Correlation of Neurodegenerative Biomarkers and Functional Outcome in Patients with Relapsing–Remitting Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Characteristics
3.2. Magnetic Resonance Imaging Data Analysis and Correlation with Disability
3.3. Cognitive Test Results
3.4. Plasma Neurofilament Light Chain Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RRMS | Relapsing–remitting multiple sclerosis |
| SDMT | Symbol Digit Modalities Test |
| BVMT-R | Brief Visuospatial Memory Test-Revised |
| pNfL | Plasma neurofilament light chain |
| NfL | Neurofilament light chain |
| EDSS | Expanded Disability Status Scale |
| MRI | Magnetic resonance imaging |
| SPMS | Secondary progressive multiple sclerosis |
| BICAMS | Brief International Cognitive Assessment for Multiple Sclerosis |
| CI | Confidence interval |
References
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Absinta, M.; Lassmann, H.; Trapp, B.D. Mechanisms underlying progression in multiple sclerosis. Curr. Opin. Neurol. 2020, 33, 277–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levin, M.C.; Douglas, J.N.; Meyers, L.; Lee, S.; Shin, Y.; Gardner, L.A. Neurodegeneration in multiple sclerosis involves multiple pathogenic mechanisms. Degener. Neurol. Neuromuscul. Dis. 2014, 4, 49–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.; Hamade, M.; Wu, Q.; Wang, Q.; Axtell, R.; Giri, S.; Mao-Draayer, Y. Current and Future Biomarkers in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 5877. [Google Scholar] [CrossRef]
- Ciubotaru, A.; Smihor, M.I.; Grosu, C.; Alexa, D.; Covali, R.; Anicăi, R.-C.; Păvăleanu, I.; Cucu, A.I.; Bobu, A.M.; Ghiciuc, C.M.; et al. Neurodegenerative Biomarkers in Multiple Sclerosis: At the Interface Between Research and Clinical Practice. Diagnostics 2025, 15, 1178. [Google Scholar] [CrossRef]
- Alirezaei, Z.; Pourhanifeh, M.H.; Borran, S.; Nejati, M.; Mirzaei, H.; Hamblin, M.R. Neurofilament Light Chain as a Biomarker, and Correlation with Magnetic Resonance Imaging in Diagnosis of CNS-Related Disorders. Mol. Neurobiol. 2020, 57, 469–491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freedman, M.S.; Gnanapavan, S.; Booth, R.A.; Calabresi, P.A.; Khalil, M.; Kuhle, J.; Lycke, J.; Olsson, T. Guidance for use of neurofilament light chain as a cerebrospinal fluid and blood biomarker in multiple sclerosis management. eBioMedicine 2024, 101, 104970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, Y.; Xu, Y.; Cao, M.; Chen, N.; Zeng, Q.; Lai, M.K.P.; Fan, D.; Sethi, G.; Cao, Y. Fluid-based biomarkers for neurodegenerative diseases. Ageing Res. Rev. 2025, 108, 102739. [Google Scholar] [CrossRef] [PubMed]
- Novakova, L.; Zetterberg, H.; Sundström, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmeström, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017, 89, 2230–2237. [Google Scholar] [CrossRef]
- Di Filippo, M.; Gaetani, L.; Centonze, D.; Hegen, H.; Kuhle, J.; Teunissen, C.E. Fluid biomarkers in multiple sclerosis: From current to future applications. Lancet 2024, 44, 101009. [Google Scholar] [CrossRef]
- Joisten, N.; Rademacher, A.; Warnke, C.; Proschinger, S.; Schenk, A.; Walzik, D.; Knoop, A.; Thevis, M.; Steffen, F.; Bittner, S.; et al. Exercise Diminishes Plasma Neurofilament Light Chain and Reroutes the Kynurenine Pathway in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflammation 2021, 8, e982. [Google Scholar] [CrossRef] [PubMed]
- Thebault, S.; Booth, R.A.; Rush, C.A.; MacLean, H.; Freedman, M.S. Serum Neurofilament Light Chain Measurement in MS: Hurdles to Clinical Translation. Front. Neurosci. 2021, 15, 654942. [Google Scholar] [CrossRef]
- Benedict, C.; Blennow, K.; Zetterberg, H.; Cedernaes, J. Effects of acute sleep loss on diurnal plasma dynamics of CNS health biomarkers in young men. Neurology 2020, 94, e1181–e1189. [Google Scholar] [CrossRef] [PubMed]
- Thebault, S.; Lee, H.; Bose, G.; Tessier, D.; Abdoli, M.; Bowman, M.; Berard, J.; Walker, L.; Rush, C.A.; MacLean, H.; et al. Neurotoxicity after hematopoietic stem cell transplant in multiple sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.D.; Martinelli, V.; Moiola, L.; Sangalli, F.; Colombo, B.; Finardi, A.; Cinque, P.; Kolb, E.; Haghikia, A.; Gold, R.; et al. Serum neurofilaments increase at progressive multifocal leukoencephalopathy onset in natalizumab-treated multiple sclerosis patients. Ann. Neurol. 2019, 85, 606–610. [Google Scholar] [CrossRef]
- Greif, E.; Wissemann, K.; Fafard, L.; Bumstead, B.; Buhse, M.; Zarif, M.; Gudesblatt, M. Multiple sclerosis: Brain atrophy and Computerized Cognitive Testing—A Cross-Sectional Pilot Investigation (P4.349). Neurology 2017, 88 (Suppl. S16). [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test (SDMT); Western Psychological Services: Los Angeles, CA, USA, 1982. [Google Scholar]
- Smestad, C.; Sandvik, L.; Landro, N.I.; Celius, E.G. Cognitive Impairment after Three Decades of Multiple Sclerosis. Eur. J. Neurol. 2010, 17, 499–505. [Google Scholar] [CrossRef]
- de Caneda, M.A.G.; Cuervo, D.L.M.; Marinho, N.E.; de Vecino, M.C.A. The Reliability of the Brief Visuospatial Memory Test—Revised in Brazilian multiple sclerosis patients. Dement. Neuropsychol. 2018, 12, 205–211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baetge, S.J.; Filser, M.; Renner, A.; Ullrich, S.; Lassek, C.; Penner, I.K. On the validity of single tests, two-test combinations and the full Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) in detecting patients with cognitive impairment. Mult. Scler. 2020, 26, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Filser, M.; Schreiber, H.; Pöttgen, J.; Ullrich, S.; Lang, M.; Penner, I.K. The Brief International Cognitive Assessment in Multiple Sclerosis (BICAMS): Results from the German validation study. J. Neurol. 2018, 265, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Giedraitiene, N.; Kaubrys, G.; Kizlaitiene, R. Cognition During and After Multiple Sclerosis Relapse as Assessed with the Brief International Cognitive Assessment for Multiple Sclerosis. Sci. Rep. 2018, 8, 8169. [Google Scholar] [CrossRef]
- Thomson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis, 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Viral, P.; Patel, L.A.S.; Walker, A.F. Deconstructing the symbol digit modalities test in multiple sclerosis: The role of memory. Mult. Scler. Relat. Disord. 2017, 17, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Strober, L.; DeLuca, J.; Benedict, R.H.; Jacobs, A.; Cohen, J.A.; Chiaravalloti, N.; Hudson, L.D.; Rudick, R.A.; LaRocca, N.G. Multiple Sclerosis Outcome Assessments Consortium (MSOAC). Symbol Digit Modalities Test: A valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult. Scler. 2019, 25, 1781–1790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benedict, R.H.; DeLuca, J.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R. Multiple Sclerosis Outcome Assessments Consortium. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. 2017, 23, 721–733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tam, J.W.; Schmitter-Edgecombe, M. The role of processing speed in the Brief Visuospatial Memory Test—Revised. Clin. Neuropsychol. 2013, 27, 962–972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schadelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62(2), 774–781. Available online: https://surfer.nmr.mgh.harvard.edu/fswiki/FreeSurferMethodsCitation (accessed on 1 January 2023).
- Eijlers, A.J.C.; Meijer, K.A.; Wassenaar, T.M.; Barkhof, F.; Steenwijk, M.D.; Uitdehaag, B.M.; Barkhof, F.; Wink, A.M.; Geurts, J.J.; Schoonheim, M.M. Increased default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology 2018, 90, e962–e970. [Google Scholar] [CrossRef]
- Sailer, M.; Fischl, B.; Salat, D.; Tempelmann, C.; Schonfeld, M.A.; Busa, E.; Bodammer, N.; Heinze, H.; Dale, A. Focal thinning of the cerebral cortex in multiple sclerosis. Brain 2003, 126, 1734–1744. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Weinstock-Guttman, B.; Fishman, I.; Sharma, J.; Tjoa, C.W.; Bakshi, R. Prediction of neuropsychological impairment in multiple sclerosis: Comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch. Neurol. 2005, 62, 65–70. [Google Scholar] [CrossRef]
- Sandry, J.; Simonet, D.V.; Brandstadter, R.; Krieger, S.; Sand, I.K.; Graney, R.A.; Buchanan, A.V.; Lall, S.; Sumowski, J.F. The Symbol Digit Modalities Test (SDMT) is sensitive but non-specific in MS: Lexical access speed, memory, and information processing speed independently contribute to SDMT performance. Mult. Scler. Relat. Disord. 2021, 51, 102950. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.M.; Leo, G.J.; Bernardin, L.; Unverzagt, F. Cognitive dysfunction in multiple sclerosis: I. Frequency, patterns, and prediction. Neurology 1991, 41, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.; Beier, M.; Benedict, R.H.B.; Charvet, L.; Costello, K.; Feinstein, A. Recommendations for cognitive screening and management in multiple sclerosis care. Mult. Scler. J. 2018, 24, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, O.; Cinar, B.P.; Ozakbas, S. Use of the BICAMS in Patients with Multiple Sclerosis During Attack Period: A Prospective Controlled Study. J. Mult. Scler. Res. 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Billot, B.; Greve, D.N.; Puonti, O.; Thielscher, A.; Van Leemput, K.; Fischl, B.; Dalca, A.V.; Iglesias, J.E. SynthSeg: Segmentation of brain MRI scans of any contrast and resolution without retraining. Med. Image Anal. 2023, 83, 102789. [Google Scholar] [CrossRef]
- Laso, P.; Cerri, S.; Sorby-Adams, A.; Guo, J.; Matteen, F.; Goebl, P.; Wu, J.; Liu, P.; Li, H.; Young, S.I.; et al. Quantifying white matter hyperintensity and brain volumes in heterogeneous clinical and low-field portable MRI. In Proceedings of the 2024 IEEE International Symposium on Biomedical Imaging (ISBI), Athens, Greece, 27–30 May 2024. [Google Scholar] [CrossRef]
- Kuhle, J.; Barro, C.; Disanto, G.; Mathias, A.; Soneson, C.; Bonnier, G.; Leppert, D. Serum neurofilament light chain in early relapsing-remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult. Scler. J. 2016, 22, 1550–1559. [Google Scholar] [CrossRef]
- Bridel, C.; Verberk, I.M.W.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; van der Flier, W.M. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Steenwijk, M.D.; Geurts, J.J.G.; Daams, M.; Tijms, B.M.; Wink, A.M.; Balk, L.J.; Tewarie, P.K.; Uitdehaag, B.M.J.; Barkhof, F.; Vrenken, H.; et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 2015, 138, 633–648. [Google Scholar] [CrossRef]
- Zivadinov, R.; Bergsland, N.; Dolezal, O.; Ramasamy, D.P.; Hagemeier, J.; Seidl, Z.; Dwyer, M.G.; Vaneckova, M.; Krasensky, J.; Potts, J.A.; et al. Evolution of cortical and thalamus atrophy and disability progression in early relapsing–remitting multiple sclerosis: A 5-year longitudinal study. Hum. Brain Mapp. 2013, 34, 2293–2301. [Google Scholar]
- Solís-Tarazona, L.; Raket, L.L.; Cabello-Murgui, J.; Reddam, S.; Navarro-Quevedo, S.; Gil-Perotin, S. Predictive value of individual serum neurofilament light chain levels in short-term disease activity in relapsing multiple sclerosis. Front. Neurol. 2024, 15, 1354431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malmeström, C.; Haghighi, S.; Rosengren, L.; Andersen, O.; Lycke, J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003, 61, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Barro, C.; Benkert, P.; Disanto, G.; Tsagkas, C.; Amann, M.; Naegelin, Y.; Leppert, D.; Gobbi, C.; Granziera, C.; Yaldizli, Ö.; et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018, 141, 2382–2391. [Google Scholar] [CrossRef]
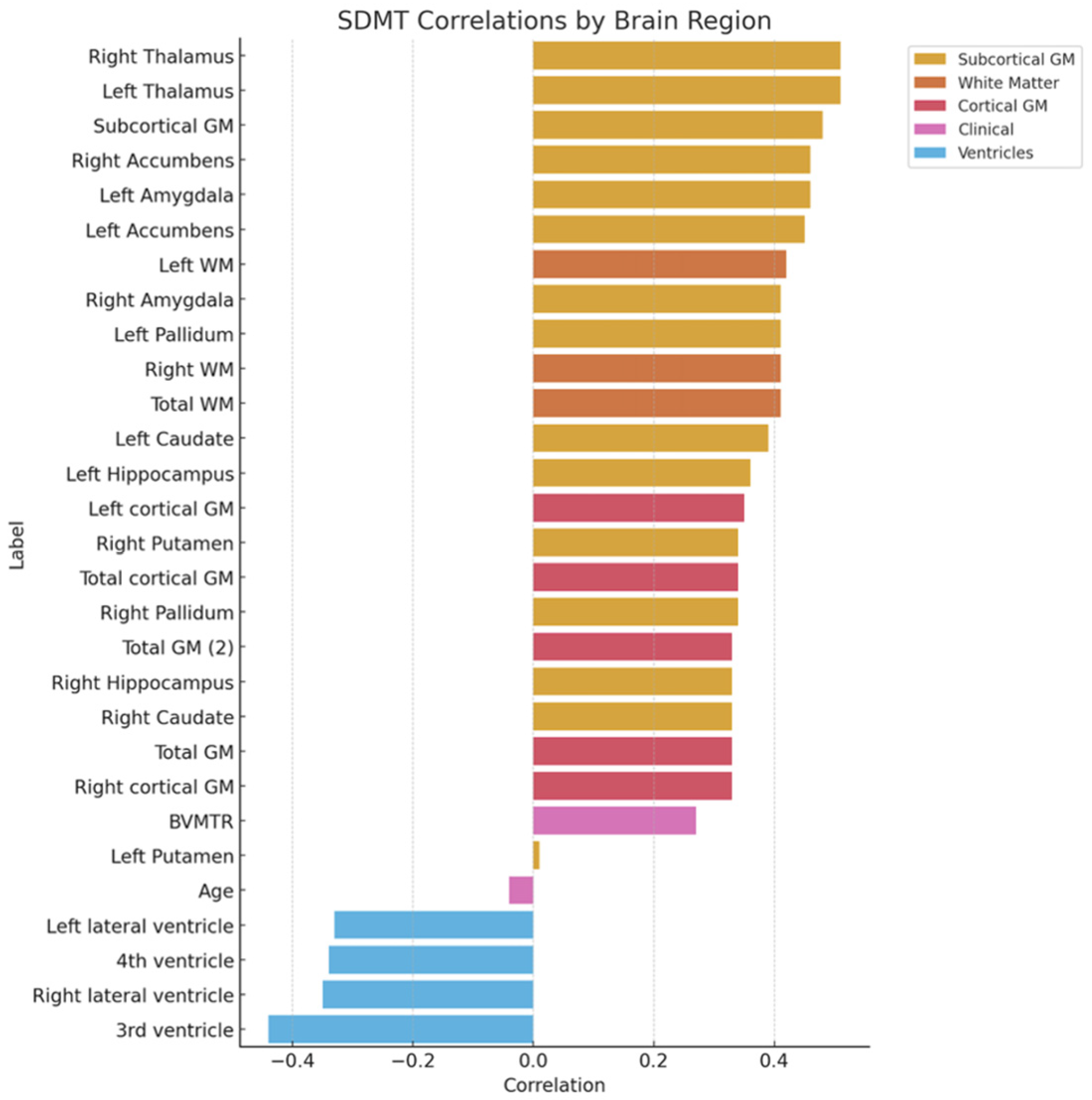
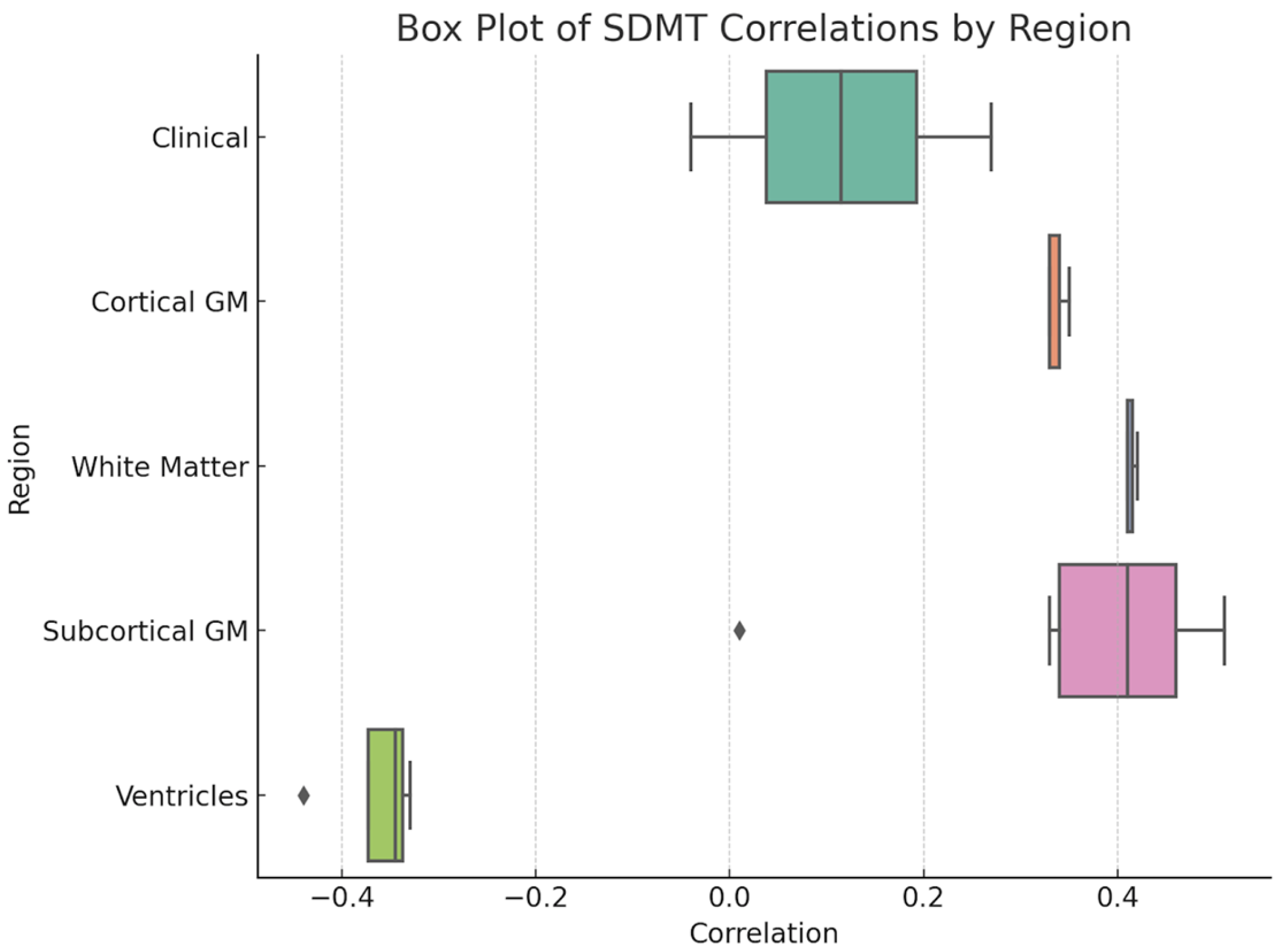

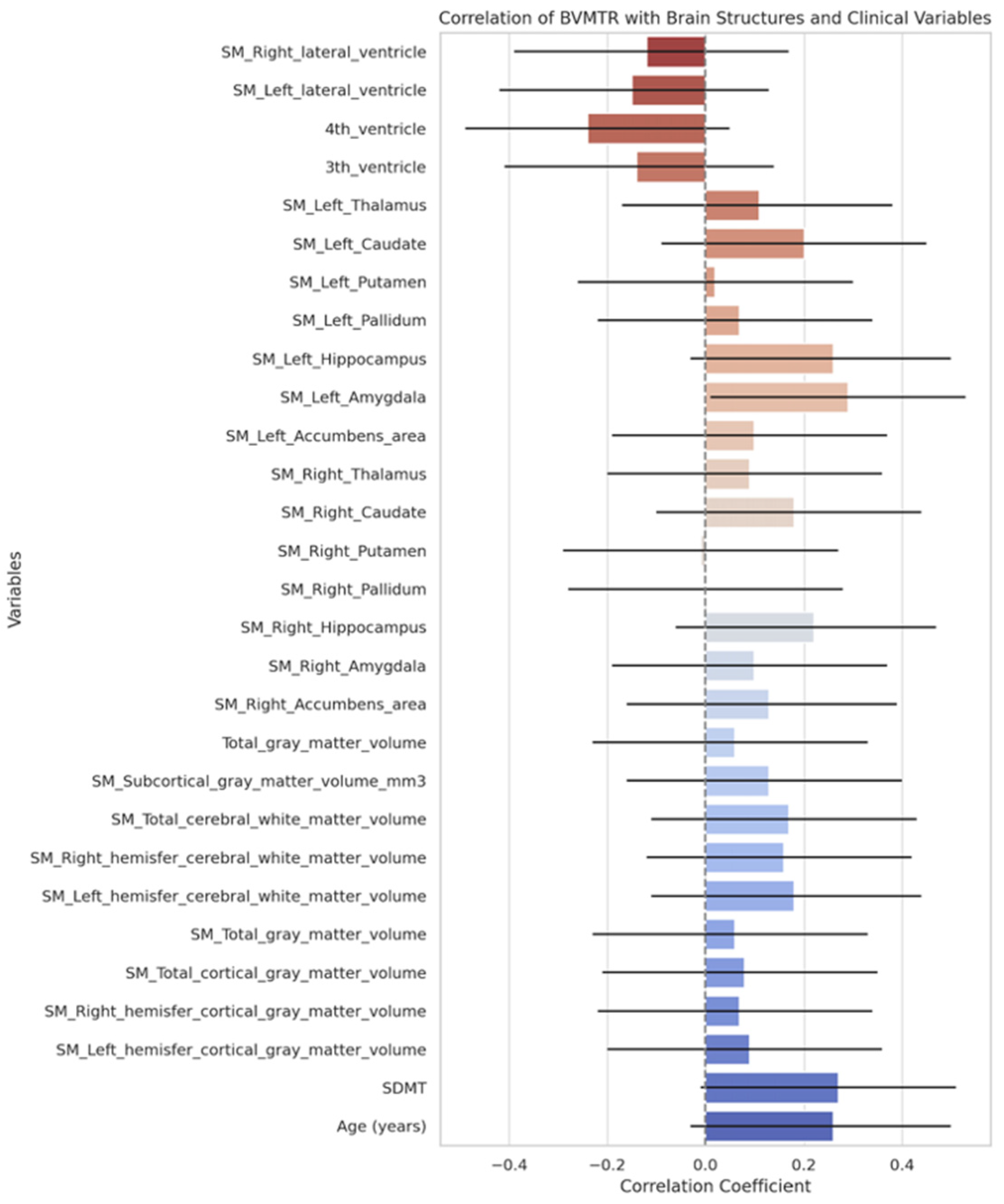

| Variable | Value |
|---|---|
| Gender, n (%) | |
| Female | 25 (51.0%) |
| Male | 24 (49.0%) |
| Age (years), median [Q1; Q3] | 38.0 [30.0; 46.0] |
| Expanded Disability Status Scale (EDSS), median [Q1; Q3] | 2.50 [2.00; 3.50] |
| Plasma NfL (pg/mL), median [Q1; Q3] | 6.30 [4.60; 10.9] |
| Duration of the disease (years), median [Q1; Q3] | 8.00 [6.00; 10.0] |
| Symbol Digit Modalities Test (SDMT), median [Q1; Q3] | 82.3 [74.0; 92.3] |
| Brief Visuospatial Memory Test-Revised (BVMT-R), median [Q1; Q3] | 3.00 [2.00; 3.00] |
| Education, n (%) | |
| Secondary school | 19 (38.8%) |
| University | 30 (61.2%) |
| Clinical relapses within the first 5 years, median [Q1; Q3] | 3.00 [3.00; 4.00] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polunosika, E.; Feldmane, M.; Pastare, D.; Simren, J.; Blennow, K.; Zdanovskis, N.; Zetterberg, H.; Erts, R.; Karelis, G. Correlation of Neurodegenerative Biomarkers and Functional Outcome in Patients with Relapsing–Remitting Multiple Sclerosis. Neurol. Int. 2025, 17, 123. https://doi.org/10.3390/neurolint17080123
Polunosika E, Feldmane M, Pastare D, Simren J, Blennow K, Zdanovskis N, Zetterberg H, Erts R, Karelis G. Correlation of Neurodegenerative Biomarkers and Functional Outcome in Patients with Relapsing–Remitting Multiple Sclerosis. Neurology International. 2025; 17(8):123. https://doi.org/10.3390/neurolint17080123
Chicago/Turabian StylePolunosika, Elina, Monta Feldmane, Daina Pastare, Joel Simren, Kaj Blennow, Nauris Zdanovskis, Henrik Zetterberg, Renars Erts, and Guntis Karelis. 2025. "Correlation of Neurodegenerative Biomarkers and Functional Outcome in Patients with Relapsing–Remitting Multiple Sclerosis" Neurology International 17, no. 8: 123. https://doi.org/10.3390/neurolint17080123
APA StylePolunosika, E., Feldmane, M., Pastare, D., Simren, J., Blennow, K., Zdanovskis, N., Zetterberg, H., Erts, R., & Karelis, G. (2025). Correlation of Neurodegenerative Biomarkers and Functional Outcome in Patients with Relapsing–Remitting Multiple Sclerosis. Neurology International, 17(8), 123. https://doi.org/10.3390/neurolint17080123







