Abstract
Background/Objectives: This study aimed to examine the influence of brain activity during motor preparation on walking ability, focusing on motor control during active ankle dorsiflexion. Methods: Participants were classified into high- and low-corticomuscular coherence (CMC), an index of neuromuscular control based on the median value. Biomechanical and neurophysiological indices of active ankle dorsiflexion and walking ability were compared between the two groups. Additionally, a machine learning model was developed to accurately predict the CMC classification using brain neural activity during motor preparation. Results: The Cz-TA CMC (beta frequency band) during active ankle dorsiflexion successfully detected significant differences in the maximum dorsiflexion angle, inversion angular velocity, brain activity localization, and variations in Cz beta power values during the transition from motor preparation to execution. Furthermore, CMC identified significant differences in dorsiflexion angle changes after toe-off and inversion angles at initial contact during gait. A support-vector machine model predicting high or low CMC demonstrated high accuracy (Accuracy: 0.96, Precision: 0.92–1.00, Recall: 0.91–1.00, F1 Score: 0.95–0.96) during motor execution based on beta power values from −500 to 0 ms prior to the initiation of active ankle dorsiflexion (representing motor preparation). Conclusions: These findings highlight that the motor preparation processes of the brain during active ankle dorsiflexion are involved in walking ability and can be used to predict it. This indicator is independent of disease severity and holds the potential to provide a clinically versatile evaluation method.
1. Introduction
Walking is a uniquely acquired bipedal motion that plays a vital role in various aspects of daily life [1]. Walking is essential for functional independence as a means of human mobility [2] and is critical for maintaining the quality of life [3]. Typical clinical evaluation indices of gait performance include gait speed, which is influenced by the gait pattern [4]. Among these, ankle movement control is indispensable for maintaining postural balance and adjusting gait speed through changes in gait patterns [4,5].
Ankle joint control coordinates the timing of ankle joint torque exertion to angular displacement during changes in gait speed [6], and is closely associated with the modulation of gait speed via kinematic alterations in ankle movement [7,8]. However, neurological degeneration due to aging, motor impairments, or other neurological conditions can lead to gait disorders stemming from decreased ankle control [9,10,11]. Improving ankle control is essential for gait rehabilitation, making its evaluation critical for the acquisition or restoration of walking ability. Currently, clinical assessments of ankle control often involve kinematic and kinetic analysis during walking. However, for individuals with severe impairments, direct assessment of walking is often not feasible [12,13]. Therefore, there is a pressing need to identify clinical indicators of ankle control that are independent of the severity and applicable to gait rehabilitation.
From a kinematic perspective, the voluntary dorsiflexion of the ankle is considered important in both the stance and swing phases of gait. Tibialis anterior (TA) muscle activity during dorsiflexion has been shown to resemble cortical motor control observed during walking [14]. Additionally, Dobkin et al. [15] demonstrated via functional magnetic resonance imaging (fMRI) measurements of brain activity that ankle dorsiflexion depends on the descending input from the primary motor cortex and can indirectly assess the spinal sensorimotor network involved in gait control. For these reasons, voluntary dorsiflexion of the ankle under non-weight-bearing conditions has been considered a potentially valuable indicator of lower-limb motor control during gait [16].
However, in clinical practice, there are cases in which the walking ability is impaired despite preserved voluntary dorsiflexion function [17,18], suggesting an inconsistent relationship between the voluntary dorsiflexion function of the ankle and gait ability. Lodha et al. [19] reported that motor control of plantar flexion and dorsiflexion rather than muscle strength may predict walking ability. One method for evaluating motor control is corticomuscular coherence (CMC), which is measured using synchronized electroencephalogram (EEG) and electromyogram (EMG) recordings of the motor areas (Cz) and muscles TA during ankle dorsiflexion [20,21,22]. The β-frequency band of Cz-TA coherence is considered an indicator of neuromuscular control of the lower limbs, with potential links to motor function and walking ability [23].
However, human movement is inherently redundant [24], and existing evaluations lack a comprehensive analysis that integrates both the kinematic and kinetic aspects of movement and the underlying neurophysiological mechanisms. Furthermore, the current evaluation methods rely on actual movements, which may not sufficiently detect foot movements in severely impaired individuals. Therefore, these methods may only be applicable to patients with relatively high levels of voluntary control.
To address this, we drew inspiration from the concept of the motor preparation stage, which involves the simulation of physical movements. Voluntary motor control involves processes such as motor program generation, postural control, and execution of fine movements [25]. Prior to motor execution, motor intention, and planning are processed in the brain during the motor preparation stage [26,27]. Evaluating the entire process from motor preparation to execution may provide insights into the essence of motor control, beyond merely capturing actual movements.
Information processing in the brain during motor preparation may serve as a versatile new indicator of ankle control related to walking ability, even in patients with severe sensorimotor disabilities in whom detecting actual movement is difficult. Furthermore, because this indicator can be measured in the supine, non-weight-bearing position, it has the potential to predict walking ability early and assist in setting clear rehabilitation goals.
The objective of this study was to comprehensively examine the effects of brain information processing during motor preparation for active ankle dorsiflexion on motor control indicators such as CMC and walking ability.
2. Materials and Methods
2.1. Participants
The participants of this study were 23 healthy adults, consisting of 18 males and 5 females (age: 22 ± 1 years, height: 1.70 ± 0.06 m, weight: 65.07 ± 14.81 kg). Exclusion criteria included cognitive impairment, sensorimotor dysfunction in the lower limbs, and visual impairment. The study procedures were explained to the participants both orally and in writing, and written informed consent was obtained prior to participation. All participants took part in the experiment after providing informed consent. This study was approved by the Kyoto Tachibana University Research Ethics Committee (Approval Number: 22-13) and conducted in accordance with the Declaration of Helsinki.
2.2. Protocol
This study measured ankle joint angles, lower-limb muscle activity, and brain activity during active ankle dorsiflexion and gait tasks. A comprehensive evaluation of ankle control requires a multifaceted approach that includes the assessment of joint angles, muscle activity, and brain activity. Therefore, we utilized the Articulation Motion Assessment System (AMAS) [28], which we developed to enable precise kinematic measurements of joint motion synchronized with other biosignals, such as muscle and brain activity.
The participants wore the AMAS, surface EMG, and scalp EEG devices on one leg. The measurements were performed during two tasks: ankle dorsiflexion and gait.
Active ankle dorsiflexion task
Each participant performed periodic ankle dorsiflexion movements for 3 s, followed by a 7 s rest, and this was repeated 10 times. The tasks were performed at a self-selected pace [23]. Their feet were suspended to allow unrestricted ankle movement (Figure 1).
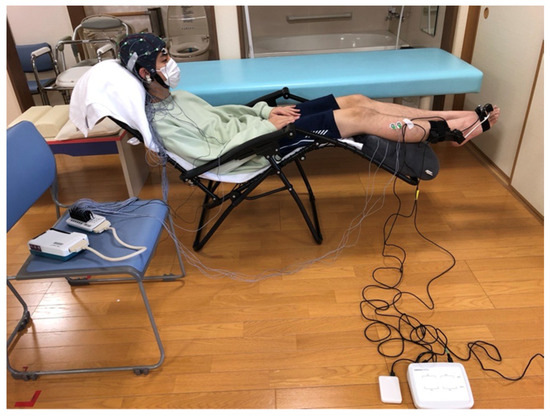
Figure 1.
Experimental Setup. Active Ankle Dorsiflexion Task: Participants sat in a chair positioned to allow a clear view of their feet. During the task, a scalp electroencephalogram device and the articulation motion assessment system were carefully set up to prevent entanglement of wires. The angle of the reclining chair was standardized across all participants.
Walking task
Each participant performed 10 round trips on an 8 m walking path with a 1.5 m acceleration zone at the start and a 1.5 m deceleration zone at the end. A sensor mat was installed on the walking path to detect the initial contact (IC) and toe-off (TO). Participants walked at a comfortable speed.
Regarding ankle movement, previous reports have indicated no significant differences between the left and right sides from both biomechanical and neurophysiological perspectives [29,30]. Therefore, the kicking foot was defined as the dominant foot during the measurements. The right foot was the dominant foot in all participants.
Visual and auditory information processing can also influence motor control [31]. To eliminate these effects, the participants were verbally instructed to look at their feet during the ankle dorsiflexion task and look straight ahead during the walking task to control for visual information processing. In addition, earplugs were used to block external environmental sounds, thereby eliminating the effects of auditory information processing (Figure 2).
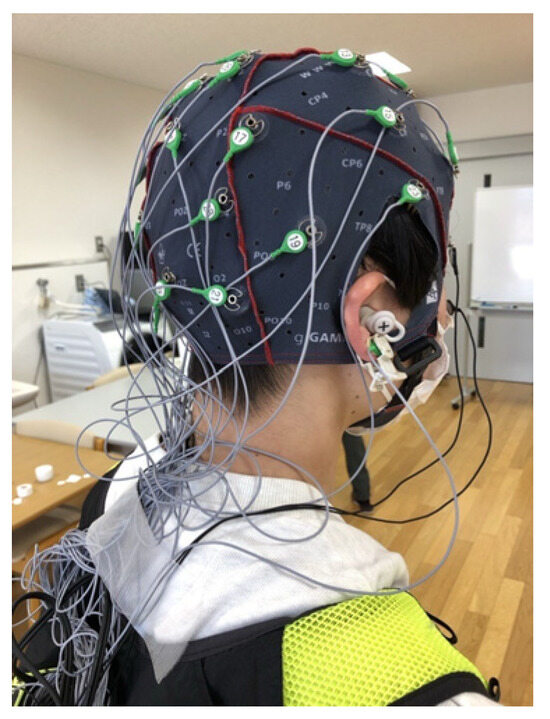
Figure 2.
Equipment Setup: Scalp electroencephalogram (EEG). Scalp EEG electrodes were attached using an EEG cap. The wires were loosely bundled to ensure they did not interfere with the movements of the participant. During the gait task, participants carried the scalp EEG device in a small backpack to allow for mobility while walking.
2.3. Instrumentation
2.3.1. AMAS
This joint angle measurement device simultaneously measures the plantar flexion/dorsiflexion and inversion/eversion angles during ankle joint motion and consists of an ankle joint orthosis, controller, and an operation application (Figure 3a). The ankle joint unit (Figure 3b) comprises two units with a built-in rotary encoder (Supertech Electronic, Rixin Street, Chiayi City, Taiwan). One unit was attached to the outer lower leg, which served as the fundamental axis, and the other unit was attached to the dorsal foot (metatarsal head), which served as the moving axis.
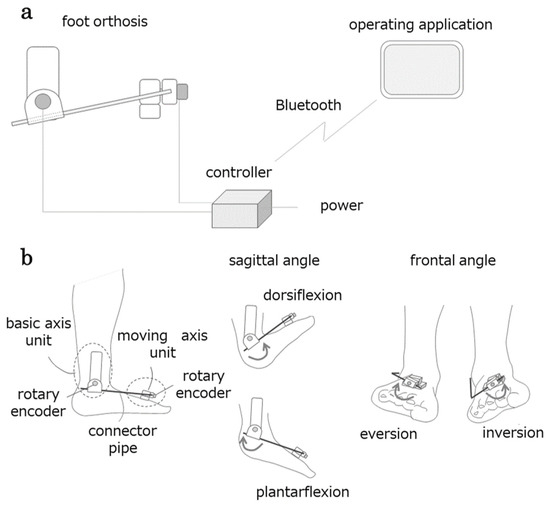
Figure 3.
Configuration of the articulation motion assessment system (AMAS). The AMAS comprises three components: a foot orthosis, a controller wirelessly connected to the operating application, and a foot orthosis (a). The foot orthosis measures the angles of the sagittal and frontal planes of the foot using a unit incorporating a rotary encoder on the basic axis and moving axis side (b).
A pipe connects both units, and the plantar dorsiflexion movement is transmitted to the basic axis-side unit via the connecting pipe, causing the rotary encoder to rotate. Similarly, the basic shaft-side unit supports the connecting pipe, and the inversion/eversion motion of the ankle is transmitted to the rotary encoder of the mobile shaft-side unit. The controller transmits the signal from the rotary encoder, resulting in ankle motion displayed in the application (tablet). The application converts the signals into angles and displays the changes in plantar flexion/dorsiflexion and inversion/eversion angles in real time. The angle data are saved in a CSV file. The system simultaneously extracts the dorsiflexion/plantar flexion and inversion/eversion angles (degrees) of the ankles. The sampling rate is set to 50 Hz.
2.3.2. Surface Electromyography Measurement
Surface EMG (sEMG) was used to measure muscle activity. The measurement followed a bipolar electrode configuration using Ag-AgCl electrodes (four electrodes), as described in previous studies [23]. Electrode placement was based on the SENIAM guidelines, with an inter-electrode distance of 2 cm. Electrodes were placed on the right TA and medialis gastrocnemius (MG) [32]. For the TA, the electrodes were positioned one-third of the distance along the line connecting the head of the fibula and the tip of the medial malleolus. For the MG, electrodes were placed at the most prominent part of the muscle belly. The sampling frequency was set to 1000 Hz.
2.3.3. EEG Measurement
EEG measurements were conducted using active electrodes and a Polymate V AP5148 biosignal acquisition system (Miyuki Giken Co., Ltd., Tokyo, Japan). The recording electrodes were positioned at 28 sites (FPz, Fz, Cz, Pz, Oz, Fp1, Fp2, F7, F8, F3, F4, C3, C4, C5, C6, P3, P4, P7, P8, O1, O2, T7, T8, CPz, CP1, CP2, PO3, and PO4) according to the international 10–10 system [33]. The ground electrode was placed on the spinous process of the seventh cervical vertebra (C7), and the reference electrode was attached to the right earlobe. In addition, Electro Oculography (EOG) electrodes were placed above and below the eyes to detect eye movement and blinking. The sampling frequency was set to 1000 Hz.
2.3.4. Video Recording and Synchronization
Each task was recorded using two 30-bit/s high-resolution digital video cameras (Panasonic, Tokyo, Japan). For the gait task, cameras were positioned to capture the frontal and sagittal planes, and the time taken to walk along the walkway was recorded. When the participants stepped outside the sensor mat, the IC and TO were visually identified from the recordings. To ensure synchronization, the video cameras and other measurement devices were triggered simultaneously when the participants stepped on the sensor mat.
2.4. Data Processing
The data for the ankle dorsiflexion task were triggered by the start of the ankle movement detected by the AMAS. Each trial consisted of a 6 s period, spanning from 3 s before movement onset to 3 s after movement onset. Data from 10 trials were extracted for the analysis (Figure 4).
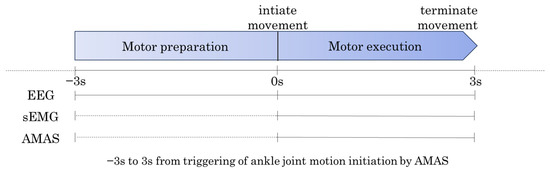
Figure 4.
Data Extraction for Ankle Dorsiflexion Task. The data for the ankle dorsiflexion task were divided into two periods. Motor Preparation Period: From 3 s before movement onset (−3 s) to 0 s (movement onset). Motor Execution Period: From 0 s (movement onset) to 3 s after movement onset (+3 s).
2.4.1. Extraction of Biomechanical Indicators
Biomechanical parameters for ankle motion were calculated for 10 repetitions in both the sagittal (dorsiflexion/plantarflexion) and frontal planes (inversion/eversion). The joint angle (°) and angular velocity (rad/s) were computed for each repetition [34,35,36]. The data extraction period was 3 s after the onset of dorsiflexion.
The biomechanical parameters for gait were calculated for 10 gait cycles, focusing on the ankle behavior during IC [37] and TO [38,39]. The joint angle and angular velocity [40,41] were analyzed during these phases. Additionally, gait performance was evaluated by extracting gait speed (m/s) as a performance metric [42,43,44].
The choice of these time windows and parameters was based on prior findings that the functional coupling between cortex and muscle in ankle joint motion is particularly pronounced during these phases [45]. Cortical activity associated with ankle dorsiflexion enhances functional connectivity with TA [46]. Moreover, variations in the ankle joint angles during the stance and swing phases of gait are characteristics of impaired ankle control [47,48,49,50]. These impairments are often accompanied by decreased joint angular velocity, which reflects deficits in muscle activation and force generation dynamics during walking [51]. Data from 10 repetitions of ankle dorsiflexion movements were averaged to obtain representative values.
2.4.2. Preprocessing of sEMG and EEG Data
The sEMG and EEG data were preprocessed using the multimodal EEG analysis program, EMSE Suite (CORETECH SOLUTIONS, Inc., Wilmington, NC, USA; Miyuki Giken Co., Ltd., Japan). First, a band-stop (notch) filter centered at 60 Hz with a bandwidth of 2 Hz was applied to the continuous sEMG and EEG data to remove power line noise from the commercial power supply frequency in western Japan (Kyoto). The sEMG data were filtered using a bandpass filter (5–300 Hz) and were full-wave rectified. The EEG data were filtered using a bandpass filter (0.5–45 Hz).
Artifacts from eye movements and blinking were removed using the EOG artifact removal function implemented in the EMSE.
The preprocessed continuous data were segmented into 6 s intervals, triggered by the onset of ankle dorsiflexion, from 3 s before to 3 s after the movement onset. The same preprocessing was applied to the data from the walking task in which the gait cycle was defined by two consecutive right-foot ICs.
2.4.3. Calculation of CMC
CMC, a neuromuscular control indicator for lower-limb movements, was calculated as a motor control index for ankle dorsiflexion [52]. The CMC was computed between Cz, TA, and MG. Coherence was derived from the normalization of the cross-spectrum. The formula for coherence is shown below (Equation (1)) [53].
: Cross-spectrum between signals and .
: Power spectral density of signal .
: Power spectral density of signal .
This metric quantifies the functional connectivity between the motor cortex and target muscles during ankle dorsiflexion movements.
The CMC is a valuable metric for analyzing the functional connectivity of the corticospinal tract between the cerebral cortex and muscles [54]. The coherence values always ranged between zero and one. A value of one indicates that the two signals are in a perfectly coherent (ideal) state. A value of zero indicates that the two signals are completely incoherent. To assess the reliability of the CMC, the confidence level () was calculated using Equation (2) [55]:
: Represents the number of data segments.
: Denotes the significance level.
If the calculated CMC value exceeds the 95% significance threshold (α = 0.95), it indicates a significant consistency between cortical and muscle activities. In this study, the total number of data segments was = 10. This resulted in a of 0.275. A CMC value above this threshold suggests statistically significant functional connectivity between the motor cortex and muscles during ankle dorsiflexion.
2.4.4. Calculation of Beta Power Value of EEG by Time-Frequency Analysis
In motor tasks, the event-related desynchronization of β-band power in the motor cortex is associated with motor cortex activation [56,57]. Event-related synchronization is related to post-movement motor evaluation phenomena [58,59]. To reflect the motor control process, fluctuations in β-band power (14–20 Hz), measured in μV2, were extracted as an indicator. The region of interest was the motor cortex corresponding to channel Cz.
A custom MATLAB(R2024b) script was used to perform the time-frequency analysis. This involved convolving a signal with a series of complex Morlet wavelets, which are defined as complex sine waves tapered using a Gaussian function.
The β power fluctuations at channel Cz were expressed as the percentage change in power within the defined time and frequency window relative to a baseline [55]. The baseline period was selected as −3000 to −2500 ms before movement onset.
The analysis used 11 segmented epochs, covering the time period from −2500 ms to +3000 ms around movement onset, as epochs (−2500~−2000 ms/−2000~−1500 ms/−1500~−1000 ms/−1000~−500 ms/−500~0 ms/0~500 ms/500~1000 ms/1000~1500 ms/1500~2000 ms/2000~2500 ms/2500~3000 ms). By setting this time window, the temporal changes in the motor control processes of EEG can be captured.
2.5. Statistical Analysis
All the statistical analyses were performed using SPSS Version 27.0 (IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test was performed to examine the normality of all data. The Cz-TA CMC (β) calculated during ankle dorsiflexion was summarized by its median. Based on this median, the participants were divided into two groups: a CMC-high group (higher CMC values) and a CMC-low group (lower CMC values). To compare the biomechanical indicators (joint angle and joint angular velocity) during active ankle dorsiflexion and walking tasks between the two groups, the following tests were used: independent t-tests (for normally distributed data) and the Mann–Whitney U test (for non-normally distributed data) were used. The effect sizes were calculated based on Cohen’s criteria. This analysis aimed to validate whether the Cz-TA CMC (β) during ankle dorsiflexion could differentiate motor control abilities during both active ankle dorsiflexion and walking tasks. To explore the brain’s information processing during motor preparation, the β power values calculated through time-frequency analysis were compared between groups. A significance level of 5% (p < 0.05) was set for all the statistical tests.
Standardized low-resolution brain electromagnetic tomography (sLORETA) has been employed to visualize and quantify brain neural activity [60]. Neural activity within brain regions was calculated as the current source density, expressed in μA/mm2 × 10−3. The regions of activity were identified using Brodmann’s area (BA) and Montreal Neurological Institute (MNI) coordinates [61]. Global Field Power (GFP) values were computed for both groups (CMC-high and CMC-low) using the sLORETA Averager software [60]. The neural activities of the two groups were compared using the sLORETA statistical non-parametric mapping [62]. Brain regions with significant differences in activity (p < 0.05) were identified and visually represented using color-coded maps.
A support-vector machine (SVM) model [63] was used to classify the motor control indicators derived from the neural activity during motor preparation [63]. The bootstrap method was applied to resample the original dataset randomly and increase the data size. The number of bootstrap samples varied from 10 to 1000 in increments of 100 to evaluate the effects of data augmentation on the model. The SVM model was trained and tested using a 5-fold cross-validation method. The data are divided into five subsets. Four subsets were used for training. One subset was used for testing. This process was repeated five times to ensure that all subsets were used for testing. To comprehensively evaluate the performance of the model, the following metrics were calculated: Accuracy, Precision, Recall, and F1 Score. Accuracy represents the proportion of correct predictions from all predictions made by the model. The Precision refers to the proportion of true positives among all instances predicted as positive by the model. The Recall measures the proportion of actual positive instances correctly identified by the model. The F1 Score is the harmonic mean of Precision and Recall, balancing the contrasting characteristics. The performance of the model was comprehensively evaluated using these metrics.
3. Results
A total of 23 healthy adults participated in this study, with no dropouts. The median Cz-TA CMC (β) value during ankle dorsiflexion was 0.26. The CMC-high group had a mean ± SD of 0.28 ± 0.02 (n = 12), while the CMC-low group had a mean ± SD of 0.23 ± 0.02 (n = 11).
3.1. Comparisons of Each Task Parameter Between CMC Groups
3.1.1. Comparison of Biomechanical Indicators in the Active Ankle Dorsiflexion Movement Task
The CMC-high group exhibited significantly greater values than the CMC-low group in dorsiflexion angle change (CMC-high: 34.26 ± 9.25 [°], CMC-low: 25.36 ± 9.43 [°]), peak dorsiflexion angular velocity (CMC-high: 8352.80 ± 3071.86 [rad/s], CMC-low: 5057.30 ± 1345.79 [rad/s]), and inversion angular velocity (CMC-high: 5594.04 ± 2017.03 [rad/s], CMC-low: 3542.88 ± 1159.39 [rad/s]) (p < 0.05) (Tables S1 and S2). The effect sizes calculated based on Cohen’s criteria indicated large effects (Cohen’s d = 0.95, 1.37, 1.23).
3.1.2. Comparison of Neurophysiological Indicators in the Active Ankle Dorsiflexion Movement Task
The CMC-high and CMC-low groups showed a significant difference in neural activity (p < 0.05) in the paracentral lobule (BA 5; MNI coordinates: X = 0, Y = −35, Z = 60) during ankle dorsiflexion (Figure 5).
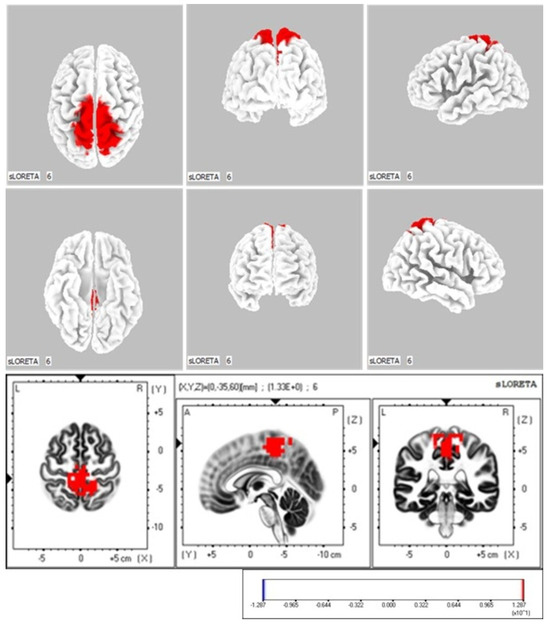
Figure 5.
sLORETA Analysis: Localization of Neural Activity During Active Ankle Dorsiflexion Execution The two groups were compared by calculating the difference in global field power (GFP) values between the CMC-high and CMC-low groups. The color-coded neural regions (in red) indicate significant differences, with the GFP values in the CMC-high group being significantly higher than those in the CMC-low group.
The β power values at Cz showed a significant difference between the groups (p < 0.001) during −0.5 to 0 ms relative to movement onset (CMC-high: −0.40 ± 0.24 [μV2], CMC-low: 1.03 ± 0.93 [μV2]) and during 0.5 to 1 ms (CMC-high: −0.06 ± 0.70 [μV2], CMC-low: 1.12 ± 0.72 [μV2]) (Figure 6).
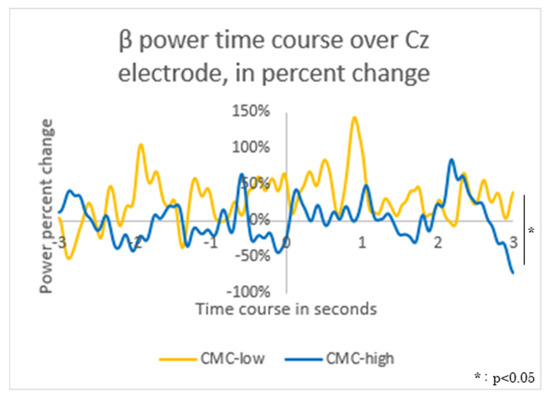
Figure 6.
Time-Frequency Analysis: β Power Fluctuations in the Motor Cortex (Cz) The average β power value during −3000 to −2500 ms relative to movement onset was used as the baseline. The fluctuations in β power values for the corticomuscular coherence (CMC)-high group (high: blue solid line) and the CMC-low group (low: yellow solid line) were visualized in a graph.
3.1.3. Comparison of Biomechanical Indicators in the Walking Task
During walking, there was no significant difference in walking performance as measured by gait speed between the two groups (CMC-high: 1.20 ± 0.17 [m/s], CMC-low: 1.08 ± 0.16 [m/s]). On the other hand, significant differences were observed in the change in dorsiflexion after TO (CMC-high: 2.23 ± 0.88 [°], CMC-low: 3.51 ± 1.75 [°]) and the inversion angle at IC (CMC-high: 2.51 ± 1.45 [°], CMC-low: 4.72 ± 2.56 [°]) (p < 0.05) (Table S3). The effect sizes calculated based on Cohen’s criteria indicated large effects (Cohen’s d = −0.89, −1.00).
3.2. Accuracy Assessment of Machine Learning Models (SVM)
The β power fluctuations during motor preparation in ankle dorsiflexion movements demonstrated a high accuracy rate of 0.70 or above in distinguishing high- and low-CMC levels. Notably, the SVM model based on β power fluctuations in the −500 to 0 ms time window achieved an accuracy of 0.96. For Class 1 (CMC-high), the model showed a precision of 1.00, a recall of 0.91, and an F1 Score of 0.95. For Class 2 (CMC-low), the precision was 0.92, the recall was 1.00, and the F1 Score was 0.96. The SVM model based on the −500 to 0 ms β power fluctuations outperformed all other models across all performance evaluation metrics (Table 1).

Table 1.
Accuracy of Predictive Models with Support-Vector Machines.
4. Discussion
This study examined the impact of brain information processing during motor preparation in active ankle dorsiflexion on the motor control indicator CMC and walking ability.
In the active ankle dorsiflexion task, the β-band Cz-TA CMC successfully detected significant differences in biomechanical and neurophysiological indicators during motor execution. The β-band CMC serves as a measure of connectivity between the sensorimotor cortex and muscles, influencing changes in the mechanical conditions of movement [64]. Impairment in neuromuscular control resulting from motor dysfunction has been associated with reduced CMC [65]. This suggests that CMC is an indicator of motor control through the connectivity between the cortex and muscles. In this study, CMC values detected differences in biomechanical indicators such as dorsiflexion angles and joint angular velocities in the sagittal and frontal planes during active ankle dorsiflexion. These biomechanical indicators have been clinically used to represent motor control [66,67]. Additionally, CMC values detected differences in neural activity in the paracentral lobule (BA 5; MNI coordinates: X = 0, Y = −35, Z = 60) during ankle dorsiflexion. The paracentral lobule is a cortical region associated with lower-limb movement and is considered a target for rehabilitation interventions for the lower limbs [68]. These findings support the notion that coherence, as functional connectivity between the midline primary motor cortex (Cz) and contracting leg muscles (TA), influences motor execution performance through motor control.
Furthermore, the CMC values during active ankle dorsiflexion detected differences in biomechanical indicators of gait, including the changes in ankle dorsiflexion angle after TO and differences in inversion angle at the IC. Ankle dorsiflexion during TO plays a critical role in preventing foot tripping and falls [36,69]. Reduced dorsiflexion angles and increased variability during the swing phase have been observed [41,70,71]. Changes in these biomechanical indicators contributed to improvements in walking ability [72,73]. Additionally, the angle of the frontal plane ankle at the IC is clinically used as an indicator of functional ankle instability and is associated with the occurrence of ankle sprains [74]. These findings suggest a relationship between motor control during active ankle dorsiflexion and walking ability, highlighting that ankle dorsiflexion influences walking ability not only through muscle strength or joint angle changes but also via motor control aspects, such as neuromuscular connectivity, as previously discussed in related studies [19]. This evaluation method, which can be performed in a non-weight-bearing supine position, provides a quantitative assessment even for individuals who are unable to walk.
However, detection of ankle dorsiflexion movements in clinical practice may not always be feasible. Therefore, this study focused on brain information processing during motor preparation to explore versatile and impairment-independent indicators of ankle control in relation to walking ability. The study revealed that during active ankle dorsiflexion, the CMC values—an indicator of ankle control related to walking ability—clearly detected differences in β-band power fluctuations in the motor cortex (Cz) during the motor preparation period. Voluntary human movement involves processes such as intention, planning, program generation, execution, adjustment, and learning of movements [75]. Neural activity during motor preparation plays a critical role in controlling voluntary movements [76,77], possibly contributing to skilled execution [78,79] and modification [80] of movements during the preparatory state before movement onset, even when no actual movement occurs. Moreover, intentional motor behavior is accompanied by a strong sense of agency arising from predictive brain information processing [81]. These findings suggest a strong link between motor preparation and execution processes in the motor control of ankle dorsiflexion.
Additionally, this study developed a machine learning model to predict the high or low levels of β-band Cz-TA CMC related to walking ability, based on β power during motor preparation in ankle dorsiflexion. The results showed that fluctuations in β power during the −500 to 0 ms period before movement onset most accurately predicted CMC levels during motor execution. Neural activity during motor preparation begins approximately 3 s before movement onset and precedes voluntary movement execution. The magnitude and duration of these fluctuations vary depending on factors such as movement area [82], limb dominance [83], attention to movement timing [84], movement intention [85], and type of movement [86]. Moreover, the significance of these indicators depends on the timing of their occurrence and the neural regions involved [87,88]. The β-band power during motor preparation, emerging from the −500 ms period, is generated by the activity of the sensorimotor cortex [88]. Compared to baseline, β-band power in the sensorimotor cortex decreases by 20–30% during the motor preparation stage [55]. Therefore, the β-band power during this period likely reflects the motor preparation process of voluntary movement. Previous studies have shown that CMC during motor execution reflects both descending and ascending pathways [89], with β-band CMC primarily transmitting descending motor control information [52]. Thus, β-band power during motor preparation for active ankle dorsiflexion is suggested to be involved in neuromuscular control during motor execution related to walking ability and is regulated through descending pathways.
Looking ahead, this indicator holds potential as a clinically versatile evaluation method, allowing the assessment of ankle control through motor intention and planning, even in patients who are unable to perform actual movements.
Limitations
One limitation of this study was that the participants were healthy young adults without sensorimotor impairments. In this study, high and low levels of CMC, as an indicator of ankle control, did not show a clear difference in gait performance metrics, such as walking speed. Gait speed is influenced by neurological disorders and motor impairment. Therefore, future studies should include participants with gait impairment for further validation. The sample size in this study was relatively small. It can be assumed that increasing the sample size would make the results more generalizable. Therefore, further validation with a larger number of participants is considered necessary in future research.
5. Conclusions
This study captured the motor control of active ankle dorsiflexion through multifaceted evaluation as a predictive assessment of walking ability. The β power fluctuations obtained from the Cz EEG electrode, which were the focus of this study, correspond to the simulation of bodily movements that occur before the execution of a movement. This neurophysiological indicator can be detected without the actual movement execution. It identifies the intention to perform a movement and has the potential to become a clinically versatile evaluation metric, independent of impairment severity.
In cases of sensorimotor dysfunction caused by stroke, abnormalities may occur in the neural system that compares motor intentions with the feedback information generated during movement. For such impairments, a neurofeedback training system has been developed and validated to improve the ability to compare intentionally generated movements with actual sensorimotor information [90]. Similarly, EEG signals combined with SVM-based classification have been used to recognize human motor behavior [91,92] and to develop brain–machine interfaces aimed at support and rehabilitation [93,94,95]. The findings of this study suggest potential applications in the field of rehabilitation, not only as an evaluation method for ankle control but also as a foundation for developing feedback systems as an intervention tool. However, this evaluation method may not be applicable to patients with impaired consciousness or cognitive decline who are unable to intend movement. When generalizing this evaluation method to clinical practice, it is necessary to consider the limitations of its applicability.
The multifaceted evaluation of movement using the AMAS, which we developed independently, integrates biomechanical evaluation of movement with neurophysiological evaluation of its underlying mechanisms. The AMAS, which captures the entire process, from movement preparation to execution, is expected to provide new insights into the mechanisms of motor control and contribute to the development of treatments tailored to specific impairments.
Supplementary Materials
Supporting information can be downloaded from https://www.mdpi.com/article/10.3390/neurolint17060093/s1, Table S1. Biomechanical parameters of active ankle dorsiflexion: This table shows the statistics of biomechanical indices in the comparison between the high- and low-CMC groups for active ankle dorsiflexion. Table S2. β frequency band CMC of active ankle dorsiflexion movement: This table shows the CMC statistics for the active ankle dorsiflexion movement for the high and low-CMC groups. Table S3. Biomechanical parameters of walking: This table shows the statistics of the biomechanical indices in the high and low-CMC groups for walking.
Author Contributions
Conceptualization, H.I. and T.K.; methodology, H.I., H.Y., R.Y., K.K., K.N. and T.K.; software, H.I., H.Y. and T.K.; validation, H.I., H.Y. and T.K.; formal analysis, H.I.; investigation, H.I.; resources, H.I.; data curation, H.I.; writing—original draft preparation, H.I.; writing—review and editing, H.I. and T.K.; visualization, H.I.; supervision, T.K.; project administration, H.I.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Kyoto Tachibana University (protocol code 22-13, date of approval 2022/06/27).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The Raw data supporting the conclusions of this study will be made available by the authors upon request.
Acknowledgments
The authors would like to thank Takayuki Kodama for his precise and careful guidance throughout the planning and execution of the research and the writing of the paper, and CARETECH as well as Hideaki Yamaguchi for his advice in devising and developing the AMAS system and in the accuracy verification experiments from the beginning of this research. We would like to thank Hideaki Yamaguchi for his advice in the design, development, and accuracy validation experiments of the AMAS system as well as his cooperation in this research over a long period of time.
Conflicts of Interest
Author Hideaki Yamaguchi was employed by the company CARETECH Plus. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| AMAS | Articulation Motion Assessment System |
| IC | Initial Contact |
| TO | Toe Off |
| sEMG | surface electromyography |
| TA | Tibialis Anterior |
| MG | Gastrocnemius Medialis |
| EEG | Electroencephalography |
| CMC | Corticomuscular Coherence |
| sLORETA | Standardized Low-Resolution Brain Electromagnetic Tomography |
| BA | Brodmann’s area |
| GFP | global field power |
| SVM | Support-Vector Machine |
References
- Schmitt, D. Insights into the evolution of human bipedalism from experimental studies of humans and other primates. J. Exp. Biol. 2003, 206, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Shema, S.; Maidan, I.; Hausdorff, J.M. Gait. Handb. Clin. Neurol. 2018, 159, 119–134. [Google Scholar] [CrossRef]
- Baker, J.M. Gait disorders. Am. J. Med. 2018, 131, 602–607. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Schmid, A.; Duncan, P.W.; Studenski, S.; Lai, S.M.; Richards, L.; Perera, S.; Wu, S.S. Improvements in speed-based gait classifications are meaningful. Stroke 2007, 38, 2096–2100. [Google Scholar] [CrossRef]
- Wade, L.; Birch, J.; Farris, D.J. Walking with increasing acceleration is achieved by tuning ankle torque onset timing and rate of torque development. J. R. Soc. Interface 2022, 19, 20220035. [Google Scholar] [CrossRef] [PubMed]
- van Hoeve, S.; Leenstra, B.; Willems, P.; Poeze, M.; Meijer, K. The effect of age and speed on foot and ankle kinematics assessed using a 4-segment foot model. Medicine 2017, 96, e7907. [Google Scholar] [CrossRef]
- Peiffer, M.; Duquesne, K.; Delanghe, M.; Van, O.A.; De, M.S.; Audenaert, E.; Burssens, A. Quantifying walking speeds in relation to ankle biomechanics on a real-time interactive gait platform: A musculoskeletal modeling approach in healthy adults. Front. Bioeng. Biotechnol. 2024, 12, 1348977. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Judge, J.O.; Ounpuu, S.; Thelen, D.G. The effect of walking speed on lower-extremity joint powers among elderly adults who exhibit low physical performance. Arch. Phys. Med. Rehabil. 2005, 86, 2177–2183. [Google Scholar] [CrossRef]
- Lamontagne, A.; Richards, C.L.; Malouin, F. Coactivation during gait as an adaptive behavior after stroke. J. Electromyogr. Kinesiol. 2000, 10, 407–415. [Google Scholar] [CrossRef]
- Allen, J.L.; Kautz, S.A.; Neptune, R.R. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin. Biomech. 2013, 28, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Fung, V.S.C. Functional gait disorder. Handb. Clin. Neurol. 2016, 139, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.R.; Huang, R.C.; Wu, A.; Girardi, F.P.; Cammisa, F.P., Jr. Evaluation of the elderly patient with an abnormal gait. J. Am. Acad. Orthop. Surg 2007, 15, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Capaday, C.; Lavoie, B.A.; Barbeau, H.; Schneider, C.; Bonnard, M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J. Neurophysiol. 1999, 81, 129–139. [Google Scholar] [CrossRef]
- Dobkin, B.H.; Firestine, A.; West, M.; Saremi, K.; Woods, R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage 2004, 23, 370–381. [Google Scholar] [CrossRef]
- Smania, N.; Gambarin, M.; Paolucci, S.; Girardi, P.; Bortolami, M.; Fiaschi, A.; Santilli, V.; Picelli, A. Active ankle dorsiflexion and the Mingazzini manoeuvre: Two clinical bedside tests related to prognosis of postural transferring, standing and walking ability in patients with stroke. Eur. J. Phys. Rehabil. Med. 2011, 47, 435–440. [Google Scholar] [PubMed]
- Chisholm, A.E.; Perry, S.D.; McIlroy, W.E. Correlations between ankle-foot impairments and dropped foot gait deviations among stroke survivors. Clin. Biomech. 2013, 28, 1049–1054. [Google Scholar] [CrossRef]
- Mizuta, N.; Hasui, N.; Nakatani, T.; Takamura, Y.; Fujii, S.; Tsutsumi, M.; Taguchi, J.; Morioka, S. Walking characteristics including mild motor paralysis and slow walking speed in post-stroke patients. Sci. Rep. 2020, 10, 11819. [Google Scholar] [CrossRef]
- Lodha, N.; Patel, P.; Casamento-Moran, A.; Hays, E.; Poisson, S.N.; Christou, E.A. Strength or motor control: What matters in high-functioning stroke? Front. Neurol. 2018, 9, 1160. [Google Scholar] [CrossRef]
- Fauvet, M.; Gasq, D.; Chalard, A.; Tisseyre, J.; Amarantini, D. Temporal dynamics of corticomuscular coherence reflects alteration of the central mechanisms of neural motor control in post-stroke patients. Front. Hum. Neurosci. 2021, 15, 682080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gwin, J.T.; Ferris, D.P. Beta- and gamma-range human lower limb corticomuscular coherence. Front. Hum. Neurosci. 2012, 6, 258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, T.H.; Willerslev-Olsen, M.; Conway, B.A.; Nielsen, J.B. The motor cortex drives the muscles during walking in human subjects. J. Physiol. 2012, 590, 2443–2452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshida, T.; Masani, K.; Zabjek, K.; Chen, R.; Popovic, M.R. Dynamic cortical participation during bilateral, cyclical ankle movements: Effects of aging. Sci. Rep. 2017, 7, 44658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernstein, N.A. The Coordination and Regulation of Movements; Pergamon Press: London, UK, 1967. [Google Scholar]
- Isomura, Y.; Harukuni, R.; Takekawa, T.; Aizawa, H.; Fukai, T. Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat. Neurosci. 2009, 12, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Churchland, M.M.; Yu, B.M.; Ryu, S.I.; Santhanam, G.; Shenoy, K.V. Neural variability in premotor cortex provides a signature of motor preparation. J. Neurosci. 2006, 26, 3697–3712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giordano, N.; Alia, C.; Fruzzetti, L.; Pasquini, M.; Palla, G.; Mazzoni, A.; Micera, S.; Fogassi, L.; Bonini, L.; Caleo, M. Fast-spiking interneurons of the premotor cortex contribute to initiation and execution of spontaneous actions. J. Neurosci. 2023, 43, 4234–4250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ito, H.; Yamaguchi, H.; Inoue, M.; Nagano, H.; Kitai, K.; Morita, K.; Kodama, T. Reliability and validity of the articulation motion assessment system using a rotary encoder. Biomechanics 2025, 5, 2. [Google Scholar] [CrossRef]
- McCurdy, K.; Langford, G. Comparison of unilateral squat strength between the dominant and non-dominant leg in men and women. J. Sports Sci. Med. 2005, 4, 153–159. [Google Scholar]
- Kapreli, E.; Athanasopoulos, S.; Papathanasiou, M.; Van Hecke, P.; Strimpakos, N.; Gouliamos, A.; Peeters, R.; Sunaert, S. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage 2006, 32, 1709–1721. [Google Scholar] [CrossRef]
- Glazebrook, C.M.; Welsh, T.N.; Tremblay, L. The processing of visual and auditory information for reaching movements. Psychol. Res. 2016, 80, 757–773. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Chatrian, G.E.; Lettich, E.; Nelson, P.L. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activities. Am. J. EEG Technol. 1985, 25, 83–92. [Google Scholar] [CrossRef]
- Su, C.; Chen, S.; Jiang, H.; Chen, Y. Ankle joint torque prediction based on surface electromyographic and angular velocity signals. IEEE Access 2020, 8, 217681–217687. [Google Scholar] [CrossRef]
- Little, V.L.; McGuirk, T.E.; Patten, C. Slower than normal walking speeds involve a pattern shift in joint and temporal coordination contributions. Exp. Brain Res. 2019, 237, 2973–2982. [Google Scholar] [CrossRef]
- Brockett, C.L.; Chapman, G.J. Biomechanics of the ankle. Orthop. Trauma 2016, 30, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, J.E.; Seo, K.J.; Lee, J. Bilateral ankle deformities affects gait kinematics in chronic stroke patients. Front. Neurol. 2023, 14, 1078064. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hong, W.H.; Wang, C.M.; Chen, C.K.; Wu, K.P.H.; Kang, C.F.; Tang, S.F. Kinematic features of rear-foot motion using anterior and posterior ankle-foot orthoses in stroke patients with hemiplegic gait. Arch. Phys. Med. Rehabil. 2010, 91, 1862–1868. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; Banky, M.; Clark, R.A.; Kahn, M.B.; Williams, G. Lower limb angular velocity during walking at various speeds. Gait Posture 2018, 65, 190–196. [Google Scholar] [CrossRef]
- Srivastava, S.; Kindred, J.H.; Seamon, B.A.; Charalambous, C.C.; Boan, A.D.; Kautz, S.A.; Bowden, M.G. A novel biomechanical indicator for impaired ankle dorsiflexion function during walking in individuals with chronic stroke. Gait Posture 2024, 107, 246–252. [Google Scholar] [CrossRef]
- Hamacher, D.; Hollander, K.; Zech, A. Effects of ankle instability on running gait ankle angles and its variability in young adults. Clin. Biomech. 2016, 33, 73–78. [Google Scholar] [CrossRef]
- Hwang, Y.I.; Park, D.J. Effects of elastic neutral ankle-foot orthoses on 3 dimensional parameters during gait training in patients with stroke: A pilot study. J. Bodyw. Mov. Ther. 2021, 27, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.J. Spectrum estimation and harmonic analysis. Proc. IEEE 1982, 70, 1055–1096. [Google Scholar] [CrossRef]
- Roeder, L.; Boonstra, T.W.; Smith, S.S.; Kerr, G.K. Dynamics of corticospinal motor control during overground and treadmill walking in humans. J. Neurophysiol. 2018, 120, 1017–1031. [Google Scholar] [CrossRef]
- Yokoyama, H.; Yoshida, T.; Zabjek, K.; Chen, R.; Masani, K. Defective corticomuscular connectivity during walking in patients with Parkinson’s disease. J. Neurophysiol. 2020, 124, 1399–1414. [Google Scholar] [CrossRef]
- Stergiou, N.; Harbourne, R.; Cavanaugh, J. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 2006, 30, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor control programs and walking. Neuroscientist 2006, 12, 339–348. [Google Scholar] [CrossRef]
- Moisan, G.; Mainville, C.; Descarreaux, M.; Cantin, V. Lower limb biomechanics in individuals with chronic ankle instability during gait: A case-control study. J. Foot Ankle Res. 2021, 14, 36. [Google Scholar] [CrossRef]
- Piming, G.; Yaming, Y.; Hai, S.; Xia, L.; Xiaobing, L. Three-dimensional ankle kinematics of the full gait cycle in patients with chronic ankle instability: A case-control study. Heliyon 2023, 9, e22265. [Google Scholar] [CrossRef]
- Granata, K.P.; Abel, M.F.; Damiano, D.L. Joint angular velocity in spastic gait and the influence of muscle-tendon lengthening. J. Bone Joint Surg. Am. 2000, 82, 174–186. [Google Scholar] [CrossRef]
- Fukuchi, C.A.; Fukuchi, R.K.; Duarte, M. Effects of walking speed on gait biomechanics in healthy participants: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 153. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Q.; Liu, X.; Dong, B.; Liu, X.; Wang, H. Identifying bidirectional total and non-linear information flow in functional corticomuscular coupling during a dorsiflexion task: A pilot study. J. Neuroeng. Rehabil. 2021, 18, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, J.; Zikereya, T.; Shao, Z.; Shi, K. The neuromechanical of Beta-band corticomuscular coupling within the human motor system. Front. Neurosci. 2024, 18, 1441002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenberg, J.R.; Amjad, A.M.; Breeze, P.; Brillinger, D.R.; Halliday, D.M. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog. Biophys. Mol. Biol. 1989, 53, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Stolk, A.; Brinkman, L.; Vansteensel, M.J.; Aarnoutse, E.; Leijten, F.S.; Dijkerman, C.H.; Knight, R.T.; de Lange, F.P.; Toni, I. Electrocorticographic dissociation of alpha and beta rhythmic activity in the human sensorimotor system. eLife 2019, 8, e48065. [Google Scholar] [CrossRef]
- Shiner, C.T.; Tang, H.; Johnson, B.W.; McNulty, P.A. Cortical beta oscillations and motor thresholds differ across the spectrum of post-stroke motor impairment, a preliminary MEG and TMS study. Brain Res. 2015, 1629, 26–37. [Google Scholar] [CrossRef]
- Koelewijn, T.; Van Schie, H.T.; Bekkering, H.; Oostenveld, R.; Jensen, O. Motor-cortical beta oscillations are modulated by correctness of observed action. Neuroimage 2008, 40, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Cardellicchio, P.; Hilt, P.M.; Dolfini, E.; Fadiga, L.; D’Ausilio, A. Beta rebound as an index of temporal integration of somatosensory and motor signals. Front. Syst. Neurosci. 2020, 14, 63. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find. Exp. Clin. Pharmacol. 2002, 24 (Suppl. D), 5–12. [Google Scholar] [PubMed]
- Collins, D.L.; Holmes, C.J.; Peters, T.M.; Evans, A.C. Automatic 3-D model-based neuroanatomical segmentation. Hum. Brain Mapp. 1995, 3, 190–208. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Instantaneous and Lagged Measurements of Linear and Nonlinear Dependence Between Groups of Multivariate Time Series: Frequency Decomposition. arXiv 2007, arXiv:0711.1455. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Wu, G.; Kelly, K.M. Evidence for beta corticomuscular coherence during human standing balance: Effects of stance width, vision, and support surface. Neuroscience 2015, 298, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, H.; Shi, X.; Liang, J.; Wan, C.; Ming, D. Lower-limb motor assessment with corticomuscular coherence of multiple muscles during ankle dorsiflexion after stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Morris, M.E.; Lord, S. Foot and ankle risk factors for falls in older people: A prospective study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 866–870. [Google Scholar] [CrossRef]
- Tabrizi, P.; McIntyre, W.M.; Quesnel, M.B.; Howard, A.W. Limited dorsiflexion predisposes to injuries of the ankle in children. J. Bone Joint Surg. Br. 2000, 82, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, H.C.; Youn, I.; Lee, S.J.; Lee, J.H. Use of functional magnetic resonance imaging to identify cortical loci for lower limb movements and their efficacy for individuals after stroke. J. Neuroeng. Rehabil. 2024, 21, 58. [Google Scholar] [CrossRef]
- Simonsen, E.B. Contributions to the understanding of gait control. Dan. Med. J. 2014, 61, B4823. [Google Scholar] [PubMed]
- Wanner, P.; Schmautz, T.; Kluge, F.; Eskofier, B.; Pfeifer, K.; Steib, S. Ankle angle variability during running in athletes with chronic ankle instability and copers. Gait Posture 2019, 68, 329–334. [Google Scholar] [CrossRef] [PubMed]
- de Ruvo, R.; Russo, G.; Lena, F.; Giovannico, G.; Neville, C.; Turolla, A.; Torre, M.; Pellicciari, L. The Effect of Manual Therapy Plus Exercise in Patients with Lateral Ankle Sprains: A Critically Appraised Topic with a Meta-Analysis. J. Clin. Med. 2022, 11, 4925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Z.; Jiang, X.; Zhong, M.; Shen, B.; Zhu, J.; Pan, Y.; Dong, J.; Xu, P.; Zhang, W.; Zhang, L. Wearable sensors measure ankle joint changes of patients with Parkinson’s disease before and after acute levodopa challenge. Parkinsons Dis. 2020, 2020, 2976535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaqueline da Cunha, M.; Rech, K.D.; Salazar, A.P.; Pagnussat, A.S. Functional electrical stimulation of the peroneal nerve improves post-stroke gait speed when combined with physiotherapy. A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2021, 64, 101388. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, E.; Monaghan, K.; Caulfield, B. Altered neuromuscular control and ankle joint kinematics during walking in subjects with functional instability of the ankle joint. Am. J. Sports Med. 2006, 34, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Sülzenbrück, S. Mind and movement. Psychol. Res. 2012, 76, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.H. The computational and neural basis of voluntary motor control and planning. Trends Cogn. Sci. 2012, 16, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Ames, K.C.; Ryu, S.I.; Shenoy, K.V. Neural dynamics of reaching following incorrect or absent motor preparation. Neuron 2014, 81, 438–451. [Google Scholar] [CrossRef]
- Churchland, M.M.; Shenoy, K.V. Preparatory activity and the expansive null-space. Nat. Rev. Neurosci. 2024, 25, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Zimnik, A.J.; Churchland, M.M. Independent generation of sequence elements by motor cortex. Nat. Neurosci. 2021, 24, 412–424, Erratum in: Nat. Neurosci. 2022, 25, 530. https://doi.org/10.1038/s41593-022-01048-y. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ames, K.C.; Ryu, S.I.; Shenoy, K.V. Simultaneous motor preparation and execution in a last-moment reach correction task. Nat. Commun. 2019, 10, 2718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haggard, P. Conscious intention and motor cognition. Trends Cogn. Sci. 2005, 9, 290–295. [Google Scholar] [CrossRef]
- Rektor, I. Parallel information processing in motor systems: Intracerebral recordings of readiness potential and CNV in human subjects. Neural Plast. 2000, 7, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Dirnberger, G.; Duregger, C.; Lindinger, G.; Lang, W. On the regularity of preparatory activity preceding movements with the dominant and non-dominant hand: A readiness potential study. Int. J. Psychophysiol. 2011, 81, 127–131. [Google Scholar] [CrossRef]
- Baker, K.S.; Piriyapunyaporn, T.; Cunnington, R. Neural activity in readiness for incidental and explicitly timed actions. Neuropsychologia 2012, 50, 715–722. [Google Scholar] [CrossRef]
- Takashima, S.; Ogawa, C.Y.; Najman, F.A.; Ramos, R.T. The volition, the mode of movement selection and the readiness potential. Exp. Brain Res. 2020, 238, 2113–2123. [Google Scholar] [CrossRef]
- Joordens, S.; van Duijn, M.; Spalek, T.M. When timing the mind one should also mind the timing: Biases in the measurement of voluntary actions. Conscious. Cogn. 2002, 11, 231–240. [Google Scholar] [CrossRef]
- Praamstra, P.; Stegeman, D.F.; Horstink, M.W.; Brunia, C.H.; Cools, A.R. Movement-related potentials preceding voluntary movement are modulated by the mode of movement selection. Exp. Brain Res. 1995, 103, 429–439. [Google Scholar] [CrossRef]
- Schurger, A.; Hu, P.B.; Pak, J.; Roskies, A.L. What Is the Readiness Potential? Trends Cogn. Sci. 2021, 25, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Witham, C.L.; Riddle, C.N.; Baker, M.R.; Baker, S.N. Contributions of descending and ascending pathways to corticomuscular co-herence in humans. J. Physiol. 2011, 589, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Katayama, O.; Nakano, H.; Ueda, T.; Murata, S. Treatment of Medial Medullary Infarction Using a Novel iNems Training: A Case Report and Literature Review. Clin. EEG Neurosci. 2019, 50, 429–435. [Google Scholar] [CrossRef]
- Mahalungkar, S.P.; Shrivastava, R.; Angadi, S. A brief survey on human activity recognition using motor imagery of EEG signals. Electromagn. Biol. Med. 2024, 43, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Mebarkia, K.; Reffad, A. Multi optimized SVM classifiers for motor imagery left and right hand movement identification. Australas. Phys. Eng. Sci. Med. 2019, 42, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Konar, A.; Tibarewala, D.N. Motor imagery, P300 and error-related EEG-based robot arm movement control for rehabilitation purpose. Med. Biol. Eng. Comput. 2014, 52, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.; Belkacem, A.N.; Ouhbi, S.; Lakas, A. Noninvasive electroencephalography equipment for assistive, adaptive, and rehabilitative brain-computer interfaces: A systematic literature review. Sensors 2021, 21, 4754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saibene, A.; Caglioni, M.; Corchs, S.; Gasparini, F. EEG-based BCIs on motor imagery paradigm using wearable technologies: A systematic review. Sensors 2023, 23, 2798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).