The Pathogenesis and Medical Treatment of Depression: Opportunity and Challenge
Abstract
1. Introduction
2. Methods of Literature Search
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection Process
2.4. Data Extraction, Synthesis and Quality Assessment
3. Pathogenesis of Depression
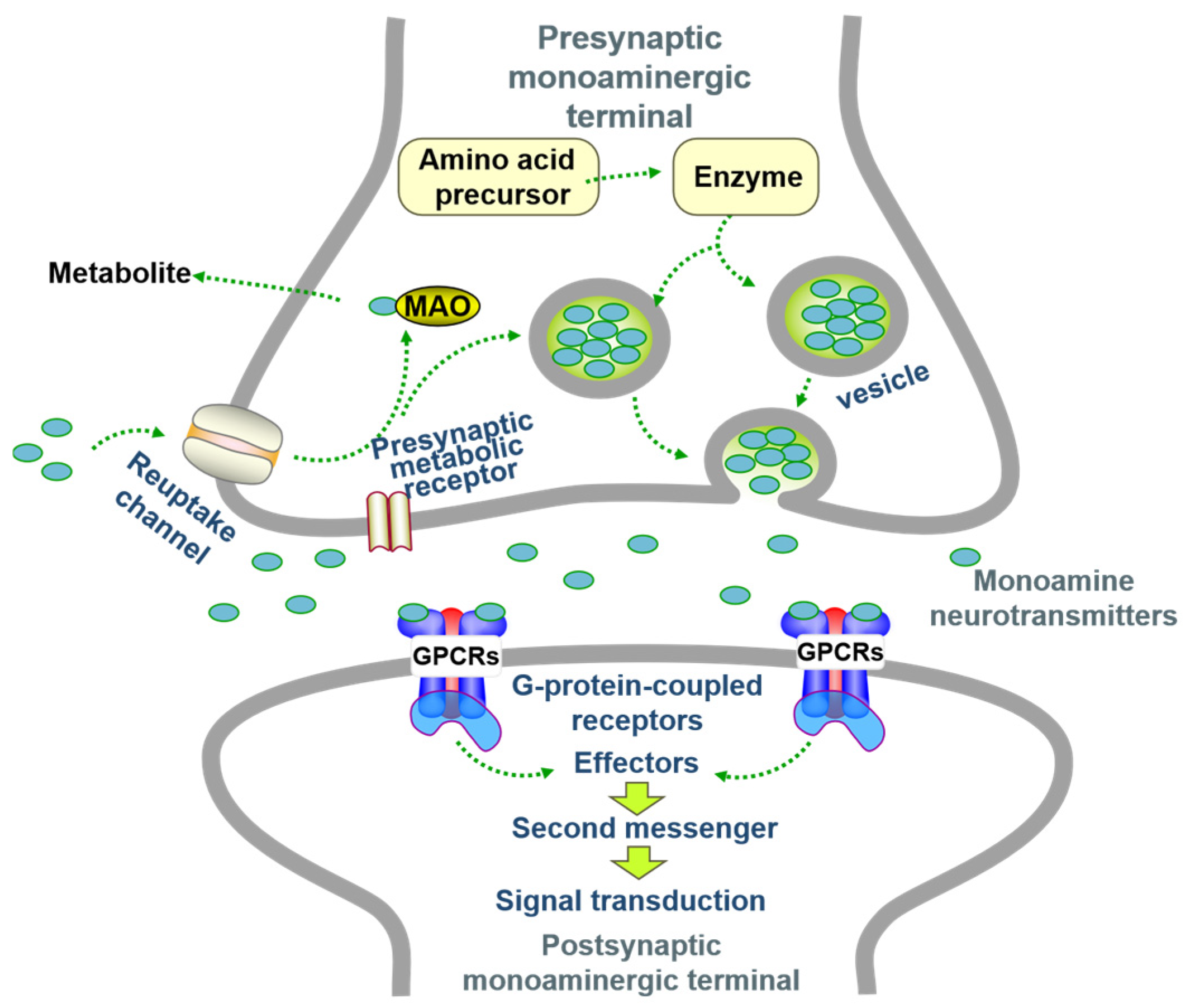
3.1. Hypothesis of Monoamine Neurotransmitters
3.2. Impairment of the HPA Axis
3.3. The Role of Inflammatory Cytokines and Oxidative Stress
3.4. Hypothesis of Neuroplasticity and Neurotrophic
3.4.1. Neuroplasticity Hypothesis
3.4.2. Neurotrophic Hypothesis
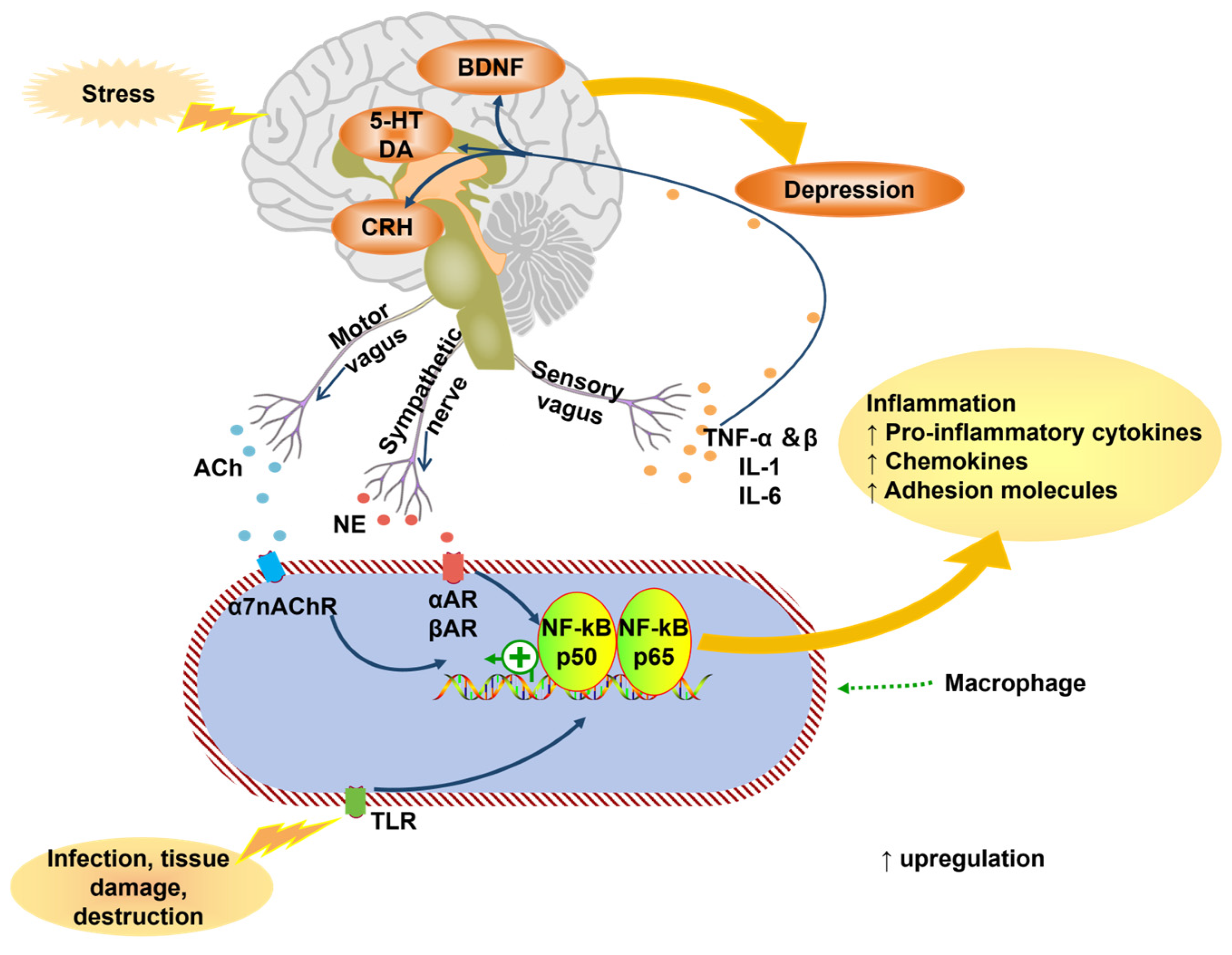
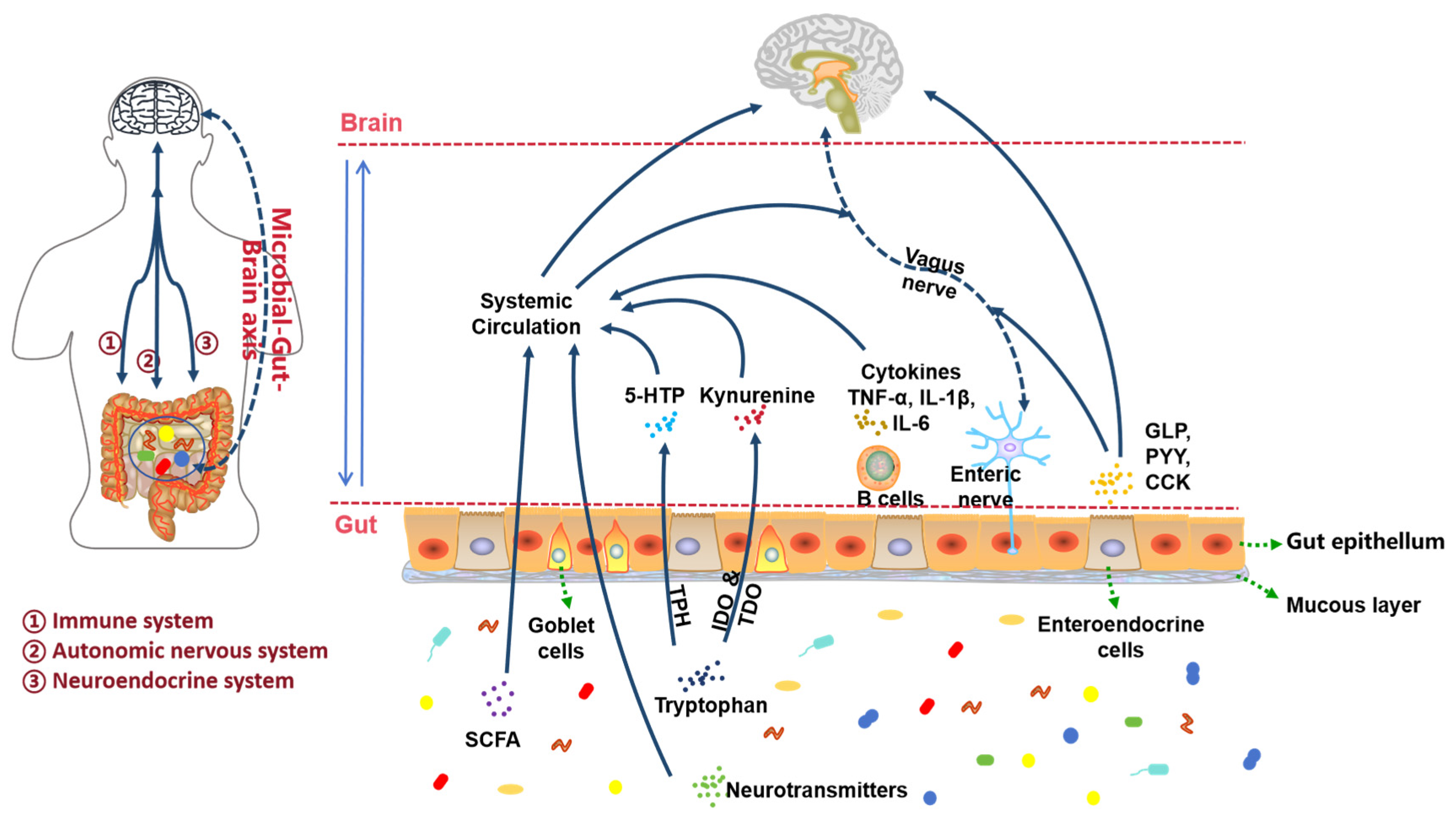
3.5. Dysfunction of Gut Microbiota
3.5.1. Autonomic Nervous System Pathway
3.5.2. Immune System Pathways
3.5.3. Endocrine System Pathways
4. Drug Therapy for Depression
4.1. Monoamine Oxidase Inhibitor and Tricyclic Antidepressants
4.2. Selective Serotonin Reuptake Inhibitors
4.3. Serotonin-Norepinephrine Reuptake Inhibitors
4.4. Atypical Antidepressants
4.5. Herbal and Emerging Agents
4.6. Novel Drug Treatment Strategies
5. Limitations and Future Directions
5.1. Critical Appraisal
5.2. Future Priorities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Wang, K.; Wang, H.; Gao, Y.; Nie, K.; Jiang, X.; Su, H.; Tang, Y.; Lu, F.; Dong, H.; et al. The therapeutic effects of saikosaponins on depression through the modulation of neuroplasticity: From molecular mechanisms to potential clinical applications. Pharmacol. Res. 2024, 201, 107090. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Wang, H.; Liu, Z.; Yu, X.; Yan, J.; Yu, Y.; Kou, C.; Xu, X.; Lu, J.; et al. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry 2019, 6, 211–224. [Google Scholar] [CrossRef]
- Walker, J.; Hansen, C.H.; Martin, P.; Symeonides, S.; Ramessur, R.; Murray, G.; Sharpe, M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014, 1, 343–350. [Google Scholar] [CrossRef]
- Scott, K.M.; Bruffaerts, R.; Tsang, A.; Ormel, J.; Alonso, J.; Angermeyer, M.C.; Benjet, C.; Bromet, E.; de Girolamo, G.; de Graaf, R.; et al. Depression–anxiety relationships with chronic physical conditions: Results from the World Mental Health surveys. J. Affect. Disord. 2007, 103, 113–120. [Google Scholar] [CrossRef]
- Stewart, W.F.; Ricci, J.A.; Chee, E.; Hahn, S.R.; Morganstein, D. Cost of Lost Productive Work Time Among US Workers With Depression. JAMA 2003, 289, 3135. [Google Scholar] [CrossRef]
- Lerner, D.; Adler, D.A.; Chang, H.; Lapitsky, L.; Hood, M.Y.; Perissinotto, C.; Reed, J.; McLaughlin, T.J.; Berndt, E.R.; Rogers, W.H. Unemployment, Job Retention, and Productivity Loss Among Employees With Depression. Psychiatr. Serv. 2004, 55, 1371–1378. [Google Scholar] [CrossRef]
- Wen, J.; Fu, C.H.Y.; Tosun, D.; Veturi, Y.; Yang, Z.; Abdulkadir, A.; Mamourian, E.; Srinivasan, D.; Skampardoni, I.; Singh, A.; et al. Characterizing Heterogeneity in Neuroimaging, Cognition, Clinical Symptoms, and Genetics Among Patients With Late-Life Depression. JAMA Psychiatry 2022, 79, 464–474. [Google Scholar] [CrossRef]
- Maletic, V.; Eramo, A.; Gwin, K.; Offord, S.J.; Duffy, R.A. The Role of Norepinephrine and Its α-Adrenergic Receptors in the Pathophysiology and Treatment of Major Depressive Disorder and Schizophrenia: A Systematic Review. Front. Psychiatry 2017, 8, 42. [Google Scholar] [CrossRef]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef]
- Lapolla, T.; Saltiel, P.F.; Silvershein, D. Major depressive disorder: Mechanism-based prescribing for personalized medicine. Neuropsychiatr. Dis. Treat. 2015, 11, 875–888. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Feng, X.; Ma, X.; Li, J.; Zhou, Q.; Liu, Y.; Song, J.; Liu, J.; Situ, Q.; Wang, L.; Zhang, J.; et al. Inflammatory Pathogenesis of Post-stroke Depression. Aging Dis. 2024, 16, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Martínez, S.; Segura-Real, L.; Gómez-García, A.P.; Tesoro-Cruz, E.; Constantino-Jonapa, L.A.; Amedei, A.; Aguirre-García, M.M. Neuroinflammation, Microbiota-Gut-Brain Axis, and Depression: The Vicious Circle. J. Integr. Neurosci. 2023, 22, 65. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef]
- Schildkraut, J.J.; Draskoczy, P.R.; Gershon, E.S.; Reich, P.; Grab, E.L. Catecholamine metabolism in affective disorders —IV. Preliminary studies of norepinephrine metabolism in depressed patients treated with amitriptyline. J. Psychiatr. Res. 1972, 9, 173–185. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The Role of Corticotropin-Releasing Factor in the Pathogenesis of Major Depression. Pharmacopsychiatry 1988, 21, 76–82. [Google Scholar] [CrossRef]
- Lesch, K.P.; Wolozin, B.L.; Murphy, D.L.; Riederer, P. Primary Structure of the Human Platelet Serotonin Uptake Site: Identity with the Brain Serotonin Transporter. J. Neurochem. 1993, 60, 2319–2322. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.W.; Ball, S.G.; Martinez, J.; Robinson, M.J.; Yang, C.R.; Russell, J.M.; Shekhar, A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress. Anxiety 2010, 27, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ma, L.; Wang, Y.-G.; Ye, F.; Wang, C.; Zhou, W.-H.; Zhao, X. Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of serotonergic system. Neurochem. Int. 2017, 108, 426–435. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Basic psychopharmacology of antidepressants, part 1: Antidepressants have seven distinct mechanisms of action. J. Clin. Psychiatry 1998, 59, 5–14. [Google Scholar]
- Chen, Y.; Xu, H.; Zhu, M.; Liu, K.; Lin, B.; Luo, R.; Chen, C.; Li, M. Stress inhibits tryptophan hydroxylase expression in a rat model of depression. Oncotarget 2017, 8, 63247. [Google Scholar] [CrossRef]
- Ayala-Lopez, N.; Watts, S.W. Physiology and Pharmacology of Neurotransmitter Transporters. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2021; pp. 2279–2295. [Google Scholar] [CrossRef]
- Bröer, S.; Gether, U. The solute carrier 6 family of transporters. Br. J. Pharmacol. 2012, 167, 256–278. [Google Scholar] [CrossRef] [PubMed]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialog- Clin. Neurosci. 2002, 4, 7–20. [Google Scholar] [CrossRef]
- Ananth, M.R.; DeLorenzo, C.; Yang, J.; Mann, J.J.; Parsey, R.V. Decreased Pretreatment Amygdalae Serotonin Transporter Binding in Unipolar Depression Remitters: A Prospective PET Study. J. Nucl. Med. 2018, 59, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Boas, G.R.V.; de Lacerda, R.B.; Paes, M.M.; Gubert, P.; Almeida, W.L.d.C.; Rescia, V.C.; de Carvalho, P.M.G.; de Carvalho, A.A.V.; Oesterreich, S.A. Molecular aspects of depression: A review from neurobiology to treatment. Eur. J. Pharmacol. 2019, 851, 99–121. [Google Scholar] [CrossRef]
- Kaufman, J.; DeLorenzo, C.; Choudhury, S.; Parsey, R.V. The 5-HT1A receptor in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2016, 26, 397–410. [Google Scholar] [CrossRef]
- Jacobsen, J.P.; Krystal, A.D.; Krishnan, K.R.R.; Caron, M.G. Adjunctive 5-Hydroxytryptophan Slow-Release for Treatment-Resistant Depression: Clinical and Preclinical Rationale. Trends Pharmacol. Sci. 2016, 37, 933–944. [Google Scholar] [CrossRef]
- Du, X.; Pang, T.Y. Is Dysregulation of the HPA-Axis a Core Pathophysiology Mediating Co-Morbid Depression in Neurodegenerative Diseases? Front. Psychiatry 2015, 6, 32. [Google Scholar] [CrossRef]
- Holsboer, F. The Corticosteroid Receptor Hypothesis of Depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Juruena, M.F. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav. 2014, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Cullinan, W.E. Neurocircuitry of stress: Central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997, 20, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, J.J.; Inda, C.; Refojo, D.; Holsboer, F.; Arzt, E.; Silberstein, S. The Corticotropin-Releasing Hormone Network and the Hypothalamic-Pituitary-Adrenal Axis: Molecular and Cellular Mechanisms Involved. Neuroendocrinology 2011, 94, 12–20. [Google Scholar] [CrossRef]
- Austin, M.C.; Janosky, J.E.; Murphy, H.A. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol. Psychiatry 2003, 8, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Nikkheslat, N.; Pariante, C.M.; Zunszain, P.A. Neuroendocrine Abnormalities in Major Depression: An Insight Into Glucocorticoids, Cytokines, and the Kynurenine Pathway. In Inflammation and Immunity in Depression; Academic Press: Cambridge, MA, USA, 2018; pp. 45–60. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Bouças, A.P.; Rheinheimer, J.; Lagopoulos, J. Why Severe COVID-19 Patients Are at Greater Risk of Developing Depression: A Molecular Perspective. Neurosci. 2020, 28, 11–19. [Google Scholar] [CrossRef]
- Machado, M.O.; Oriolo, G.; Bortolato, B.; Köhler, C.A.; Maes, M.; Solmi, M.; Grande, I.; Martín-Santos, R.; Vieta, E.; Carvalho, A.F. Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: A critical systematic review. J. Affect. Disord. 2017, 209, 235–245. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef]
- Han, K.-M.; Ham, B.-J. How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies. J. Clin. Neurol. 2021, 17, 503–515. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Klabnik, J.; Donnell, J.O. Novel Therapeutic Targets in Depression and Anxiety: Antioxidants as a Candidate Treatment. Curr. Neuropharmacol. 2014, 12, 108–119. [Google Scholar] [CrossRef]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and Oxidative Stress. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Tobe, E.H. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr. Dis. Treat. 2013, 9, 567–573. [Google Scholar] [CrossRef]
- Chen, L.-M.; Bao, C.-H.; Wu, Y.; Liang, S.-H.; Wang, D.; Wu, L.-Y.; Huang, Y.; Liu, H.-R.; Wu, H.-G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflammation 2021, 18, 135. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.; Myint, A.; Kubera, M.; Verkerk, R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chen, L.; Lim, G.; Sung, B.; Wang, S.; McCabe, M.F.; Rusanescu, G.; Yang, L.; Tian, Y.; Mao, J. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J. Clin. Investig. 2012, 122, 2940–2954. [Google Scholar] [CrossRef] [PubMed]
- Usui-Kawanishi, F.; Kani, K.; Karasawa, T.; Honda, H.; Takayama, N.; Takahashi, M.; Takatsu, K.; Nagai, Y. Isoliquiritigenin inhibits NLRP3 inflammasome activation with CAPS mutations by suppressing caspase-1 activation and mutated NLRP3 aggregation. Genes Cells 2024, 29, 423–431. [Google Scholar] [CrossRef]
- Chai, Y.; Cai, Y.; Fu, Y.; Wang, Y.; Zhang, Y.; Zhang, X.; Zhu, L.; Miao, M.; Yan, T. Salidroside Ameliorates Depression by Suppressing NLRP3-Mediated Pyroptosis via P2X7/NF-κB/NLRP3 Signaling Pathway. Front. Pharmacol. 2022, 13, 812362. [Google Scholar] [CrossRef]
- Xia, C.-Y.; Guo, Y.-X.; Lian, W.-W.; Yan, Y.; Ma, B.-Z.; Cheng, Y.-C.; Xu, J.-K.; He, J.; Zhang, W.-K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol. Res. 2023, 187, 106625. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Liu, Y.-Z.; Shen, X.-L.; Wu, T.-Y.; Zhang, T.; Wang, W.; Wang, Y.-X.; Jiang, C.-L. NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. Int. J. Neuropsychopharmacol. 2015, 18, pyv006. [Google Scholar] [CrossRef]
- Han, C.; Pei, H.; Shen, H.; Zhai, L.; Yang, Y.; Li, W.; Wang, J. Antcin K targets NLRP3 to suppress neuroinflammation and improve the neurological behaviors of mice with depression. Int. Immunopharmacol. 2023, 117, 109908. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Liu, Z.; Li, X.; Liu, M.; Wang, Y.; Zhang, K.; Sun, N. Association of the TLR4 gene with depressive symptoms and antidepressant efficacy in major depressive disorder. Neurosci. Lett. 2020, 736, 135292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Wang, C.; He, C.; Ma, Q.; Li, J.; Wang, W.; Xu, Y.-T.; Wang, T. Qingwenzhike Prescription Alleviates Acute Lung Injury Induced by LPS via Inhibiting TLR4/NF-kB Pathway and NLRP3 Inflammasome Activation. Front. Pharmacol. 2021, 12, 790072. [Google Scholar] [CrossRef]
- Kéri, S.; Szabó, C.; Kelemen, O. Expression of Toll-Like Receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain, Behav. Immun. 2014, 40, 235–243. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Liu, L.; Zhang, W.; Zhang, Y.; Liu, Y.-Z.; Shen, X.-L.; Gong, H.; Yang, Y.-Y.; Bi, X.-Y.; Jiang, C.-L.; et al. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J. Psychiatr. Res. 2015, 64, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Moylan, S.; Berk, M.; Dean, O.M.; Samuni, Y.; Williams, L.J.; O’nEil, A.; Hayley, A.C.; Pasco, J.A.; Anderson, G.; Jacka, F.N.; et al. Oxidative & nitrosative stress in depression: Why so much stress? Neurosci. Biobehav. Rev. 2014, 45, 46–62. [Google Scholar] [CrossRef]
- Wigner, P.; Czarny, P.; Galecki, P.; Su, K.-P.; Sliwinski, T. The molecular aspects of oxidative & nitrosative stress and the tryptophan catabolites pathway (TRYCATs) as potential causes of depression. Psychiatry Res. 2018, 262, 566–574. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Fındıklı, E.; İzci, F.; Kurutaş, E.B.; Tuman, T.C. Evaluation of malondialdehyde, superoxide dismutase and catalase activity and their diagnostic value in drug naïve, first episode, non-smoker major depression patients and healthy controls. Psychiatry Res. 2016, 238, 81–85. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yao, J.K. Oxidative stress and therapeutic implications in psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 197–199. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Duman, R.S.; Malberg, J.; Thome, J. Neural plasticity to stress and antidepressant treatment. Biol. Psychiatry 1999, 46, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Pilar-Cuéllar, F.; Vidal, R.; Díaz, A.; Castro, E.; dos Anjos, S.; Pascual-Brazo, J.; Linge, R.; Vargas, V.; Blanco, H.; Martínez-Villayandre, B.; et al. Neural Plasticity and Proliferation in the Generation of Antidepressant Effects: Hippocampal Implication. Neural Plast. 2013, 2013, 537265. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; Voss, G.T.; Rodrigues, K.d.C.; Pinz, M.P.; Biondi, J.V.; Becker, N.P.; Blodorn, E.; Domingues, W.B.; Larroza, A.; Campos, V.F.; et al. Prospecting for a quinoline containing selenium for comorbidities depression and memory impairment induced by restriction stress in mice. Psychopharmacology 2022, 239, 59–81. [Google Scholar] [CrossRef]
- Ruiz, N.A.L.; del Ángel, D.S.; Olguín, H.J.; Silva, M.L. Neuroprogression: The hidden mechanism of depression. Neuropsychiatr. Dis. Treat. 2018, 14, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Zelada, M.I.; Garrido, V.; Liberona, A.; Jones, N.; Zúñiga, K.; Silva, H.; Nieto, R.R. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Major Depressive Disorder (MDD): A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14810. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Srivastava, P.; Bungau, S. Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. J. Mol. Neurosci. 2021, 71, 2008–2021. [Google Scholar] [CrossRef]
- Sambataro, F.; Murty, V.P.; Lemaitre, H.S.; Reed, J.D.; Das, S.; Goldberg, T.E.; Callicott, J.H.; Weinberger, D.R.; Mattay, V.S. BNDF modulates normal human hippocampal ageing. Mol. Psychiatry 2010, 15, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Han, R.; Liu, Z.; Sun, N.; Liu, S.; Li, L.; Shen, Y.; Xiu, J.; Xu, Q. BDNF Alleviates Neuroinflammation in the Hippocampus of Type 1 Diabetic Mice via Blocking the Aberrant HMGB1/RAGE/NF-κB Pathway. Aging Dis. 2019, 10, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Dranovsky, A.; Hen, R. The when and where of BDNF and the antidepressant response. Biol. Psychiatry 2008, 63, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.O.; Martins, L.F.; Tahiri, E.; Duarte, C.B. Brain-derived neurotrophic factor-induced regulation of RNA metabolism in neuronal development and synaptic plasticity. Wiley Interdiscip. Rev. RNA 2022, 13, e1713. [Google Scholar] [CrossRef]
- Ambrus, L.; Lindqvist, D.; Träskman-Bendz, L.; Westrin, Å. Hypothalamic–pituitary–adrenal axis hyperactivity is associated with decreased brain-derived neurotrophic factor in female suicide attempters. Nord. J. Psychiatry 2016, 70, 575–581. [Google Scholar] [CrossRef]
- Lepack, A.E.; Fuchikami, M.; Dwyer, J.M.; Banasr, M.; Duman, R.S. BDNF Release Is Required for the Behavioral Actions of Ketamine. Int. J. Neuropsychopharmacol. 2014, 18, pyu033. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.; Cui, L.; Zhou, B.; Liu, W.; Xu, F.; Hayashi, T.; Hattori, S.; Ushiki-Kaku, Y.; Tashiro, S.-I.; et al. Silibinin ameliorates anxiety/depression-like behaviors in amyloid β-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physiol. Behav. 2017, 179, 487–493. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. Sesquiterpenoids from the Root of Panax ginseng Attenuates Lipopolysaccharide-Induced Depressive-Like Behavior through the Brain-Derived Neurotrophic Factor/Tropomyosin-Related Kinase B and Sirtuin Type 1/Nuclear Factor-κB Signaling Pathways. J. Agric. Food Chem. 2017, 66, 265–271. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, J.; Zheng, J.; Li, X.; Zhao, F. Deterministic transition of enterotypes shapes the infant gut microbiome at an early age. Genome Biol. 2021, 22, 243. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef]
- Zheng, P.; Yang, J.; Li, Y.; Wu, J.; Liang, W.; Yin, B.; Tan, X.; Huang, Y.; Chai, T.; Zhang, H.; et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv. Sci. 2020, 7, 1902862. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Microbiota and neuroimmune signalling—Metchnikoff to microglia. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Fischbach, M.A. Small molecules from the human microbiota. Science 2015, 349, 1254766. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Maes, M. The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Mol. Neurobiol. 2020, 57, 4269–4295. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2014, 29, 1395–1403. [Google Scholar] [CrossRef]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2009, 11, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, srep43859. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; del Mármol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Wilson, S.J.; Bailey, M.L.; Andridge, R.; Peng, J.; Jaremka, L.M.; Fagundes, C.P.; Malarkey, W.B.; Laskowski, B.; Belury, M.A. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98, 52–60. [Google Scholar] [CrossRef]
- Sarkar, S.R.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Tian, P.; Zou, R.; Song, L.; Zhang, X.; Jiang, B.; Wang, G.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Ingestion of Bifidobacterium longum subspecies infantis strain CCFM687 regulated emotional behavior and the central BDNF pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019, 10, 7588–7598. [Google Scholar] [CrossRef]
- Winther, G.; Jørgensen, B.M.P.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef]
- Jang, H.-M.; Lee, K.-E.; Kim, D.-H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.-L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, D.; Caso, J.R.; Bris, Á.G.; Maus, S.R.; Madrigal, J.L.M.; García-Bueno, B.; MacDowell, K.S.; Alou, L.; Gómez-Lus, M.L.; Leza, J.C. Bacterial translocation affects intracellular neuroinflammatory pathways in a depression-like model in rats. Neuropharmacology 2016, 103, 122–133. [Google Scholar] [CrossRef]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part II–contemporary contextual research. Gut Pathog. 2013, 5, 3. [Google Scholar] [CrossRef]
- Bhandari, S.; Larson, M.E.; Kumar, N.; Stein, D. Association of Inflammatory Bowel Disease (IBD) with Depressive Symptoms in the United States Population and Independent Predictors of Depressive Symptoms in an IBD Population: A NHANES Study. Gut Liver 2017, 11, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor Necrosis Factor-α Mediates the Blood–Brain Barrier Dysfunction Induced by Activated Microglia in Mouse Brain Microvascular Endothelial Cells. J. Pharmacol. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.-C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Park, A.J.; Collins, J.; Blennerhassett, P.A.; Ghia, J.E.; Verdu, E.F.; Bercik, P.; Collins, S.M. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 2013, 25, 733-e575. [Google Scholar] [CrossRef]
- Ichimura, A.; Hirasawa, A.; Hara, T.; Tsujimoto, G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009, 89, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Ihara, S.; Katayama, N.; Nogami, W.; Amano, M.; Noda, S.; Kurata, C.; Kobayashi, Y.; Sasaki, Y.; Mitsuda, D.; Ozawa, M.; et al. Comparison of changes in stress coping strategies between cognitive behavioral therapy and pharmacotherapy. Front. Psychiatry 2024, 15, 1343637. [Google Scholar] [CrossRef]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Zhao, J.; Jia, Y.; Zhao, W.; Chen, H.; Zhang, X.; Ngo, F.Y.; Luo, D.; Song, Y.; Lao, L.; Rong, J.; et al. Botanical Drug Puerarin Ameliorates Liposaccharide-Induced Depressive Behaviors in Mice via Inhibiting RagA/mTOR/p70S6K Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 7716201. [Google Scholar] [CrossRef]
- Gao, L.-N.; Yan, M.; Zhou, L.; Wang, J.; Sai, C.; Fu, Y.; Liu, Y.; Ding, L. Puerarin Alleviates Depression-Like Behavior Induced by High-Fat Diet Combined With Chronic Unpredictable Mild Stress via Repairing TLR4-Induced Inflammatory Damages and Phospholipid Metabolism Disorders. Front. Pharmacol. 2021, 12, 767333. [Google Scholar] [CrossRef]
- Kuhn, R. The treatment of depressive states with G 22355 (imipramine hydrochloride). Am. J. Psychiatry 1958, 115, 459–464. [Google Scholar] [CrossRef]
- Boyce, P.; Judd, F. The Place for the Tricyclic Antidepressants in the Treatment of Depression. Aust. N. Z. J. Psychiatry 1999, 33, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I.; Herrmann, N.; Walker, S.E. Current Place of Monoamine Oxidase Inhibitors in the Treatment of Depression. CNS Drugs 2013, 27, 789–797. [Google Scholar] [CrossRef]
- Henkel, V.; Mergl, R.; Allgaier, A.-K.; Kohnen, R.; Möller, H.-J.; Hegerl, U. Treatment of depression with atypical features: A meta-analytic approach. Psychiatry Res. 2006, 141, 89–101. [Google Scholar] [CrossRef]
- Orsolini, L.; Vellante, F.; Valchera, A.; Fornaro, M.; Carano, A.; Pompili, M.; Perna, G.; Serafini, G.; Di Nicola, M.; Martinotti, G.; et al. Atypical Antipsychotics in Major Depressive Disorder. In Understanding Depression; Springer: Singapore, 2018; pp. 257–268. [Google Scholar] [CrossRef]
- de Filippis, R.; Foysal, A.A. Case Report: The Role of Monoamine Oxidase Inhibitors in Treating Resistant Depression. Open Access Libr. J. 2024, 11, 1–12. [Google Scholar] [CrossRef]
- Feighner, J.P. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999, 60, 4–13. [Google Scholar] [PubMed]
- Pannu, A.; Goyal, R.K. From Evidence to Practice: A Comprehensive Analysis of Side Effects in Synthetic Anti-Depressant Therapy. Curr. Drug Saf. 2025, 20, 120–147. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef]
- E Murphy, S.; Capitão, L.P.; Giles, S.L.C.; Cowen, P.J.; Stringaris, A.; Harmer, C.J. The knowns and unknowns of SSRI treatment in young people with depression and anxiety: Efficacy, predictors, and mechanisms of action. Lancet Psychiatry 2021, 8, 824–835. [Google Scholar] [CrossRef]
- Den Boer, J.A.; Bosker, F.J.; Slaap, B.R. Serotonergic drugs in the treatment of depressive and anxiety disorders. Hum. Psychopharmacol. Clin. Exp. 2000, 15, 315–336. [Google Scholar] [CrossRef]
- Lurati, A.R. Management of Antidepressant Therapy–Induced Sexual Dysfunction in Women. J. Nurse Pract. 2022, 18, 522–524. [Google Scholar] [CrossRef]
- Horowitz, M.A.; Taylor, D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry 2019, 6, 538–546. [Google Scholar] [CrossRef]
- Lambert, O.; Bourin, M. SNRIs: Mechanism of action and clinical features. Expert Rev. Neurother. 2014, 2, 849–858. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Gao, Z.; Feng, Y.; Luo, H.; Lu, T.; Sun, X.; Hu, J.; Luo, Y. SNRIs achieve faster antidepressant effects than SSRIs by elevating the concentrations of dopamine in the forebrain. Neuropharmacology 2020, 177, 108237. [Google Scholar] [CrossRef] [PubMed]
- Zemanova, N.; Anzenbacher, P.; Anzenbacherova, E. The role of cytochromes P450 in the metabolism of selected antidepressants and anxiolytics under psychological stress. Biomed. Pap. 2022, 166, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Prost, J.F.; Solles, A.; Briley, M. Efficacy and tolerability of milnacipran: An overview. Int. Clin. Psychopharmacol. 1996, 11, 47–51. [Google Scholar] [CrossRef]
- Puech, A.; Montgomery, S.A.; Prost, J.F.; Solles, A.; Briley, M. Milnacipran, a new serotonin and noradrenaline reuptake inhibitor: An overview of its antidepressant activity and clinical tolerability. Int. Clin. Psychopharmacol. 1997, 12, 99–108. [Google Scholar] [CrossRef]
- Sobieraj, D.M.; Martinez, B.K.; Hernandez, A.V.; Coleman, C.I.; Ross, J.S.; Berg, K.M.; Steffens, D.C.; Baker, W.L. Adverse Effects of Pharmacologic Treatments of Major Depression in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Grinchii, D.; Dremencov, E. Mechanism of Action of Atypical Antipsychotic Drugs in Mood Disorders. Int. J. Mol. Sci. 2020, 21, 9532. [Google Scholar] [CrossRef]
- Morais, M.; Patrício, P.; Mateus-Pinheiro, A.; Alves, N.D.; Machado-Santos, A.R.; Correia, J.S.; Pereira, J.; Pinto, L.; Sousa, N.; Bessa, J.M. The modulation of adult neuroplasticity is involved in the mood-improving actions of atypical antipsychotics in an animal model of depression. Transl. Psychiatry 2017, 7, e1146. [Google Scholar] [CrossRef]
- Fasipe, O.J. The emergence of new antidepressants for clinical use: Agomelatine paradox versus other novel agents. IBRO Rep. 2019, 6, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Pruckner, N.; Holthoff-Detto, V. Antidepressant pharmacotherapy in old-age depression—A review and clinical approach. Eur. J. Clin. Pharmacol. 2017, 73, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Carboni, L.; Rullo, L.; Caputi, F.F.; Stamatakos, S.; Candeletti, S.; Romualdi, P. Chronic Trazodone and Citalopram Treatments Increase Trophic Factor and Circadian Rhythm Gene Expression in Rat Brain Regions Relevant for Antidepressant Efficacy. Int. J. Mol. Sci. 2022, 23, 14041. [Google Scholar] [CrossRef]
- Howland, R. Critical appraisal and update on the clinical utility of agomelatine, a melatonergic agonist, for the treatment of major depressive disease in adults. Neuropsychiatr. Dis. Treat. 2009, 5, 563–576. [Google Scholar] [CrossRef]
- Jha, M.K.; Mathew, S.J. Pharmacotherapies for Treatment-Resistant Depression: How Antipsychotics Fit in the Rapidly Evolving Therapeutic Landscape. Am. J. Psychiatry 2023, 180, 190–199. [Google Scholar] [CrossRef]
- Panocka, I.; Perfumi, M.; Angeletti, S.; Ciccocioppo, R.; Massi, M. Effects of Hypericum perforatum Extract on Ethanol Intake, and on Behavioral Despair: A Search for the Neurochemical Systems Involved. Pharmacol. Biochem. Behav. 2000, 66, 105–111. [Google Scholar] [CrossRef]
- Brattström, A. Long-term effects of St. John’s wort (Hypericum perforatum) treatment: A 1-year safety study in mild to moderate depression. Phytomedicine 2009, 16, 277–283. [Google Scholar] [CrossRef]
- Seifritz, E.; Hatzinger, M.; Holsboer-Trachsler, E. Efficacy of Hypericum extract WS® 5570 compared with paroxetine in patients with a moderate major depressive episode—A subgroup analysis. Int. J. Psychiatry Clin. Pract. 2016, 20, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Vance, K.M.; Ribnicky, D.M.; Hermann, G.E.; Rogers, R.C. St. John’s Wort enhances the synaptic activity of the nucleus of the solitary tract. Nutrition 2014, 30, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.-A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef]
- Yang, S.-J.; Yu, H.-Y.; Kang, D.-Y.; Ma, Z.-Q.; Qu, R.; Fu, Q.; Ma, S.-P. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol. Biochem. Behav. 2014, 124, 451–457. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Miao, M.; Yan, T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015, 606, 1–6. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Zee, J.; Soeller, I.; Li, Q.S.; Rockwell, K.; Amsterdam, J.D. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine 2015, 22, 394–399. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, R.; Zhao, L.; Fan, J.; Li, B. Mechanisms of Panax ginseng action as an antidepressant. Cell Prolif. 2019, 52, e12696. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Yang, L.; Wang, Y.; Lu, P.; Li, W.; Miao, J.; Gong, Y.; Zhang, B.; Yin, Y. Ginseng total saponins rescue susceptibility to adult depression-like behaviors in mice induced by early-life stress via regulating CREB/BDNF/TrkB signaling. CyTA J. Food 2023, 21, 701–710. [Google Scholar] [CrossRef]
- Chen, L.; Dai, J.; Wang, Z.; Zhang, H.; Huang, Y.; Zhao, Y.; Rahman, K. Ginseng Total Saponins Reverse Corticosterone-Induced Changes in Depression-Like Behavior and Hippocampal Plasticity-Related Proteins by Interfering with GSK-3β-CREB Signaling Pathway. Evid.-Based Complement. Altern. Med. 2014, 2014, 506735. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Wang, J.-J.; Cheng, P.; Chen, L.-X.; Hu, J.-M.; Zhu, G.-Q. Ginsenoside Rg1 in neurological diseases: From bench to bedside. Acta Pharmacol. Sin. 2022, 44, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-X.; Qi, Z.; Shao, Z.-J.; Li, S.-S.; Qi, Y.-L.; Gao, K.; Liu, S.-X.; Li, Z.; Sun, Y.-S.; Li, P.-Y. Study on Antidepressant Activity of Pseudo-Ginsenoside HQ on Depression-Like Behavior in Mice. Molecules 2019, 24, 870. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, L.; Ma, J.; Yuan, J.; Pi, C.; Xiong, L.; Chen, J.; Liu, H.; Tang, J.; Zhong, Y.; et al. Molecular mechanisms of rapid-acting antidepressants: New perspectives for developing antidepressants. Pharmacol. Res. 2023, 194, 106837. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef]
- A Cristea, I.; Naudet, F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 2019, 6, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Akbar, D.; Rhee, T.G.; Ceban, F.; Ho, R.; Teopiz, K.M.; Cao, B.; Subramaniapillai, M.; Kwan, A.T.H.; Rosenblat, J.D.; McIntyre, R.S. Dextromethorphan-Bupropion for the Treatment of Depression: A Systematic Review of Efficacy and Safety in Clinical Trials. CNS Drugs 2023, 37, 867–881. [Google Scholar] [CrossRef]
- Fava, M.; Stahl, S.M.; Pani, L.; De Martin, S.; Cutler, A.J.; Maletic, V.; Gorodetzky, C.W.; Vocci, F.J.; Sapienza, F.L.; Kosten, T.R.; et al. Efficacy and safety of esmethadone (REL-1017) in patients with major depressive disorder and inadequate response to standard antidepressants: A phase 3 randomized controlled trial. J. Clin. Psychiatry 2024, 85, 24m15265. [Google Scholar] [CrossRef] [PubMed]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D.; et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021, 589, 474–479. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Cariprazine: First Global Approval. Drugs 2015, 75, 2035–2043. [Google Scholar] [CrossRef]
- Ali, E.; Latif, F.; Mashkoor, Y.; Sheikh, A.; Iqbal, A.; Owais, R.; Ahmed, J.; Naveed, S.; Moeed, A.; Ullah, I.; et al. Role of Adjunctive Cariprazine for Treatment-Resistant Depression in patients with Major Depressive Disorder: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Asian J. Psychiatry 2024, 95, 104005. [Google Scholar] [CrossRef]
- Papp, M.; Gruca, P.; Litwa, E.; Lason, M.; Newman-Tancredi, A.; Depoortère, R. The 5-HT1A receptor biased agonists, NLX-204 and NLX-101, like ketamine, elicit rapid-acting antidepressant activity in the rat chronic mild stress model via cortical mechanisms. J. Psychopharmacol. 2024, 38, 661–671. [Google Scholar] [CrossRef]
- Efthimiou, O.; Taipale, H.; Radua, J.; Schneider-Thoma, J.; Pinzón-Espinosa, J.; Ortuño, M.; Vinkers, C.H.; Mittendorfer-Rutz, E.; Cardoner, N.; Tanskanen, A.; et al. Efficacy and effectiveness of antipsychotics in schizophrenia: Network meta-analyses combining evidence from randomised controlled trials and real-world data. Lancet Psychiatry 2024, 11, 102–111. [Google Scholar] [CrossRef]
- Chowdhury, A.; Boukezzi, S.; Costi, S.; Hameed, S.; Jacob, Y.; Salas, R.; Iosifescu, D.V.; Han, M.-H.; Swann, A.; Mathew, S.J.; et al. Effects of the KCNQ (Kv7) Channel Opener Ezogabine on Resting-State Functional Connectivity of Striatal Brain Reward Regions, Depression, and Anhedonia in Major Depressive Disorder: Results From a Randomized Controlled Trial. Biol. Psychiatry 2025. [Google Scholar] [CrossRef] [PubMed]
- Arcella, A.; Alborghetti, M.; Traficante, A.; Oliva, M.A.; Staffieri, S.; Russo, V.; Caridi, M.; Battaglia, G. Pharmacological Blockade of Group II Metabotropic Glutamate Receptors Reduces the Incidence of Brain Tumors Induced by Prenatal Exposure to N-ethyl-N-nitrosourea in Rats. Curr. Neuropharmacol. 2024, 23, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Zelek-Molik, A.; Litwa, E. Trends in research on novel antidepressant treatments. Front. Pharmacol. 2025, 16, 1544795. [Google Scholar] [CrossRef] [PubMed]



| Class | Examples | Primary Mechanism | Clinical Evidence | Key Limitations | References |
|---|---|---|---|---|---|
| MAOIs | Phenelzine, Isocarboxazid | MAO inhibition → ↑ monoamines | Established efficacy in atypical depression | Tyramine restrictions, hypertensive risk | [126,127,128,143] |
| TCAs | Amitriptyline, Imipramine | 5-HT/NE reuptake inhibition | Broad efficacy, low cost | Anticholinergic effects, cardiotoxicity | [125,130,131,132] |
| SSRIs | Fluoxetine, Sertraline | Selective 5-HT reuptake inhibition | First-line for mild-mod MDD | Sexual dysfunction, withdrawal | [133,134,135,136,144] |
| SNRIs | Venlafaxine, Duloxetine | Dual 5-HT/NE reuptake inhibition | Rapid onset (some agents) | Hypertension, nausea | [137,139,140,141,142] |
| Atypical | Bupropion, Agomelatine | Varied (e.g., NDRI, melatonergic agonism) | Fewer anticholinergic effects | Sedation, activation | [143,144,145,146,147,148,149] |
| Herbal Agents | Hypericum perforatum (Hypericin) | SERT/NET inhibition, GABA modulation | Established: Efficacy vs. placebo (mild-mod MDD) | CYP450 interactions | [150,151,152,153] |
| Rhodiola rosea (Salidroside) | MAO inhibition, ↓IL-6, ↑BDNF | Probable: Improved HAM-D scores (RCTs) | Limited long-term data | [154,155,156,157] | |
| Panax ginseng (Ginsenosides) | HPA modulation, ↑BDNF/TrkB | Preclinical: Stress resilience models | No robust human RCTs | [158,159,160,161,162] | |
| Novel Agents | (R,S)-Ketamine | NMDA antagonism → ↑ AMPAR activation | FDA-approved: TRD, acute suicidality | Transient dissociation, abuse potential | [163,164,165,166] |
| Psilocybin | 5-HT2A agonism → neural plasticity | Phase II: Breakthrough therapy (TRD) | Hallucinogenic effects | [167,168] | |
| Cariprazine (adjunct) | D2/D3 partial agonism | FDA-approved: Adjunct for MDD/BD | Akathisia, metabolic effects | [169,170,171] |
| Evidence Domain | Key Limitations | Evidence Quality | Conflicting Findings/Gaps | References |
|---|---|---|---|---|
| Biomarker Heterogeneity | High inter-study variability in cytokine/oxidative stress levels; lack of standardized assays. | L (Inconsistency 1) | IL-6 elevation in depression: 67% studies vs. 33% null (n = 45 meta-analyses) | [39,40,41,43,44,45,61,62] |
| IDO Inhibitor Translation | Compensatory TDO activation in humans; low BBB permeability of candidates; no predictive biomarkers. | VL (Indirectness 2) | Preclinical efficacy (rodent despair tests) vs. null human RCTs (n = 3) | [42,43,44,45,46,47,48,97] |
| Microbiome Reproducibility | Geographical/dietary confounders; low agreement on depression-associated taxa (F/B ratio, Bacteroides spp.). | L (Imprecision 3) | FMT-induced depressive phenotypes: replicable in rodents (n = 5) but not primates | [82,83,84,85,86,87,97,105,106,107] |
| Neuroplasticity Targets | BDNF as treatment biomarker: inconsistent correlation with symptom improvement (HDRS ∆). | M (Publication bias 4) | BDNF ↑ post-SSRI: 71% studies (95% CI: 62–79%); no ∆ in 29% | [70,71,72,73,74,75,79,80,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhang, Z.; Zhang, Z.; Liu, D.; Shang, Y.; Tang, C.; Wang, W.; Li, H.; You, B.; Ying, H.; et al. The Pathogenesis and Medical Treatment of Depression: Opportunity and Challenge. Neurol. Int. 2025, 17, 120. https://doi.org/10.3390/neurolint17080120
Xu M, Zhang Z, Zhang Z, Liu D, Shang Y, Tang C, Wang W, Li H, You B, Ying H, et al. The Pathogenesis and Medical Treatment of Depression: Opportunity and Challenge. Neurology International. 2025; 17(8):120. https://doi.org/10.3390/neurolint17080120
Chicago/Turabian StyleXu, Mengjiao, Zhiyu Zhang, Zhoudong Zhang, Dong Liu, Yanguo Shang, Chenglun Tang, Weipeng Wang, Huanqiu Li, Bengang You, Hanjie Ying, and et al. 2025. "The Pathogenesis and Medical Treatment of Depression: Opportunity and Challenge" Neurology International 17, no. 8: 120. https://doi.org/10.3390/neurolint17080120
APA StyleXu, M., Zhang, Z., Zhang, Z., Liu, D., Shang, Y., Tang, C., Wang, W., Li, H., You, B., Ying, H., & Shen, T. (2025). The Pathogenesis and Medical Treatment of Depression: Opportunity and Challenge. Neurology International, 17(8), 120. https://doi.org/10.3390/neurolint17080120






