984, 42, 166–174. [Google Scholar] [CrossRef]
- Morrison, R.S.; De Vellis, J.; Lee, Y.L.; Bradshaw, R.A.; Eng, L.F. Hormones and growth factors induce the synthesis of glial fibrillary acidic protein in rat brain astrocytes. J. Neurosci. Res. 1985, 14, 167–176. [Google Scholar] [CrossRef]
- Papa, L.; Brophy, G.M.; Welch, R.D.; Lewis, L.M.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Lopez, M.A.; Haeussler, C.A.; Mendez Giordano, D.I.; et al. Time Course and Diagnostic Accuracy of Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 in a Large Cohort of Trauma Patients with and Without Mild Traumatic Brain Injury. JAMA Neurol. 2016, 73, 551–560. [Google Scholar] [CrossRef]
- Smith, R.; Chepisheva, M.; Cronin, T.; Seemungal, B.M. Chapter 16—Diagnostic Approaches Techniques in Concussion/Mild Traumatic Brain Injury: Where are we? In Neurosensory Disorders in Mild Traumatic Brain Injury; Hoffer, M.E., Balaban, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 247–277. [Google Scholar]
- Messing, A. Refining the concept of GFAP toxicity in Alexander disease. J. Neurodev. Disord. 2019, 11, 27. [Google Scholar] [CrossRef]
- Moody, L.R.; Barrett-Wilt, G.A.; Sussman, M.R.; Messing, A. Glial fibrillary acidic protein exhibits altered turnover kinetics in a mouse model of Alexander disease. J. Biol. Chem. 2017, 292, 5814–5824. [Google Scholar] [CrossRef]
- Prins, M.; Greco, T.; Alexander, D.; Giza, C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Model Mech. 2013, 6, 1307–1315. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Turner, R.C.; Logsdon, A.F.; Bailes, J.E.; Huber, J.D.; Rosen, C.L. Linking traumatic brain injury to chronic traumatic encephalopathy: Identification of potential mechanisms leading to neurofibrillary tangle development. J. Neurotrauma 2014, 31, 1129–1138. [Google Scholar] [CrossRef]
- Delic, V.; Beck, K.D.; Pang, K.C.H.; Citron, B.A. Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol. Commun. 2020, 8, 45. [Google Scholar] [CrossRef]
- Gardner, R.C.; Burke, J.F.; Nettiksimmons, J.; Kaup, A.; Barnes, D.E.; Yaffe, K. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol. 2014, 71, 1490–1497. [Google Scholar] [CrossRef]
- Fann, J.R.; Ribe, A.R.; Pedersen, H.S.; Fenger-Grøn, M.; Christensen, J.; Benros, M.E.; Vestergaard, M. Long-term risk of dementia among people with traumatic brain injury in Denmark: A population-based observational cohort study. Lancet Psychiatry 2018, 5, 424–431. [Google Scholar] [CrossRef]
- Barnes, D.E.; Kaup, A.; Kirby, K.A.; Byers, A.L.; Diaz-Arrastia, R.; Yaffe, K. Traumatic brain injury and risk of dementia in older veterans. Neurology 2014, 83, 312–319. [Google Scholar] [CrossRef]
- Guo, Z.; Cupples, L.A.; Kurz, A.; Auerbach, S.H.; Volicer, L.; Chui, H.; Green, R.C.; Sadovnick, A.D.; Duara, R.; DeCarli, C.; et al. Head injury and the risk of AD in the MIRAGE study. Neurology 2000, 54, 1316–1323. [Google Scholar] [CrossRef]
- O’Meara, E.S.; Kukull, W.A.; Sheppard, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Pfanschmidt, M.; Thompson, J.D.; Schellenberg, G.D.; Larson, E.B. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am. J. Epidemiol. 1997, 146, 373–384. [Google Scholar] [CrossRef]
- Mielke, M.M.; Ransom, J.E.; Mandrekar, J.; Turcano, P.; Savica, R.; Brown, A.W. Traumatic Brain Injury and Risk of Alzheimer’s Disease and Related Dementias in the Population. J. Alzheimers Dis. 2022, 88, 1049–1059. [Google Scholar] [CrossRef]
- Graves, A.B.; White, E.; Koepsell, T.D.; Reifler, B.V.; van Belle, G.; Larson, E.B.; Raskind, M. The association between head trauma and Alzheimer’s disease. Am. J. Epidemiol. 1990, 131, 491–501. [Google Scholar] [CrossRef]
- Wu, D.; Kumal, J.P.P.; Lu, X.; Li, Y.; Mao, D.; Tang, X.; Nie, M.; Liu, X.; Sun, L.; Liu, B.; et al. Traumatic Brain Injury Accelerates the Onset of Cognitive Dysfunction and Aggravates Alzheimer’s-Like Pathology in the Hippocampus by Altering the Phenotype of Microglia in the APP/PS1 Mouse Model. Front. Neurol. 2021, 12, 666430. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G., 3rd; Zhao, J.; Dash, P.K.; Soto, C.; Moreno-Gonzalez, I. Traumatic Brain Injury Induces Tau Aggregation and Spreading. J. Neurotrauma 2020, 37, 80–92. [Google Scholar] [CrossRef]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016, 275 Pt 3, 305–315. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 6418. [Google Scholar] [CrossRef]
- Ben-Gigi, L.; Sweetat, S.; Besser, E.; Fellig, Y.; Wiederhold, T.; Polakiewicz, R.D.; Behar, O. Astrogliosis Induced by Brain Injury Is Regulated by Sema4B Phosphorylation. Eneuro 2015, 2, ENEURO.0078-0014.2015. [Google Scholar] [CrossRef]
- Muñoz-Ballester, C.; Robel, S. Astrocyte-mediated mechanisms contribute to traumatic brain injury pathology. WIREs Mech. Dis. 2023, 15, e1622. [Google Scholar] [CrossRef]
- Shandra, O.; Winemiller, A.R.; Heithoff, B.P.; Munoz-Ballester, C.; George, K.K.; Benko, M.J.; Zuidhoek, I.A.; Besser, M.N.; Curley, D.E.; III, G.F.E.; et al. Repetitive Diffuse Mild Traumatic Brain Injury Causes an Atypical Astrocyte Response and Spontaneous Recurrent Seizures. J. Neurosci. 2019, 39, 1944–1963. [Google Scholar] [CrossRef]
- Mathewson, A.J.; Berry, M. Observations on the astrocyte response to a cerebral stab wound in adult rats. Brain Res. 1985, 327, 61–69. [Google Scholar] [CrossRef]
- Metting, Z.; Wilczak, N.; Rodiger, L.A.; Schaaf, J.M.; van der Naalt, J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 2012, 78, 1428–1433. [Google Scholar] [CrossRef]
- Papa, L.; Ladde, J.G.; O’Brien, J.F.; Thundiyil, J.G.; Tesar, J.; Leech, S.; Cassidy, D.D.; Roa, J.; Hunter, C.; Miller, S.; et al. Evaluation of Glial and Neuronal Blood Biomarkers Compared with Clinical Decision Rules in Assessing the Need for Computed Tomography in Patients with Mild Traumatic Brain Injury. JAMA Netw. Open 2022, 5, e221302. [Google Scholar] [CrossRef]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef]
- Middleton, J. UCH-L1 and GFAP Testing (i-STAT TBI Plasma) for the Detection of Intracranial Injury Following Mild Traumatic Brain Injury. Am. Fam. Physician 2022, 105, 313–314. [Google Scholar] [PubMed]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Gunnar Brolinson, P.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Zonfrillo, M.R.; Welch, R.D.; Lewis, L.M.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Lopez, M.A.; Haeussler, C.A.; Giordano, D.M.; et al. Evaluating glial and neuronal blood biomarkers GFAP and UCH-L1 as gradients of brain injury in concussive, subconcussive and non-concussive trauma: A prospective cohort study. BMJ Paediatr. Open 2019, 3, e000473. [Google Scholar] [CrossRef]
- Morris, M.C.; Bercz, A.; Niziolek, G.M.; Kassam, F.; Veile, R.; Friend, L.A.; Pritts, T.A.; Makley, A.T.; Goodman, M.D. UCH-L1 is a Poor Serum Biomarker of Murine Traumatic Brain Injury After Polytrauma. J. Surg. Res. 2019, 244, 63–68. [Google Scholar] [CrossRef]
- Mondello, S.; Linnet, A.; Buki, A.; Robicsek, S.; Gabrielli, A.; Tepas, J.; Papa, L.; Brophy, G.M.; Tortella, F.; Hayes, R.L.; et al. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery 2012, 70, 666–675. [Google Scholar]
- Frankel, M.; Fan, L.; Yeatts, S.D.; Jeromin, A.; Vos, P.E.; Wagner, A.K.; Wolf, B.J.; Pauls, Q.; Lunney, M.; Merck, L.H.; et al. Association of Very Early Serum Levels of S100B, Glial Fibrillary Acidic Protein, Ubiquitin C-Terminal Hydrolase-L1, and Spectrin Breakdown Product with Outcome in ProTECT III. J. Neurotrauma 2019, 36, 2863–2871. [Google Scholar] [CrossRef]
- Lei, J.; Gao, G.; Feng, J.; Jin, Y.; Wang, C.; Mao, Q.; Jiang, J. Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: A prospective cohort study. Crit. Care 2015, 19, 362. [Google Scholar] [CrossRef]
- Vos, P.E.; Jacobs, B.; Andriessen, T.M.; Lamers, K.J.; Borm, G.F.; Beems, T.; Edwards, M.; Rosmalen, C.F.; Vissers, J.L. GFAP and S100B are biomarkers of traumatic brain injury: An observational cohort study. Neurology 2010, 75, 1786–1793. [Google Scholar] [CrossRef]
- Su, Y.S.; Schuster, J.M.; Smith, D.H.; Stein, S.C. Cost-Effectiveness of Biomarker Screening for Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2083–2091. [Google Scholar] [CrossRef]
- Anderson, T.N.; Hinson, H.E. Damaged: Elevated GFAP and UCH-L1 as the Black Flag of Brain Injury. Resuscitation 2020, 154, 110–111. [Google Scholar] [CrossRef]
- Plog, B.A.; Dashnaw, M.L.; Hitomi, E.; Peng, W.; Liao, Y.; Lou, N.; Deane, R.; Nedergaard, M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 2015, 35, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.C.; Lukens, J.R. Neuroimmune cleanup crews in brain injury. Trends Immunol. 2021, 42, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.C.; Dutta, A.B.; Hurt, M.E.; Smirnov, I.; Kovacs, M.A.; McKee, C.A.; Ennerfelt, H.E.; Shapiro, D.; Nguyen, B.H.; Frost, E.L.; et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 2020, 11, 4524. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Jiwaji, Z.; Hardingham, G.E. Good, bad, and neglectful: Astrocyte changes in neurodegenerative disease. Free Radic. Biol. Med. 2022, 182, 93–99. [Google Scholar] [CrossRef]
- Masliah, E.; Hansen, L.; Alford, M.; Deteresa, R.; Mallory, M. Deficient glutamate tranport is associated with neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1996, 40, 759–766. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef]
- Chao, C.C.; Hu, S.; Sheng, W.S.; Bu, D.; Bukrinsky, M.I.; Peterson, P.K. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia 1996, 16, 276–284. [Google Scholar] [CrossRef]
- Li, S.; Mallory, M.; Alford, M.; Tanaka, S.; Masliah, E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J. Neuropathol. Exp. Neurol. 1997, 56, 901–911. [Google Scholar] [CrossRef]
- Louneva, N.; Cohen, J.W.; Han, L.Y.; Talbot, K.; Wilson, R.S.; Bennett, D.A.; Trojanowski, J.Q.; Arnold, S.E. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am. J. Pathol. 2008, 173, 1488–1495. [Google Scholar] [CrossRef]
- Cotman, C.W.; Poon, W.W.; Rissman, R.A.; Blurton-Jones, M. The Role of Caspase Cleavage of Tau in Alzheimer Disease Neuropathology. J. Neuropathol. Exp. Neurol. 2005, 64, 104–112. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Stoops, E.; Goozee, K.; Villemagne, V.L.; Asih, P.R.; Verberk, I.M.W.; Dave, P.; Taddei, K.; Sohrabi, H.R.; et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl. Psychiatry 2021, 11, 27. [Google Scholar] [CrossRef]
- Verberk, I.M.W.; Thijssen, E.; Koelewijn, J.; Mauroo, K.; Vanbrabant, J.; de Wilde, A.; Zwan, M.D.; Verfaillie, S.C.J.; Ossenkoppele, R.; Barkhof, F.; et al. Combination of plasma amyloid beta((1-42/1-40)) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res. Ther. 2020, 12, 118. [Google Scholar] [CrossRef]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J.; et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021, 78, 1471–1483. [Google Scholar] [CrossRef]
- Jesse, S.; Steinacker, P.; Cepek, L.; von Arnim, C.A.; Tumani, H.; Lehnert, S.; Kretzschmar, H.A.; Baier, M.; Otto, M. Glial fibrillary acidic protein and protein S-100B: Different concentration pattern of glial proteins in cerebrospinal fluid of patients with Alzheimer’s disease and Creutzfeldt-Jakob disease. J. Alzheimers Dis. 2009, 17, 541–551. [Google Scholar] [CrossRef]
- Cristóvão, J.S.; Gomes, C.M. S100 Proteins in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 463. [Google Scholar] [CrossRef]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, Cd003154. [Google Scholar] [CrossRef]
- Wang, Y. An insider’s perspective on FDA approval of aducanumab. Alzheimers Dement. 2023, 9, e12382. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Kishi, T.; Iwata, N. Memantine monotherapy for Alzheimer’s disease: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0123289. [Google Scholar] [CrossRef]
- Cicognola, C.; Janelidze, S.; Hertze, J.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res. Ther. 2021, 13, 68. [Google Scholar] [CrossRef]
- Oeckl, P.; Anderl-Straub, S.; Von Arnim, C.A.F.; Baldeiras, I.; Diehl-Schmid, J.; Grimmer, T.; Halbgebauer, S.; Kort, A.M.; Lima, M.; Marques, T.M.; et al. Serum GFAP differentiates Alzheimer’s disease from frontotemporal dementia and predicts MCI-to-dementia conversion. J. Neurol. Neurosurg. Psychiatry 2022, 93, 659–667. [Google Scholar] [CrossRef]
- Rajan, K.B.; Aggarwal, N.T.; McAninch, E.A.; Weuve, J.; Barnes, L.L.; Wilson, R.S.; DeCarli, C.; Evans, D.A. Remote Blood Biomarkers of Longitudinal Cognitive Outcomes in a Population Study. Ann. Neurol. 2020, 88, 1065–1076. [Google Scholar] [CrossRef]
- Asken, B.M.; VandeVrede, L.; Rojas, J.C.; Fonseca, C.; Staffaroni, A.M.; Elahi, F.M.; Lindbergh, C.A.; Apple, A.C.; You, M.; Weiner-Light, S.; et al. Lower White Matter Volume and Worse Executive Functioning Reflected in Higher Levels of Plasma GFAP among Older Adults with and Without Cognitive Impairment. J. Int. Neuropsychol. Soc. 2022, 28, 588–599. [Google Scholar] [CrossRef]
- Banks, W.A. Drug delivery to the brain in Alzheimer’s disease: Consideration of the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 629–639. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Salvadó, G.; Milà-Alomà, M.; Shekari, M.; Ashton, N.J.; Operto, G.; Falcon, C.; Cacciaglia, R.; Minguillon, C.; Fauria, K.; Niñerola-Baizán, A.; et al. Reactive astrogliosis is associated with higher cerebral glucose consumption in the early Alzheimer’s continuum. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4567–4579. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef]
- Deane, R.; Zlokovic, B.V. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 191–197. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Lan, G.; Lai, Y.J.; Wang, Q.H.; Chen, Y.; Xiao, Z.S.; Chen, X.; Bu, X.L.; Liu, Y.H.; et al. Diagnostic accuracy of plasma p-tau217/Aβ42 for Alzheimer’s disease in clinical and community cohorts. Alzheimers Dement. 2025, 21, e70038. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, Q.; Yang, M.; Lin, G.; Yao, K.; Wu, Z.; Xu, D.; Zhou, H.; Chen, B.; Shi, H.; et al. Plasma p-tau217 and p-tau217/Aβ1-42 are effective biomarkers for identifying CSF- and PET imaging-diagnosed Alzheimer’s disease: Insights for research and clinical practice. Alzheimer’s Dement. 2025, 21, e14536. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Edelmayer, R.M.; Boxer, A.L.; Carrillo, M.C.; Mielke, M.M.; Rabinovici, G.D.; Salloway, S.; Sperling, R.; Zetterberg, H.; Teunissen, C.E. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 2669–2686. [Google Scholar] [CrossRef]
- Zou, Y.; Li, L.; Guan, L.; Ma, C.; Yu, S.; Ma, X.; Mao, C.; Gao, J.; Qiu, L. Research trends and hotspots of glial fibrillary acidic protein within the area of Alzheimer’s disease: A bibliometric analysis. Front. Aging Neurosci. 2023, 15, 1196272. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanuri, S.H.; Sirrkay, P.J. Deciphering the Structural Biology of GFAP: Connotations of Its Potency in Presaging the Diagnosis for Traumatic Brain Injury and AD. Neurol. Int. 2025, 17, 134. https://doi.org/10.3390/neurolint17090134
Kanuri SH, Sirrkay PJ. Deciphering the Structural Biology of GFAP: Connotations of Its Potency in Presaging the Diagnosis for Traumatic Brain Injury and AD. Neurology International. 2025; 17(9):134. https://doi.org/10.3390/neurolint17090134
Chicago/Turabian StyleKanuri, Sri Harsha, and Prapthi Jayesh Sirrkay. 2025. "Deciphering the Structural Biology of GFAP: Connotations of Its Potency in Presaging the Diagnosis for Traumatic Brain Injury and AD" Neurology International 17, no. 9: 134. https://doi.org/10.3390/neurolint17090134
APA StyleKanuri, S. H., & Sirrkay, P. J. (2025). Deciphering the Structural Biology of GFAP: Connotations of Its Potency in Presaging the Diagnosis for Traumatic Brain Injury and AD. Neurology International, 17(9), 134. https://doi.org/10.3390/neurolint17090134




