Ketamine in Status Epilepticus: How Soon Is Now?
Abstract
1. Introduction
2. Methods and Research Outputs
Risk of Bias Assessment
3. Evidence for Early Use of NMDA Receptor Antagonists
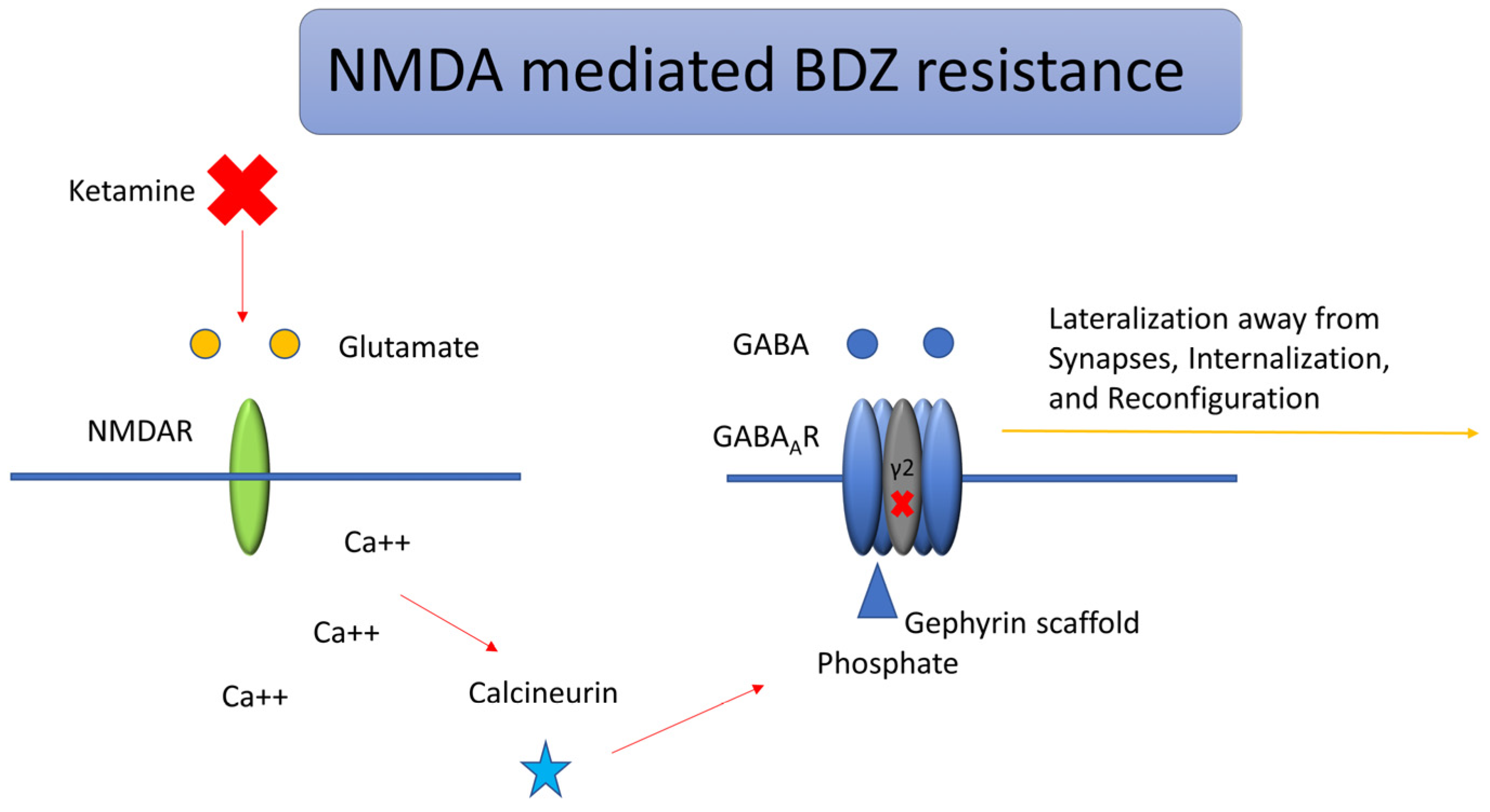
3.1. Trafficking of GABA Receptors and Etiology
3.2. Model of SE Response to NMDA Antagonists
3.3. Other NMDA Receptor Antagonists
3.4. Translational Challenges from Animal Models to Clinical Application
4. Ketamine: Properties and Advantages
4.1. Properties
4.2. Adverse Effects Associated with Ketamine and Frail Patients
5. Discussion of Available Evidence
| No. | Author | Population | SE Type | Ketamine Timing | Initial Dose | Infusion Rate | Response Rate | Adverse Events | Median Age | Pre-Existing Epilepsy | ASMs Before Ketamine |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Othman et al., 2025 [16] | Children (144) | Early SE | Immediately (34 min) | 2 mg/kg bolus | NA (Bolus only) | 76% (Keta-Mid) vs. 21% (Pla-Mid) | 12.5% (Ket-Mid) vs. 35.7% (Pla-Mid) | 2.5 years | 24% | None |
| 2 | Jacobwitz et al., 2024 [69] | Pediatrics (117) | RSE | 11.8 h | 1 mg/kg | 1–6 mg/kg/h | 61% (Keta) vs. 28% (Mid) | 3% (Keta) vs. 24% (Mid) | 0.8 years | 28% | None |
| 3 | Horvat et al., 2025 [10] | Pediatric cardiac patients (34) | RSE | 24.7 h | NA | 10 mg/kg/h | 73% Keta vs. 63% Mid | 54% Keta 68% Mid (No difference) | 0.24 years | 32% | 2+ |
| 4 | Jacobwitz et al., 2022 [70] | Neonates & children (67) | RSE | 20 h | 1 mg/kg | 0.5–7 mg/kg/h | 46% cessation (28% reduction) | 4% | 0.7 days | 25% | 3 |
| 5 | Fletman et al., 2024 [71] | Adults (73) | RSE | Concurrent with midazolam (27 h) | 1.5 mg/kg | 1.2–10 mg/kg/h | Improved SE duration | None noted | 57 years | 36% | 4 |
| 6 | Harnicher et al., 2024 [72] | Adults (51) | RSE | 44.8 h | NA | 0.6–3 mg/kg/h | 43% full cessation | 5.9% | 58 years | 13% | 4 |
| 7 | Gaspard et al., 2013 [60] | Mostly Adults (60 episodes, 46 adults, 12 children) | RSE | <12 h likely response to Ketamine, >10 days no response | 1.5–5 mg/kg | 2.75–10 mg/kg/h | 57% total; 32% Ketane-attributed | 7% | 24 ears | 15% | 6 |
| 8 | Kimmons et al., 2024 [73] | Adults (28) | RSE | 19.6 h response, 36 h no response | 1 mg/kg | 0.6–2 mg/kg/h | 71.4% | Hpertension 39.3%, Hypotentsion 31.8% | 62 years | 53.6% | 3 |
| 9 | Srinivas et al., 2023 [74] | Mostly Adults (9 adults, 2 children) | RSE | 2.8 days | 2–10 mg/kg | 2.43–6.66 mg/kg/h | 60.5%; 44.4% attributed to Keta | 28.4% Hypertension; 11% Hypotension | 55 years | 34.6% | 3 |
5.1. Adverse Effects Reported in the Included Studies
5.2. Limitation of Generalizability
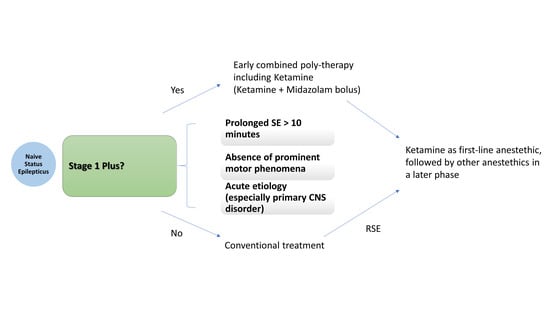
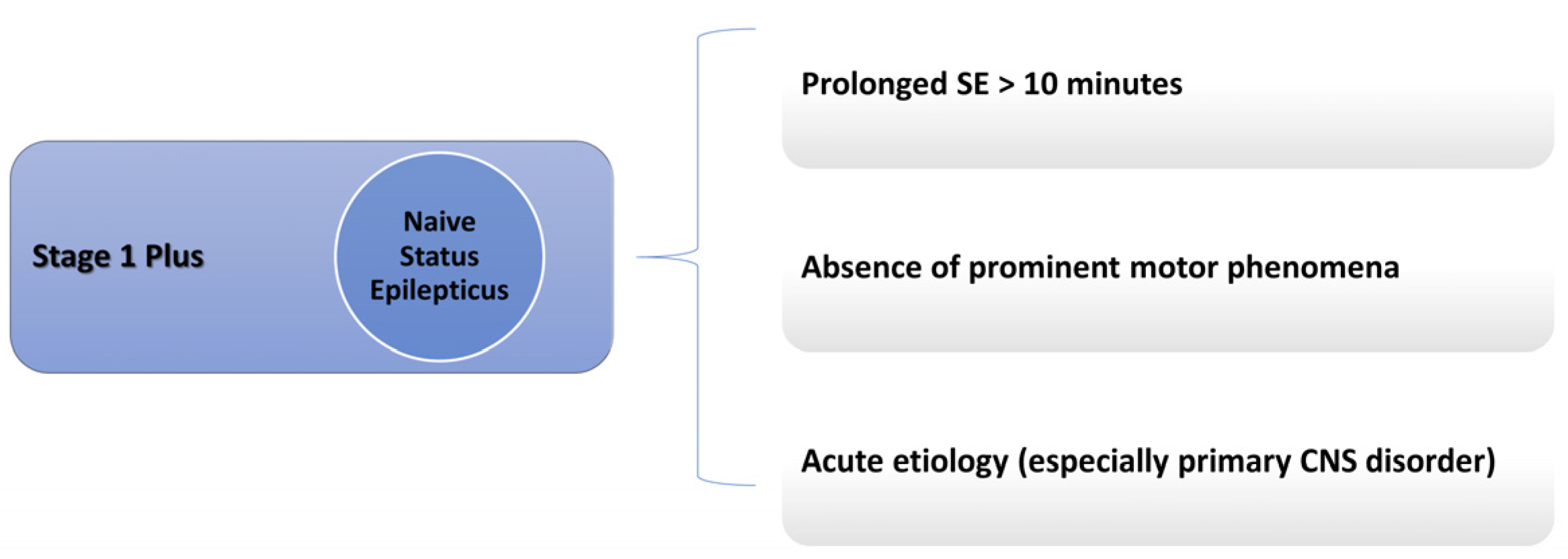
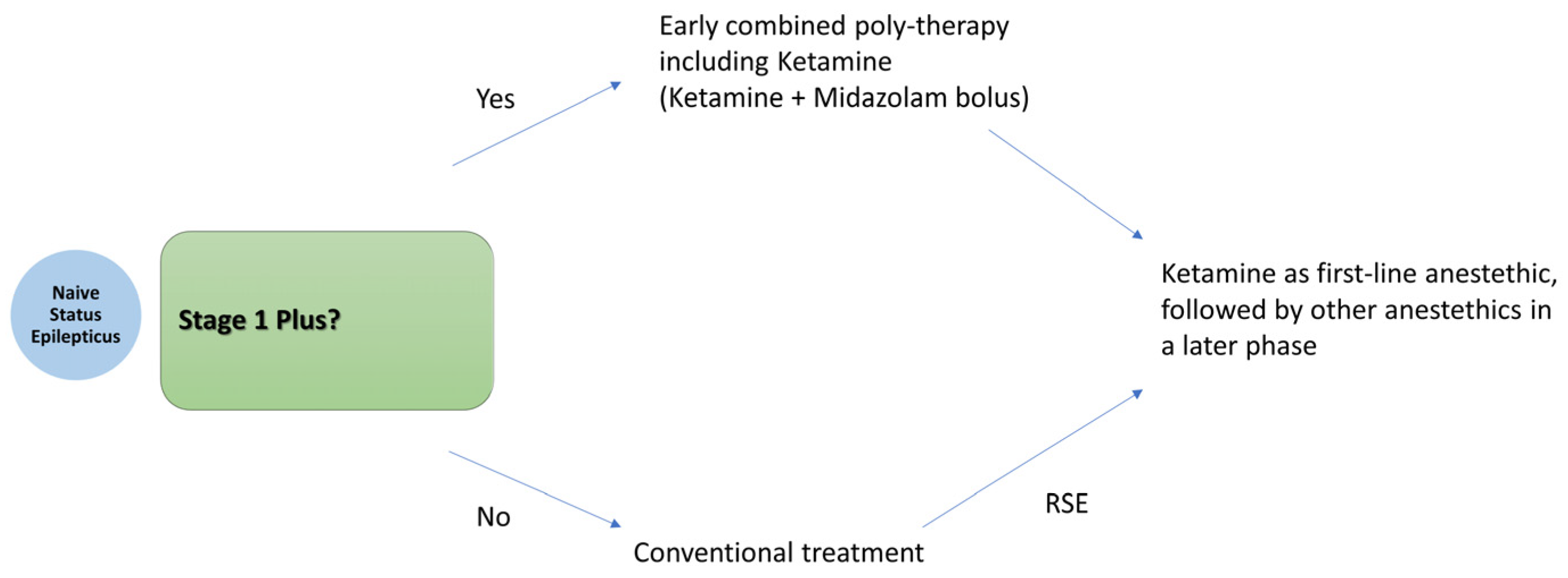
5.3. How Soon Can It Be Administered?
6. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Early SE | A status epilepticus stage requiring first-line therapy for ongoing seizure activity, typically with a benzodiazepine |
| Established SE | A stage with seizure activity persisting despite first line of treatment |
| SE | status epilepticus |
| RSE | Refractory status epilepticus |
| SRSE | Super-refractory status epilepticus |
| Anesthetics | Agents commonly used in continuous infusion after failure of at least one anti-seizure medication |
| ASM | Anti-seizure medication |
| BDZ | Benzodiazepine |
| NMDA | N-methyl-D-aspartate (NMDA) receptor of glutamate |
| AMPA | Alpha-amino-3-hydroxy-5-methyl-4-isooxazole-propionic acid glutamate receptors |
| CNS | Central nervous system |
References
- Schubert-Bast, S.; Zöllner, J.P.; Ansorge, S.; Hapfelmeier, J.; Bonthapally, V.; Eldar-Lissai, A.; Rosenow, F.; Strzelczyk, A. Burden and epidemiology of status epilepticus in infants, children, and adolescents: A population-based study on German health insurance data. Epilepsia 2019, 60, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Faure, M.; Bergamasco, A.; Spalding, W.; Benitez, A.; Moride, Y.; Fournier, M. Epidemiology of status epilepticus in the United States: A systematic review. Epilepsy Behav. 2020, 112, 107459. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, M.; Trinka, E.; Zimmermann, G.; Granbichler, C.A.; Kobulashvili, T.; Siebert, U. Epidemiology of status epilepticus in adults: Apples, pears, and oranges—A critical review. Epilepsy Behav. 2020, 103, 106720. [Google Scholar] [CrossRef] [PubMed]
- Bleck, T.P. The future treatment of status epilepticus. Epilepsy Behav. 2024, 161, 110146. [Google Scholar] [CrossRef]
- Vossler, D.G. First Seizures, Acute Repetitive Seizures, and Status Epilepticus. Continuum 2025, 31, 95–124. [Google Scholar] [CrossRef]
- Trinka, E.; Leitinger, M. Management of Status Epilepticus, Refractory Status Epilepticus, and Super-refractory Status Epilepticus. Continuum 2022, 28, 559–602. [Google Scholar] [CrossRef]
- Glauser, T.; Shinnar, S.; Gloss, D.; Alldredge, B.; Arya, R.; Bainbridge, J.; Bare, M.; Bleck, T.; Dodson, W.E.; Garrity, L.; et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016, 16, 48–61. [Google Scholar] [CrossRef]
- Vossler, D.G.; Bainbridge, J.L.; Boggs, J.G.; Novotny, E.J.; Loddenkemper, T.; Faught, E.; Amengual-Gual, M.; Fischer, S.N.; Gloss, D.S.; Olson, D.M.; et al. Treatment of Refractory Convulsive Status Epilepticus: A Comprehensive Review by the American Epilepsy Society Treatments Committee. Epilepsy Curr. 2020, 20, 245–264. [Google Scholar] [CrossRef]
- Cornwall, C.D.; Krøigård, T.; Kristensen, J.S.S.; Callesen, H.E.; Beier, C.P. Outcomes and Treatment Approaches for Super-Refractory Status Epilepticus: A Systematic Review and Meta-Analysis. JAMA Neurol. 2023, 80, 959–968. [Google Scholar] [CrossRef]
- Horvat, D.E.; Keenan, J.S.; Javadian, S.; Liu, Y.T.; Voleti, S.; Staso, K.; Conley, C.; Schlatterer, S.D.; Sansevere, A.J.; Harrar, D.B. Ketamine Versus Midazolam as the First-Line Continuous Infusion for Status Epilepticus in Children with Cardiac Disease. Neurocrit. Care 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Caranzano, L.; Novy, J.; Rossetti, A.O. Ketamine in adult super-refractory status epilepticus: Efficacy analysis on a prospective registry. Acta Neurol. Scand. 2022, 145, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Alkhachroum, A.; Der-Nigoghossian, C.A.; Mathews, E.; Massad, N.; Letchinger, R.; Doyle, K.; Chiu, W.T.; Kromm, J.; Rubinos, C.; Velazquez, A.; et al. Ketamine to treat super-refractory status epilepticus. Neurology 2020, 95, e2286–e2294. [Google Scholar] [CrossRef] [PubMed]
- Richards, N.D.; Howell, S.J.; Bellamy, M.C.; Beck, J. The diverse effects of ketamine, jack-of-all-trades: A narrative review. Br. J. Anaesth. 2025, 134, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Buratti, S.; Giacheri, E.; Palmieri, A.; Tibaldi, J.; Brisca, G.; Riva, A.; Striano, P.; Mancardi, M.M.; Nobili, L.; Moscatelli, A. Ketamine as advanced second-line treatment in benzodiazepine-refractory convulsive status epilepticus in children. Epilepsia 2023, 64, 797–810. [Google Scholar] [CrossRef]
- Chiriboga, N.; Spentzas, T.; Abu-Sawwa, R. A systematic review and meta-analysis of ketamine in pediatric status epilepticus. Epilepsia 2024, 65, 2200–2212. [Google Scholar] [CrossRef]
- Othman, A.A.; Sadek, A.A.; Ahmed, E.A.; Abdelkreem, E. Combined Ketamine and Midazolam Versus Midazolam Alone for Initial Treatment of Pediatric Generalized Convulsive Status Epilepticus (Ket-Mid Study): A Randomized Controlled Trial. Pediatr. Neurol. 2025, 167, 24–32. [Google Scholar] [CrossRef]
- Burman, R.J.; Rosch, R.E.; Wilmshurst, J.M.; Sen, A.; Ramantani, G.; Akerman, C.J.; Raimondo, J.V. Why won’t it stop? The dynamics of benzodiazepine resistance in status epilepticus. Nat. Rev. Neurol. 2022, 18, 428–441. [Google Scholar] [CrossRef]
- Joshi, S.; Rajasekaran, K.; Hawk, K.M.; Chester, S.J.; Goodkin, H.P. Status epilepticus: Role for etiology in determining response to benzodiazepines. Ann. Neurol. 2018, 83, 830–841. [Google Scholar] [CrossRef]
- Kapur, J.; Elm, J.; Chamberlain, J.M.; Barsan, W.; Cloyd, J.; Lowenstein, D.; Shinnar, S.; Conwit, R.; Meinzer, C.; Cock, H.; et al. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N. Engl. J. Med. 2019, 381, 2103–2113. [Google Scholar] [CrossRef]
- Llauradó, A.; Quintana, M.; Ballvé, A.; Campos, D.; Fonseca, E.; Abraira, L.; Toledo, M.; Santamarina, E. Factors associated with resistance to benzodiazepines in status epilepticus. J. Neurol. Sci. 2021, 423, 117368. [Google Scholar] [CrossRef]
- Rollo, E.; Romozzi, M.; Dono, F.; Bernardo, D.; Consoli, S.; Anzellotti, F.; Ricciardi, L.; Paci, L.; Sensi, S.L.; Della Marca, G.; et al. Treatment of benzodiazepine-refractory status epilepticus: A retrospective, cohort study. Epilepsy Behav. 2023, 140, 109093. [Google Scholar] [CrossRef] [PubMed]
- Magro, G. Early Polytherapy for Probably Benzodiazepine Refractory Naïve Status Epilepticus (Stage 1 Plus). Neurol. Int. 2025, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Magro, G.; Laterza, V. Status epilepticus: Is there a Stage 1 plus? Epilepsia 2024, 65, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.E.; Liu, H.; Niquet, J.; Wasterlain, C.G. Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol. Dis. 2013, 54, 225–238. [Google Scholar] [CrossRef]
- Niquet, J.; Nguyen, D.; de Araujo Furtado, M.; Lumley, L. Treatment of cholinergic-induced status epilepticus with polytherapy targeting GABA and glutamate receptors. Epilepsia Open 2023, 8, S117–S140. [Google Scholar] [CrossRef]
- Naylor, D.E. In the fast lane: Receptor trafficking during status epilepticus. Epilepsia Open 2023, 8 (Suppl. S1), S35–S65. [Google Scholar] [CrossRef]
- Nusser, Z.; Sieghart, W.; Somogyi, P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998, 18, 1693–1703. [Google Scholar] [CrossRef]
- Venkatachalan, S.P.; Czajkowski, C. Structural link between γ-aminobutyric acid type A (GABAA) receptor agonist binding site and inner β-sheet governs channel activation and allosteric drug modulation. J. Biol. Chem. 2012, 287, 6714–6724. [Google Scholar] [CrossRef]
- Muir, J.; Arancibia-Carcamo, I.L.; MacAskill, A.F.; Smith, K.R.; Griffin, L.D.; Kittler, J.T. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the γ2 subunit. Proc. Natl. Acad. Sci. USA 2010, 107, 16679–16684. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Chen, Z.; Chen, S.; Zhu, F.; Zhou, L. Long-term expressional changes of Na+-K+-Cl− co-transporter 1 (NKCC1) and K+-Cl− co-transporter 2 (KCC2) in CA1 region of hippocampus following lithium-pilocarpine induced status epilepticus (PISE). Brain Res. 2008, 1221, 141–146. [Google Scholar] [CrossRef]
- Lee, H.H.; Jurd, R.; Moss, S.J. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol. Cell. Neurosci. 2010, 45, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Deeb, T.Z.; Walker, J.A.; Davies, P.A.; Moss, S.J. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat. Neurosci. 2011, 14, 736–743. [Google Scholar] [CrossRef]
- Kapur, J.; Coulter, D.A. Experimental status epilepticus alters gamma-aminobutyric acid type A receptor function in CA1 pyramidal neurons. Ann. Neurol. 1995, 38, 893–900. [Google Scholar] [CrossRef]
- Naylor, D.E.; Liu, H.; Wasterlain, C.G. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J. Neurosci. 2005, 25, 7724–7733. [Google Scholar] [CrossRef]
- Goodkin, H.P.; Yeh, J.L.; Kapur, J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J. Neurosci. 2005, 25, 5511–5520. [Google Scholar] [CrossRef]
- Joshi, S.; Rajasekaran, K.; Hawk, K.M.; Brar, J.; Ross, B.M.; Tran, C.A.; Chester, S.J.; Goodkin, H.P. Phosphatase inhibition prevents the activity-dependent trafficking of GABAA receptors during status epilepticus in the young animal. Epilepsia 2015, 56, 1355–1365. [Google Scholar] [CrossRef]
- Lattanzi, S.; Giovannini, G.; Brigo, F.; Orlandi, N.; Trinka, E.; Meletti, S. Acute symptomatic status epilepticus: Splitting or lumping? A proposal of classification based on real-world data. Epilepsia 2023, 64, e200–e206. [Google Scholar] [CrossRef] [PubMed]
- Urzì Brancati, V.; Pinto Vraca, T.; Minutoli, L.; Pallio, G. Polymorphisms Affecting the Response to Novel Antiepileptic Drugs. Int. J. Mol. Sci. 2023, 24, 2535. [Google Scholar] [CrossRef] [PubMed]
- Niquet, J.; Baldwin, R.; Norman, K.; Suchomelova, L.; Lumley, L.; Wasterlain, C.G. Midazolam-ketamine dual therapy stops cholinergic status epilepticus and reduces Morris water maze deficits. Epilepsia 2016, 57, 1406–1415. [Google Scholar] [CrossRef]
- Niquet, J.; Baldwin, R.; Norman, K.; Suchomelova, L.; Lumley, L.; Wasterlain, C.G. Simultaneous triple therapy for the treatment of status epilepticus. Neurobiol. Dis. 2017, 104, 41–49. [Google Scholar] [CrossRef]
- Dorandeu, F.; Dhote, F.; Barbier, L.; Baccus, B.; Testylier, G. Treatment of status epilepticus with ketamine, are we there yet? CNS Neurosci. Ther. 2013, 19, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.B.; Saraçoğlu, K.T.; Aykın, U.; Akça, M.; Demirtaş, C.; Saraçoğlu, A.; Yıldırım, M. Efficacy of Low-Dose Ketamine and Propofol in the Treatment of Experimental Refractory Status Epilepticus on Male Rats. J. Neurosci. Res. 2024, 102, e25393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, Y.; Cao, X.; Li, Z.; Yu, J. Diazepam Monotherapy or Diazepam-Ketamine Dual Therapy at Different Time Points Terminates Seizures and Reduces Mortality in a Status Epilepticus Animal Model. Med. Sci. Monit. 2021, 27, e934043. [Google Scholar] [CrossRef]
- Olney, J.W.; Labruyere, J.; Wang, G.; Wozniak, D.F.; Price, M.T.; Sesma, M.A. NMDA antagonist neurotoxicity: Mechanism and prevention. Science 1991, 254, 1515–1518. [Google Scholar] [CrossRef]
- Paule, M.G.; Li, M.; Allen, R.R.; Liu, F.; Zou, X.; Hotchkiss, C.; Hanig, J.P.; Patterson, T.A.; Slikker, W., Jr.; Wang, C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol. Teratol. 2011, 33, 220–230. [Google Scholar] [CrossRef]
- Ikonomidou, C.; Bosch, F.; Miksa, M.; Bittigau, P.; Vöckler, J.; Dikranian, K.; Tenkova, T.I.; Stefovska, V.; Turski, L.; Olney, J.W. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999, 283, 70–74. [Google Scholar] [CrossRef]
- Huang, T.-H.; Lai, M.-C.; Chen, Y.-S.; Huang, C.-W. The Roles of Glutamate Receptors and Their Antagonists in Status Epilepticus, Refractory Status Epilepticus, and Super-Refractory Status Epilepticus. Biomedicines 2023, 11, 686. [Google Scholar] [CrossRef]
- The Eclampsia Trial Collaborative, G. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet 1995, 345, 1455–1463. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Matuszczak, M.; Teitelbaum, J.; Gillman, L.M.; Kazina, C.J. Magnesium sulfate for non-eclamptic status epilepticus. Seizure Eur. J. Epilepsy 2015, 32, 100–108. [Google Scholar] [CrossRef]
- Kornhuber, J.; Bormann, J.; Hübers, M.; Rusche, K.; Riederer, P. Effects of the 1-amino-adamantanes at the MK-801-binding site of the NMDA-receptor-gated ion channel: A human postmortem brain study. Eur. J. Pharmacol. Mol. Pharmacol. 1991, 206, 297–300. [Google Scholar] [CrossRef]
- Kalemenev, S.V.; Zubareva, O.E.; Sizov, V.V.; Lavrent’eva, V.V.; Lukomskaya, N.Y.; Kim, K.K.; Zaitsev, A.V.; Magazanik, L.G. Memantine attenuates cognitive impairments after status epilepticus induced in a lithium–pilocarpine model. Dokl. Biol. Sci. 2016, 470, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Thomas, K.L.; Lucke-Wold, B.P.; Cavendish, J.Z.; Crowe, M.S.; Matsumoto, R.R. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol. Ther. 2016, 159, 1–22. [Google Scholar] [CrossRef]
- Tetz, L.M.; Rezk, P.E.; Ratcliffe, R.H.; Gordon, R.K.; Steele, K.E.; Nambiar, M.P. Development of a rat pilocarpine model of seizure/status epilepticus that mimics chemical warfare nerve agent exposure. Toxicol. Ind. Health 2006, 22, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Mody, I.; Pearce, R.A. Diversity of inhibitory neurotransmission through GABA-A receptors. Trends Neurosci. 2004, 27, 569–575. [Google Scholar] [CrossRef]
- Lévesque, M.; Avoli, M. The kainic acid model of temporal lobe epilepsy. Neurosci. Biobehav. Rev. 2013, 37, 2887–2899. [Google Scholar] [CrossRef]
- Akeju, O.; Song, A.H.; Hamilos, A.E.; Pavone, K.J.; Flores, F.J.; Brown, E.N.; Purdon, P.L. Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin. Neurophysiol. 2016, 127, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Green, S.M.; Roback, M.G.; Kennedy, R.M.; Krauss, B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann. Emerg. Med. 2011, 57, 449–461. [Google Scholar] [CrossRef]
- Chang, L.C.; Raty, S.R.; Ortiz, J.; Bailard, N.S.; Mathew, S.J. The emerging use of ketamine for anesthesia and sedation in traumatic brain injuries. CNS Neurosci. Ther. 2013, 19, 390–395. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Teitelbaum, J.; West, M.; Gillman, L.M. The ketamine effect on intracranial pressure in nontraumatic neurological illness. J. Crit. Care 2014, 29, 1096–1106. [Google Scholar] [CrossRef]
- Gaspard, N.; Foreman, B.; Judd, L.M.; Brenton, J.N.; Nathan, B.R.; McCoy, B.M.; Al-Otaibi, A.; Kilbride, R.; Fernández, I.S.; Mendoza, L.; et al. Intravenous ketamine for the treatment of refractory status epilepticus: A retrospective multicenter study. Epilepsia 2013, 54, 1498–1503. [Google Scholar] [CrossRef]
- Bredmose, P.P.; Grier, G.; Davies, G.E.; Lockey, D.J. Pre-hospital use of ketamine in paediatric trauma. Acta Anaesthesiol. Scand. 2009, 53, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Ilvento, L.; Rosati, A.; Marini, C.; L’Erario, M.; Mirabile, L.; Guerrini, R. Ketamine in refractory convulsive status epilepticus in children avoids endotracheal intubation. Epilepsy Behav. 2015, 49, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Wellington, N.J.; Boųcas, A.P.; Lagopoulos, J.; Quigley, B.L.; Kuballa, A.V. Molecular pathways of ketamine: A systematic review of immediate and sustained effects on PTSD. Psychopharmacology 2025, 242, 1197–1243. [Google Scholar] [CrossRef]
- Yan, M.; Sun, T.; Liu, J.; Chang, Q. The efficacy and safety of ketamine in the treatment of super-refractory status epilepticus: A systematic review. J. Neurol. 2024, 271, 3942–3952. [Google Scholar] [CrossRef]
- Ubogu, E.E.; Sagar, S.M.; Lerner, A.J.; Maddux, B.N.; Suarez, J.I.; Werz, M.A. Ketamine for refractory status epilepticus: A case of possible ketamine-induced neurotoxicity. Epilepsy Behav. 2003, 4, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Prüss, H.; Holtkamp, M. Ketamine successfully terminates malignant status epilepticus. Epilepsy Res. 2008, 82, 219–222. [Google Scholar] [CrossRef]
- Strous, J.F.M.; Weeland, C.J.; van der Draai, F.A.; Daams, J.G.; Denys, D.; Lok, A.; Schoevers, R.A.; Figee, M. Brain Changes Associated With Long-Term Ketamine Abuse, A Systematic Review. Front. Neuroanat. 2022, 16, 795231. [Google Scholar] [CrossRef]
- Fujikawa, D.G. Starting ketamine for neuroprotection earlier than its current use as an anesthetic/antiepileptic drug late in refractory status epilepticus. Epilepsia 2019, 60, 373–380. [Google Scholar] [CrossRef]
- Jacobwitz, M.; Mulvihill, C.; Kaufman, M.C.; Gonzalez, A.K.; Resendiz, K.; Francoeur, C.; Helbig, I.; Topjian, A.A.; Abend, N.S. A Comparison of Ketamine and Midazolam as First-Line Anesthetic Infusions for Pediatric Status Epilepticus. Neurocrit. Care 2024, 40, 984–995. [Google Scholar] [CrossRef]
- Jacobwitz, M.; Mulvihill, C.; Kaufman, M.C.; Gonzalez, A.K.; Resendiz, K.; MacDonald, J.M.; Francoeur, C.; Helbig, I.; Topjian, A.A.; Abend, N.S. Ketamine for Management of Neonatal and Pediatric Refractory Status Epilepticus. Neurology 2022, 99, e1227–e1238. [Google Scholar] [CrossRef]
- Fletman, E.W.; Cleymaet, S.; Salvatore, A.; Devlin, K.; Pickard, A.; Shah, S.O. Ketamine plus midazolam compared to midazolam infusion for the management of refractory status epilepticus. Clin. Neurol. Neurosurg. 2024, 246, 108592. [Google Scholar] [CrossRef] [PubMed]
- Harnicher, B.; Murray, N.M.; Dresbach, J.; Collingridge, D.S.; Reachi, B.; Bair, J.; Hoang, Q.; Fontaine, G.V. Ketamine reduces seizure and interictal continuum activity in refractory status epilepticus: A multicenter in-person and teleneurocritical care study. Neurol. Sci. 2024, 45, 5449–5456. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, L.A.; Alzayadneh, M.; Metter, E.J.; Alsherbini, K. Safety and Efficacy of Ketamine Without Intubation in the Management of Refractory Seizures: A Case Series. Neurocrit. Care 2024, 40, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Parker, D.; Millis, S.; Marawar, R.; Zutshi, D.; Basha, M.M. Factors Associated with Refractory Status Epilepticus Termination Following Ketamine Initiation: A Multivariable Analysis Model. Neurocrit. Care 2023, 38, 235–241. [Google Scholar] [CrossRef]
- Höfler, J.; Trinka, E. Intravenous ketamine in status epilepticus. Epilepsia 2018, 59 (Suppl. S2), 198–206. [Google Scholar] [CrossRef]
- Dzhala, V.I.; Talos, D.M.; Sdrulla, D.A.; Brumback, A.C.; Mathews, G.C.; Benke, T.A.; Delpire, E.; Jensen, F.E.; Staley, K.J. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 2005, 11, 1205–1213. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magro, G. Ketamine in Status Epilepticus: How Soon Is Now? Neurol. Int. 2025, 17, 83. https://doi.org/10.3390/neurolint17060083
Magro G. Ketamine in Status Epilepticus: How Soon Is Now? Neurology International. 2025; 17(6):83. https://doi.org/10.3390/neurolint17060083
Chicago/Turabian StyleMagro, Giuseppe. 2025. "Ketamine in Status Epilepticus: How Soon Is Now?" Neurology International 17, no. 6: 83. https://doi.org/10.3390/neurolint17060083
APA StyleMagro, G. (2025). Ketamine in Status Epilepticus: How Soon Is Now? Neurology International, 17(6), 83. https://doi.org/10.3390/neurolint17060083