Evaluation of Sensory and Motor Function in Spinal and Bulbar Muscular Atrophy Using Quiet Stance and Reactive Postural Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Heavy Reliance on Visual Input with Sensory Control of Balance Testing
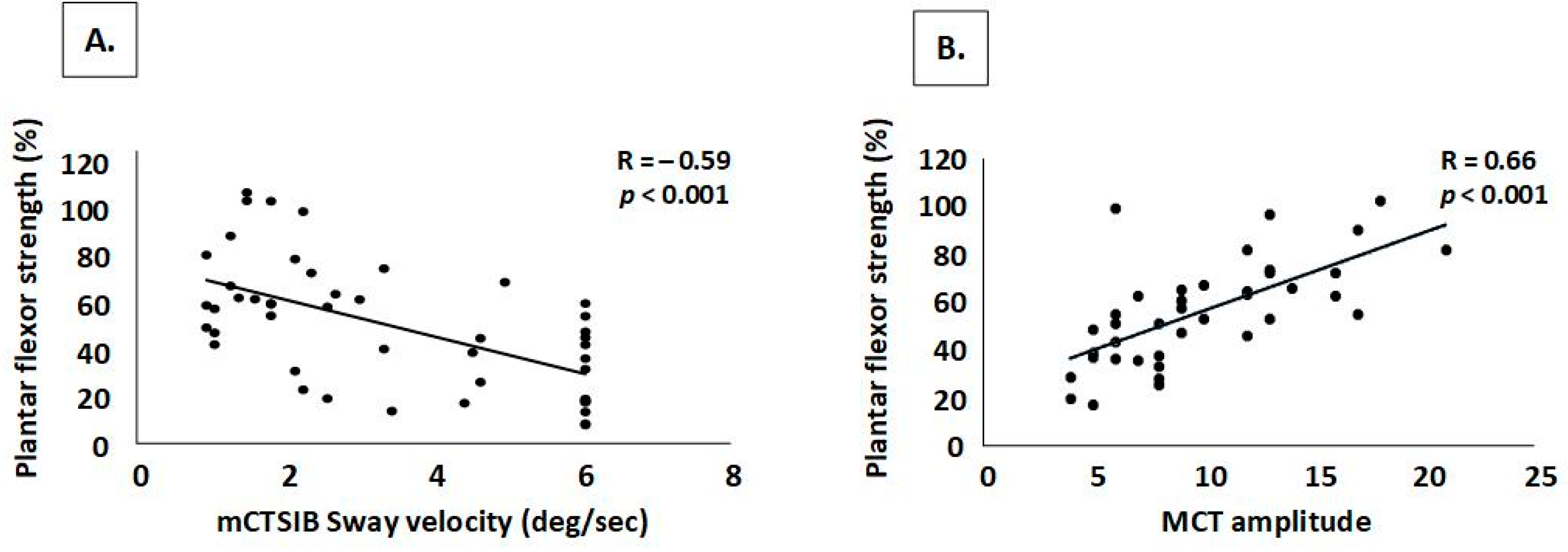
4.2. Strength and Sensory Deficits Impact Automatic Postural Reactions
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, W.R.; Alter, M.; Sung, J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 1968, 18, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.E.; Freeman, B.K.; Auh, S.; Kokkinis, A.D.; La Pean, A.; Chen, C.; Lehky, T.J.; Shrader, J.A.; Levy, E.W.; Harris-Love, M.; et al. Clinical features of spinal and bulbar muscular atrophy. Brain 2009, 132, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Shrader, J.A.; Sansare, A.; Shieh, V.; Woolstenhulme, J.G.; Rekant, J.; Jiménez-Silva, R.; Joe, G.O.; Kokkinis, A.; Fischbeck, K.H.; Grunseich, C.; et al. Dynamic Balance in Spinal and Bulbar Muscular Atrophy: Relationship between Strength and Performance of Forward Lunge, Step Up and Over, and Step Quick Turn. Rehabil. Res. Pract. 2021, 2021, 2540324. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, E.; Zachou, A.; Breza, M.; Kladi, A.; Karadima, G.; Koutsis, G. Disentangling balance impairments in spinal and bulbar muscular atrophy. Neurosci. Lett. 2019, 705, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Horak, F.B. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabil. Med. 2010, 46, 239–248. [Google Scholar] [PubMed]
- Cameron, M.H.; Horak, F.B.; Herndon, R.R.; Bourdette, D. Imbalance in multiple sclerosis: A result of slowed spinal somatosensory conduction. Somatosens. Mot. Res. 2008, 25, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.A. Balance abilities in individuals with moderate and severe traumatic brain injury. Brain Inj. 1995, 9, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Shrader, J.A.; Kats, I.; Kokkinis, A.; Zampieri, C.; Levy, E.; Joe, G.O.; Woolstenhulme, J.G.; Drinkard, B.E.; Smith, M.R.; Ching, W.; et al. A randomized controlled trial of exercise in spinal and bulbar muscular atrophy. Ann. Clin. Transl. Neurol. 2015, 2, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Fernandez-Rhodes, L.; Joe, G.; Shrader, J.A.; Kokkinis, A.; La Pean Kirschner, A.; Auh, S.; Chen, C.; Li, L.; Levy, E.; et al. Assessing function and endurance in adults with spinal and bulbar muscular atrophy: Validity of the adult myopathy assessment tool. Rehabil. Res. Pr. 2014, 2014, 873872. [Google Scholar] [CrossRef] [PubMed]
- Nashner, L.M.; Peters, J.F. Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol. Clin. 1990, 8, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Vanicek, N.; King, S.A.; Gohil, R.; Chetter, I.C.; Coughlin, P.A. Computerized dynamic posturography for postural control assessment in patients with intermittent claudication. J. Vis. Exp. 2013, 82, e51077. [Google Scholar]
- The National Isometric Muscle Strength (NIMS) Database Consortium. Muscular weakness assessment: Use of normal isometric strength data. Arch. Phys. Med. Rehabil. 1996, 77, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Portney, L. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2015. [Google Scholar]
- Peterka, R.; Loughlin, P. Dynamic regulation of sensoriomotor integration in human postural control. J. Neurophysiol. 2004, 91, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.P.; Newman, C.W.; Kartush, J.M. Handbook of Balance Function Testing, 1st ed.; Singular Publishing Group: San Diego, CA, USA, 1997. [Google Scholar]
- Robinovitch, S.N.; Heller, B.; Lui, A.; Cortez, J. Effect of strength and speed of torque development on balance recovery with the ankle strategy. J. Neurophysiol. 2002, 88, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, T.E.; Woldstad, J.C.; Smith, J.L.; Ramsey, J.D. Effects of age-related sensory degradation on perception of floor slipperiness and associated slip parameters. Saf. Sci. 2002, 40, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.T.; Horak, F.B.; Shupert, C.L.; Jones-Rycewicz, C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp. Brain Res. 1994, 101, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, L.; Ghosh, A.; Leadbetter, R.; Lance, S.; Rodrigues, M.; Roxburgh, R. Nerve ultrasound detects abnormally small nerves in patients with spinal and bulbar muscular atrophy. Muscle Nerve 2022, 65, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sobue, G.; Doyu, M.; Mukai, E.; Hashizume, Y.; Mitsuma, T. Primary sensory neurons in X-linked recessive bulbospinal neuropathy: Histopathology and androgen receptor gene expression. Muscle Nerve 1995, 18, 301–308. [Google Scholar] [CrossRef] [PubMed]
| MVIC | Mean ± SD | p Value | Cohen’s d | ES | |
|---|---|---|---|---|---|
| Muscle Group Tested | Able | Unable | |||
| N = 38 | N = 12 | ||||
| Knee extensors | 42.3 ± 22.2 | 20.1 ± 10.5 | 0.002 | 1.10 | Large |
| Ankle dorsiflexors | 45.4 ± 18.3 | 30.2 ± 14.6 | 0.012 | 0.87 | Large |
| Ankle plantarflexors | 59.3 ± 22.1 | 25.8 ± 18.5 | <0.001 | 1.57 | Large |
| Hip Extensors | 102.6 ± 38.4 | 77.3 ± 23.5 | 0.045 | 0.71 | Moderate |
| Lower extremity composite | 62.0 ± 19.4 | 37.4 ± 13.6 | <0.001 | 1.35 | Large |
| Test (Unit of Measure) | Age (Years) | Condition | Mean ± SD | p Value | Cohen’s d | ES | |
|---|---|---|---|---|---|---|---|
| Patient | Control | ||||||
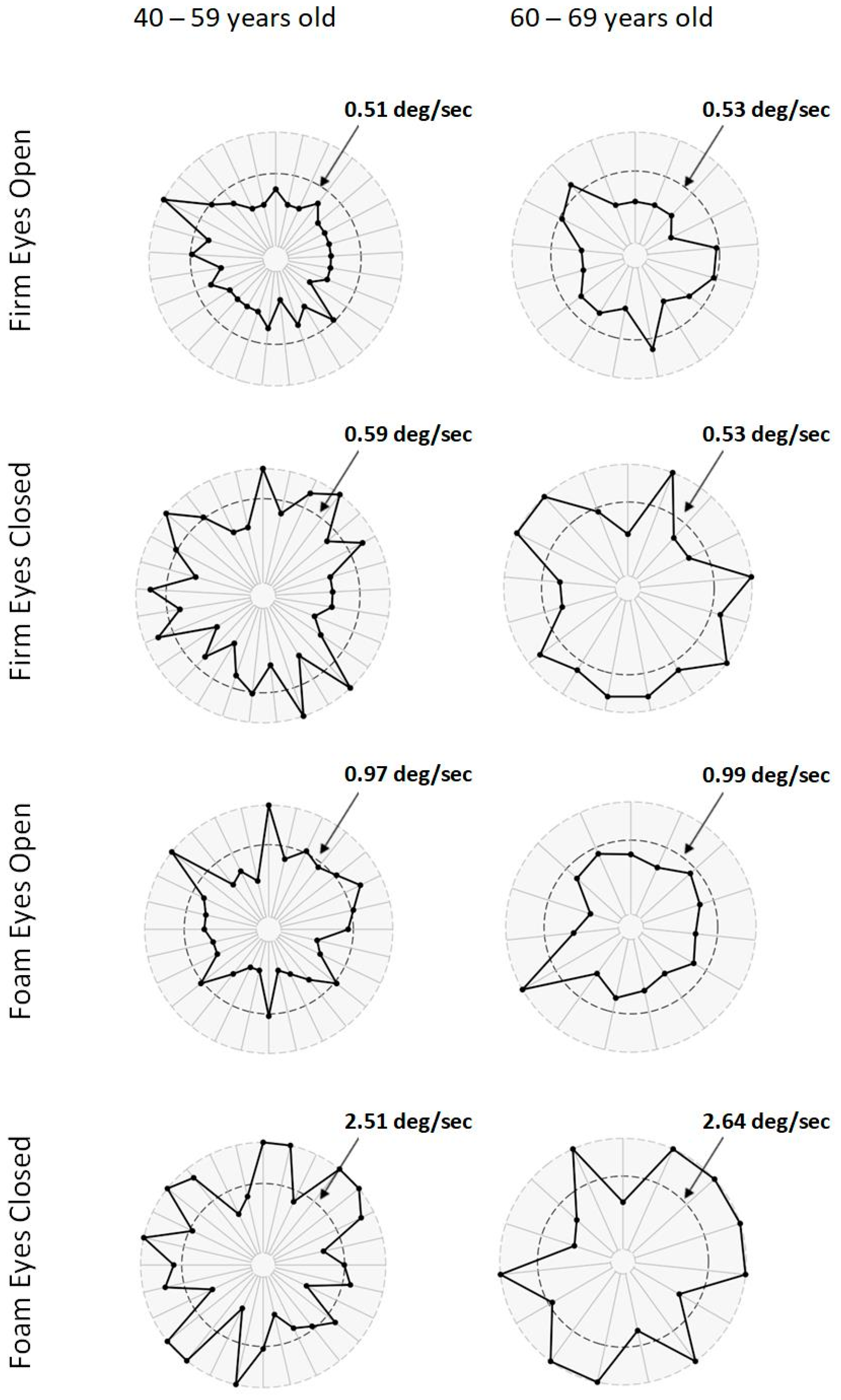
| mCTSIB | 40–59 | N = 29 | N = 29 | ||||
| (degrees/second) | Firm Eyes Open | 0.36 ± 0.12 | 0.27 ± 0.12 | 0.003 | 0.72 | Medium | |
| Firm Eyes Closed | 0.78 ± 0.99 | 0.33 ± 0.13 | 0.020 | 0.73 | Medium | ||
| Foam Eyes Open | 1.05 ± 1.20 | 0.63 ± 0.17 | 0.076 | 0.57 | Medium | ||
| Foam Eyes Closed | 3.40 ± 1.74 | 1.61 ± 0.45 | 0.000 | 1.60 | Large | ||
| 60–69 | N = 17 | N = 26 | |||||
| (degrees/second) | Firm Eyes Open | 0.38 ± 0.12 | 0.28 ± 0.12 | 0.009 | 0.86 | Large | |
| Firm Eyes Closed | 0.68 ± 0.30 | 0.31 ± 0.11 | 0.000 | 1.80 | Large | ||
| Foam Eyes Open | 0.83 ± 0.45 | 0.69 ± 0.15 | 0.252 | 0.48 | Small | ||
| Foam Eyes Closed | 4.11 ± 1.94 | 1.60 ± 0.52 | 0.000 | 2.03 | Large | ||
| MCT | 20–59 | N = 25 | N = 29 | ||||
| (milliseconds) | Latency Left | 143.6 ± 11.9 | 117.0 ± 19.8 | 0.000 | 1.60 | Large | |
| Latency Right | 140.0 ± 13.5 | 117.0 ± 19.8 | 0.000 | 1.34 | Large | ||
| (unitless) | Strength Left | 10.92 ± 4.43 | 8.60 ± 4.30 | 0.057 | 0.53 | Medium | |
| Strength Right | 9.24 ± 3.53 | 8.60 ± 4.30 | 0.550 | 0.16 | Small | ||
| 60–69 | N = 11 | N = 54 | |||||
| (milliseconds) | Latency Left | 150.0 ± 14.8 | 124.0 ± 15.6 | 0.000 | 1.68 | Large | |
| Latency Right | 152.7 ± 14.2 | 124.0 ± 15.6 | 0.000 | 1.87 | Large | ||
| (unitless) | Strength Left | 8.27 ± 4.03 | 9.90 ± 3.40 | 0.231 | 0.47 | Small | |
| Strength Right | 7.73 ± 4.15 | 9.90 ± 3.40 | 0.127 | 0.62 | Medium | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrader, J.A.; Sansare, A.; Niemic, A.C.; Jiménez-Silva, R.; Woolstenhulme, J.G.; Joe, G.O.; Jacobs, U.; Kokkinis, A.; Fischbeck, K.; Grunseich, C.; et al. Evaluation of Sensory and Motor Function in Spinal and Bulbar Muscular Atrophy Using Quiet Stance and Reactive Postural Control. Neurol. Int. 2025, 17, 79. https://doi.org/10.3390/neurolint17060079
Shrader JA, Sansare A, Niemic AC, Jiménez-Silva R, Woolstenhulme JG, Joe GO, Jacobs U, Kokkinis A, Fischbeck K, Grunseich C, et al. Evaluation of Sensory and Motor Function in Spinal and Bulbar Muscular Atrophy Using Quiet Stance and Reactive Postural Control. Neurology International. 2025; 17(6):79. https://doi.org/10.3390/neurolint17060079
Chicago/Turabian StyleShrader, Joseph A., Ashwini Sansare, Allison C. Niemic, Rafael Jiménez-Silva, Joshua G. Woolstenhulme, Galen O. Joe, Uma Jacobs, Angela Kokkinis, Kenneth Fischbeck, Chris Grunseich, and et al. 2025. "Evaluation of Sensory and Motor Function in Spinal and Bulbar Muscular Atrophy Using Quiet Stance and Reactive Postural Control" Neurology International 17, no. 6: 79. https://doi.org/10.3390/neurolint17060079
APA StyleShrader, J. A., Sansare, A., Niemic, A. C., Jiménez-Silva, R., Woolstenhulme, J. G., Joe, G. O., Jacobs, U., Kokkinis, A., Fischbeck, K., Grunseich, C., & Zampieri, C. (2025). Evaluation of Sensory and Motor Function in Spinal and Bulbar Muscular Atrophy Using Quiet Stance and Reactive Postural Control. Neurology International, 17(6), 79. https://doi.org/10.3390/neurolint17060079






