High-Level Exposure of Testosterone During Mouse Pregnancy Impairs the Offspring Social Behavior by Interrupting Neurexin–Neuroligin Binding
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibodies and Chemicals
2.3. Brain Sample Preparation and Co-IP with Nlgn
2.4. Western Blotting
2.5. Behavioral Test
2.5.1. Three-Chamber Social Test
2.5.2. Novel Object Recognition Test
2.5.3. Y-Maze Test
2.6. Statistical Analysis
3. Results
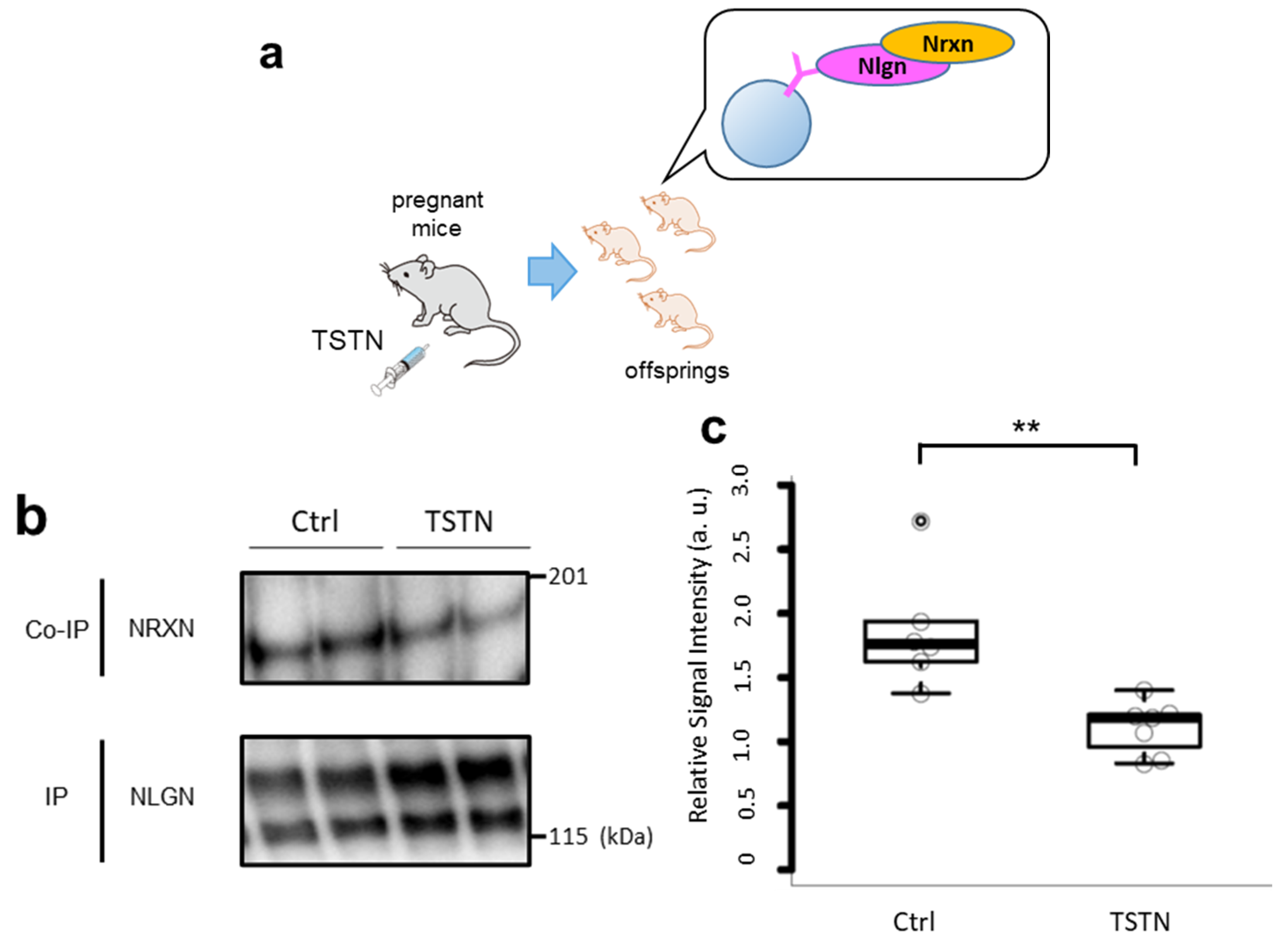
3.1. Nrxn and Nlgn Binding Is Interrupted by TSTN Injection
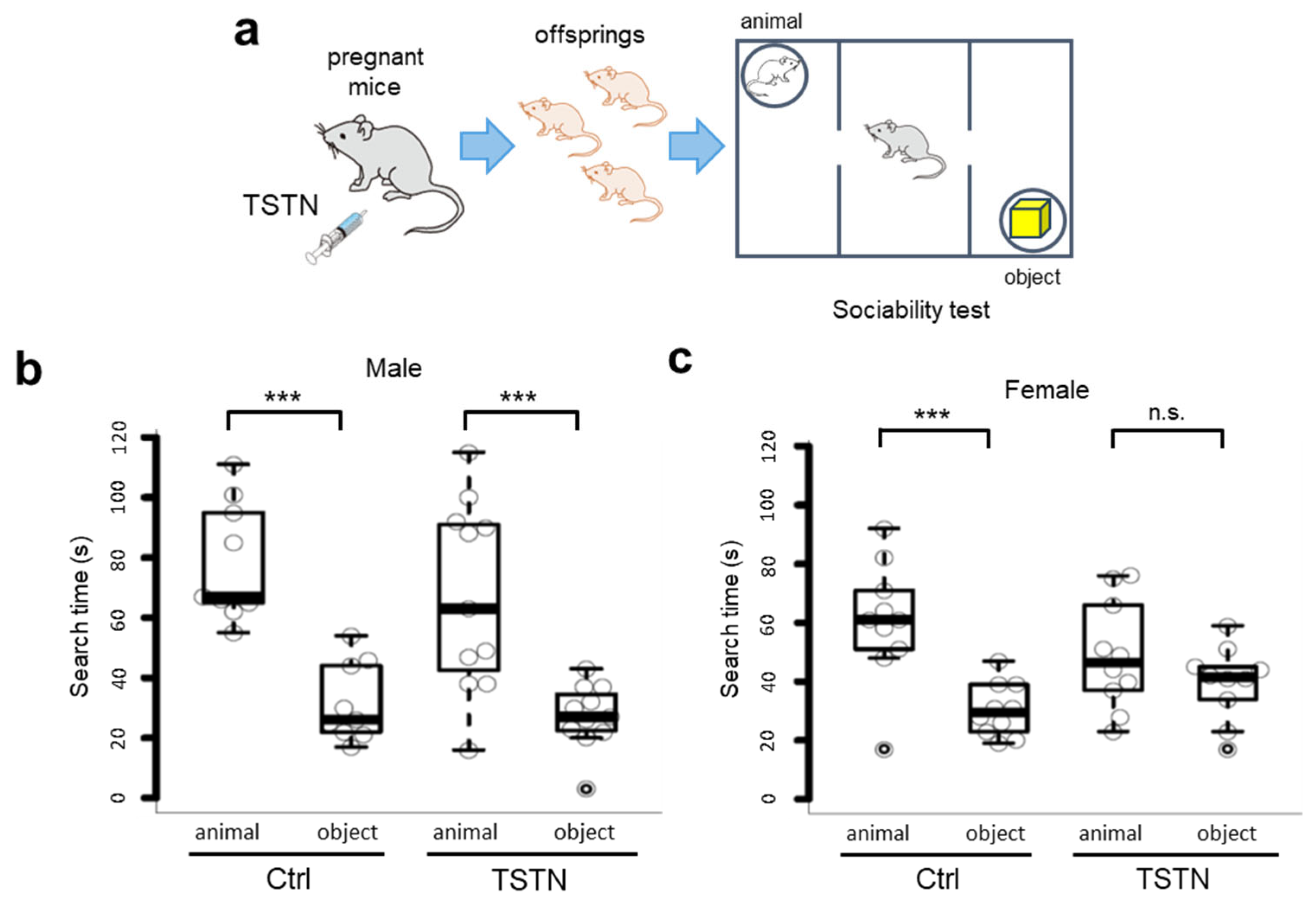
3.2. Sociability Searching Time Does Not Differ in Female Offspring from TSTN-Injected Mother
3.3. Social Novelty Searching Time Does Not Differ in Offspring from TSTN-Injected Mother
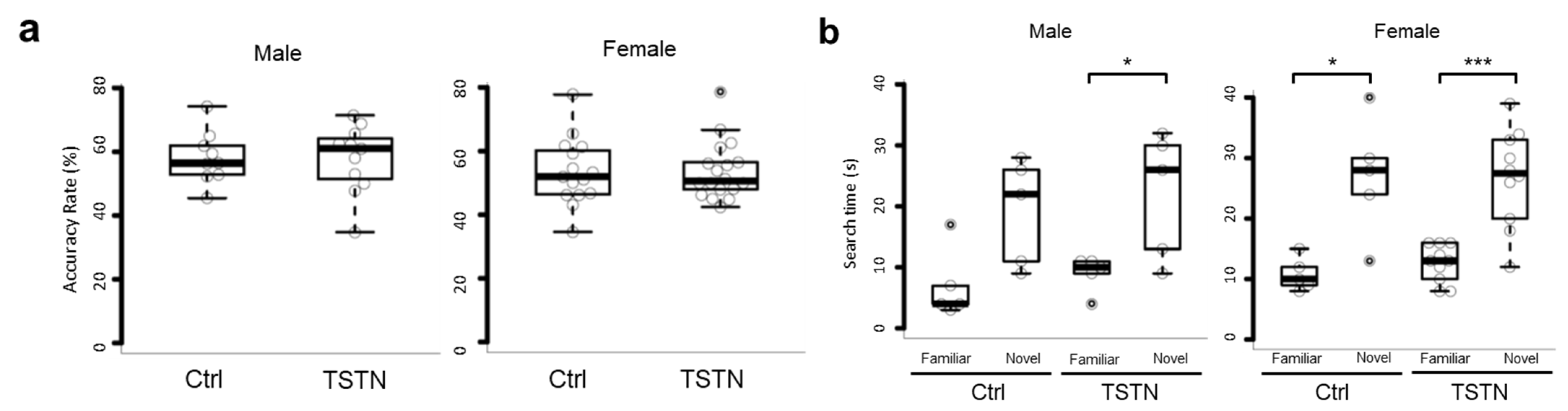
3.4. TSTN Does Not Affect Working Memory in Offspring
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism spectrum disorder |
| TSTN | Testosterone |
| Nrxn | Neurexin |
| Nlgn | Neuroligin |
| IP | Immunoprecipitation |
References
- Collignon, A.; Dion-Albert, L.; Ménard, C.; Coelho-Santos, V. Sex, hormones and cerebrovascular function: From development to disorder. Fluids Barriers CNS 2024, 21, 2. [Google Scholar] [CrossRef]
- Lutchmaya, S.; Baron-Cohen, S.; Raggatt, P. Foetal testosterone and eye contact in 12 month old infants. Infant. Behav. Dev. 2002, 25, 327–335. [Google Scholar] [CrossRef]
- Knickmeyer, R.; Baron-Cohen, S.; Raggatt, P.; Taylor, K. Foetal testosterone, social cognition, and restricted interests in children. J. Child Psychol. Psych. 2005, 46, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Zhang, H.F.; Shou, X.J.; Li, J.; Jing, W.L.; Zhou, Y.; Qian, Y.; Han, S.P.; Zhang, R.; Han, J.S. Prenatal hyperandrogenic environment induced autistic-like behavior in rat offspring. Physiol. Behav. 2015, 138, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011, 6, e1001081. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Cohen, A.S.; Chakrabarti, B.; Ruta, L.; Lombardo, M.V. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2015, 20, 369–376. [Google Scholar] [CrossRef]
- Yagishita-Kyo, N.; Ikari, Y.; Uekita, T.; Shinohara, A.; Koshimoto, C.; Yoshikawa, K.; Maruyama, K.; Yagishita, S. Testosterone interrupts binding of Neurexin and Neuroligin that are expressed in a highly socialized rodent, Octodon degus. Biochem. Biophys. Res. Commun. 2021, 551, 54–62. [Google Scholar] [CrossRef]
- Graf, E.R.; Zhang, X.; Jin, S.X.; Linhoff, M.W.; Craig, A.M. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 2004, 119, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Prange, O.; Wong, T.P.; Gerrow, K.; Wang, Y.T.; El-Husseini, A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl. Acad. Sci. USA 2004, 101, 13915–13920. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, X.; Dobie, F.; Wu, H.; Craig, A.M. Induction of GABAergic postsynaptic differentiation by alpha-neurexins. J. Biol. Chem. 2008, 283, 2323–2334. [Google Scholar] [CrossRef]
- Ko, J.; Zhang, C.; Araç, D.; Boucard, A.A.; Brunger, A.T.; Südhof, T.C. Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. EMBO J. 2009, 28, 3244–3255. [Google Scholar] [CrossRef] [PubMed]
- Gokce, O.; Südhof, T.C. Membrane-tethered monomeric neurexin LNS-domain triggers synapse formation. J. Neurosci. 2013, 33, 14617–14628. [Google Scholar] [CrossRef]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Paris Autism Research International Sibpair Study. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef]
- Laumonnier, F.; Bonnet-Brilhault, F.; Gomot, M.; Blanc, R.; David, A.; Moizard, M.P.; Raynaud, M.; Ronce, N.; Lemonnier, E.; Calvas, P.; et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am. J. Hum. Genet. 2004, 74, 552–557. [Google Scholar] [CrossRef]
- Julie, G.; Tabrez, J.S.; Peng, H.; Daisaku, Y.; Fadi, F.H.; Nathalie, C.; Mathieu, L.; Dan, S.; Anne, N.; Ronald, G.L.; et al. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum. Genet. 2011, 130, 563–573. [Google Scholar] [CrossRef]
- Sanders, S.J.; Ercan-Sencicek, A.G.; Hus, V.; Luo, R.; Murtha, M.T.; Moreno-De-Luca, D.; Chu, S.H.; Moreau, M.P.; Gupta, A.R.; Thomson, S.A.; et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 2011, 70, 863–885. [Google Scholar] [CrossRef]
- Pohl, T.T.; Hörnberg, H. Neuroligins in neurodevelopmental conditions: How mouse models of de novo mutations can help us link synaptic function to social behavior. Neuronal Signal. 2022, 6, NS20210030. [Google Scholar]
- Radyushkin, K.; Hammerschmidt, K.; Boretius, S.; Varoqueaux, F.; El-Kordi, A.; Ronnenberg, A.; Winter, D.; Frahm, J.; Fischer, J.; Brose, N.; et al. Neuroligin-3-deficient mice: Model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009, 8, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Radyushkin, K.; Hammerschmidt, K.; Granon, S.; Boretius, S.; Varoqueaux, F.; Ramanantsoa, N.; Gallego, J.; Ronnenberg, A.; Winter, D.; et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA 2008, 105, 1710–1715. [Google Scholar] [CrossRef]
- Connor, S.A.; Ammendrup-Johnsen, I.; Chan, A.W.; Kishimoto, Y.; Murayama, C.; Kurihara, N.; Tada, A.; Ge, Y.; Lu, H.; Yan, R.; et al. Altered Cortical Dynamics and Cognitive Function upon Haploinsufficiency of the Autism-Linked Excitatory Synaptic Suppressor MDGA2. Neuron 2016, 91, 1052–1068. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Wada, K.; Kabuta, T. Abnormal instability, excess density, and aberrant morphology of dendritic spines in prenatally testosterone-exposed mice. Neurochem. Int. 2015, 85–86, 53–58. [Google Scholar] [CrossRef]
- Kaidanovich-Beilin, O.; Lipina, T.; Vukobradovic, I.; Roder, J.; Woodgett, J.R. Assessment of social interaction behaviors. J. Vis. Exp. 2011, 48, 2473. [Google Scholar]
- Nomoto, M.; Ohkawa, N.; Nishizono, H.; Yokose, J.; Suzuki, A.; Matsuo, M.; Tsujimura, S.; Takahashi, Y.; Nagase, M.; Watabe, A.M.; et al. Cellular tagging as a neural network mechanism for behavioural tagging. Nat. Commun. 2016, 7, 12319. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, S.; Suzuki, S.; Yoshikawa, K.; Iida, K.; Hirata, A.; Suzuki, M.; Takashima, A.; Maruyama, K.; Hirasawa, A.; Awaji, T. Treatment of intermittent hypoxia increases phosphorylated tau in the hippocampus via biological processes common to aging. Mol. Brain 2017, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, G.; Hohenester, E.; Muller, Y.A. LG/LNS domains: Multiple functionsone business end? Trends Biochem. Sci. 2001, 26, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Pointis, G.; Latreille, M.T.; Mignot, T.M.; Janssens, Y.; Cedard, L. Regulation of testosterone synthesis in the fetal mouse testis. J. Steroid Biochem. 1979, 11, 1609–1612. [Google Scholar] [CrossRef]
- Romano, E.; Cosentino, L.; Laviola, G.; De Filippis, B. Genes and sex hormones interaction in neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2016, 67, 9–24. [Google Scholar] [CrossRef]
- Baum, M.J. Differentiation of coital behavior in mammals: A comparative analysis. Neurosci. Biobehav. Rev. 1979, 3, 265–284. [Google Scholar] [CrossRef]
- MacLusky, N.J.; Naftolin, F. Sexual differentiation of the central nervous system. Science 1981, 211, 1294–1303. [Google Scholar] [CrossRef]
- Bakker, J.; Brand, T.; van Ophemert, J.; Slob, A.K. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav. Neurosci. 1993, 107, 480–487. [Google Scholar] [CrossRef]
- Erdogan, M.A.; Bozkurt, M.F.; Erbas, O. Effects of prenatal testosterone exposure on the development of autism-like behaviours in offspring of Wistar rats. Int. J. Dev. Neurosci. 2023, 83, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Jean-Faucher, C.; Berger, M.; de Turckheim, M.; Veyssiere, G.; Jean, C. Developmental patterns of plasma and testicular testosterone in mice from birth to adulthood. Acta Endocrinol. 1978, 89, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.J.; Berho, M.; Korach, K.; Doublier, S.; Lupia, E.; Striker, G.E.; Karl, M. Gender-specific effects of endogenous testosterone: Female α-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007, 72, 464–472. [Google Scholar] [CrossRef]
- Cruz, C.D.; Kinnear, H.M.; Hashim, P.H.; Wandoff, A.; Nimmagadda, L.; Chang, F.L.; Padmanabhan, V.; Shikanov, A.; Moravek, M.B. A mouse model mimicking gender-affirming treatment with pubertal suppression followed by testosterone in transmasculine youth. Hum. Reprod. 2023, 38, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Baron-Cohen, S.; Buxbaum, J.D. Understanding autism in the light of sex/gender. Mol. Autism. 2015, 6, 24. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagishita-Kyo, N.; Yagishita, S. High-Level Exposure of Testosterone During Mouse Pregnancy Impairs the Offspring Social Behavior by Interrupting Neurexin–Neuroligin Binding. Neurol. Int. 2025, 17, 129. https://doi.org/10.3390/neurolint17080129
Yagishita-Kyo N, Yagishita S. High-Level Exposure of Testosterone During Mouse Pregnancy Impairs the Offspring Social Behavior by Interrupting Neurexin–Neuroligin Binding. Neurology International. 2025; 17(8):129. https://doi.org/10.3390/neurolint17080129
Chicago/Turabian StyleYagishita-Kyo, Nan, and Sosuke Yagishita. 2025. "High-Level Exposure of Testosterone During Mouse Pregnancy Impairs the Offspring Social Behavior by Interrupting Neurexin–Neuroligin Binding" Neurology International 17, no. 8: 129. https://doi.org/10.3390/neurolint17080129
APA StyleYagishita-Kyo, N., & Yagishita, S. (2025). High-Level Exposure of Testosterone During Mouse Pregnancy Impairs the Offspring Social Behavior by Interrupting Neurexin–Neuroligin Binding. Neurology International, 17(8), 129. https://doi.org/10.3390/neurolint17080129






